 Found 57 hits Enz. Inhib. hit(s) with all data for entry = 50033010
Found 57 hits Enz. Inhib. hit(s) with all data for entry = 50033010 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50338033
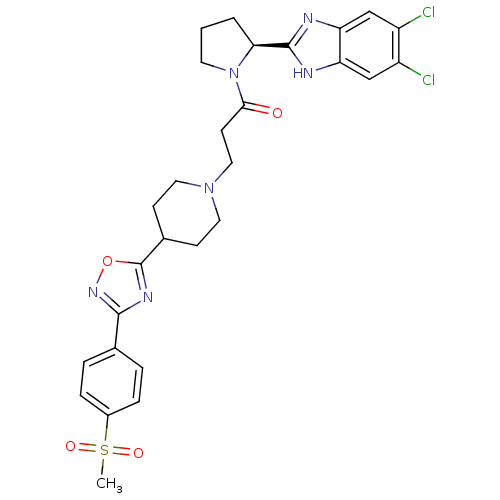
((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1noc(n1)C1CCN(CCC(=O)N2CCC[C@H]2c2nc3cc(Cl)c(Cl)cc3[nH]2)CC1 |r| Show InChI InChI=1S/C28H30Cl2N6O4S/c1-41(38,39)19-6-4-17(5-7-19)26-33-28(40-34-26)18-8-12-35(13-9-18)14-10-25(37)36-11-2-3-24(36)27-31-22-15-20(29)21(30)16-23(22)32-27/h4-7,15-16,18,24H,2-3,8-14H2,1H3,(H,31,32)/t24-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50338029
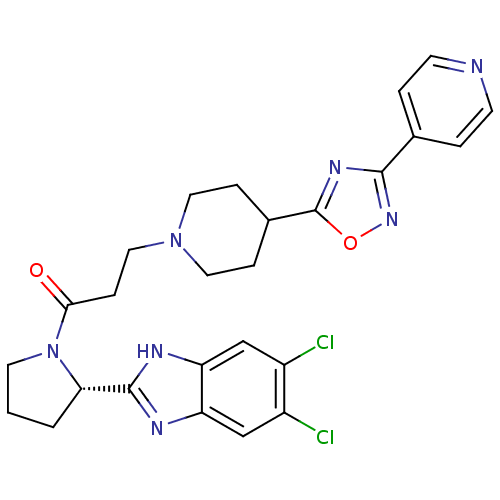
((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES Clc1cc2nc([nH]c2cc1Cl)[C@@H]1CCCN1C(=O)CCN1CCC(CC1)c1nc(no1)-c1ccncc1 |r| Show InChI InChI=1S/C26H27Cl2N7O2/c27-18-14-20-21(15-19(18)28)31-25(30-20)22-2-1-10-35(22)23(36)7-13-34-11-5-17(6-12-34)26-32-24(33-37-26)16-3-8-29-9-4-16/h3-4,8-9,14-15,17,22H,1-2,5-7,10-13H2,(H,30,31)/t22-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50338007

((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES Cn1nc(cc1-c1ccncc1)C1CCN(CCC(=O)N2CCC[C@H]2c2nc3cc(Cl)c(Cl)cc3[nH]2)CC1 |r| Show InChI InChI=1S/C28H31Cl2N7O/c1-35-26(19-4-9-31-10-5-19)17-22(34-35)18-6-12-36(13-7-18)14-8-27(38)37-11-2-3-25(37)28-32-23-15-20(29)21(30)16-24(23)33-28/h4-5,9-10,15-18,25H,2-3,6-8,11-14H2,1H3,(H,32,33)/t25-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338007

((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES Cn1nc(cc1-c1ccncc1)C1CCN(CCC(=O)N2CCC[C@H]2c2nc3cc(Cl)c(Cl)cc3[nH]2)CC1 |r| Show InChI InChI=1S/C28H31Cl2N7O/c1-35-26(19-4-9-31-10-5-19)17-22(34-35)18-6-12-36(13-7-18)14-8-27(38)37-11-2-3-25(37)28-32-23-15-20(29)21(30)16-24(23)33-28/h4-5,9-10,15-18,25H,2-3,6-8,11-14H2,1H3,(H,32,33)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338029

((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES Clc1cc2nc([nH]c2cc1Cl)[C@@H]1CCCN1C(=O)CCN1CCC(CC1)c1nc(no1)-c1ccncc1 |r| Show InChI InChI=1S/C26H27Cl2N7O2/c27-18-14-20-21(15-19(18)28)31-25(30-20)22-2-1-10-35(22)23(36)7-13-34-11-5-17(6-12-34)26-32-24(33-37-26)16-3-8-29-9-4-16/h3-4,8-9,14-15,17,22H,1-2,5-7,10-13H2,(H,30,31)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338033

((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1noc(n1)C1CCN(CCC(=O)N2CCC[C@H]2c2nc3cc(Cl)c(Cl)cc3[nH]2)CC1 |r| Show InChI InChI=1S/C28H30Cl2N6O4S/c1-41(38,39)19-6-4-17(5-7-19)26-33-28(40-34-26)18-8-12-35(13-9-18)14-10-25(37)36-11-2-3-24(36)27-31-22-15-20(29)21(30)16-23(22)32-27/h4-7,15-16,18,24H,2-3,8-14H2,1H3,(H,31,32)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50338027
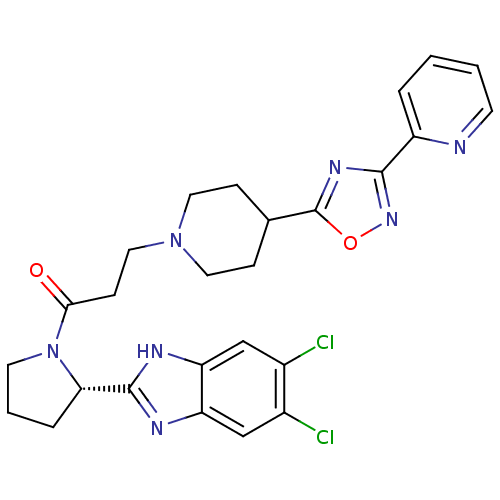
((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES Clc1cc2nc([nH]c2cc1Cl)[C@@H]1CCCN1C(=O)CCN1CCC(CC1)c1nc(no1)-c1ccccn1 |r| Show InChI InChI=1S/C26H27Cl2N7O2/c27-17-14-20-21(15-18(17)28)31-25(30-20)22-5-3-10-35(22)23(36)8-13-34-11-6-16(7-12-34)26-32-24(33-37-26)19-4-1-2-9-29-19/h1-2,4,9,14-16,22H,3,5-8,10-13H2,(H,30,31)/t22-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338032
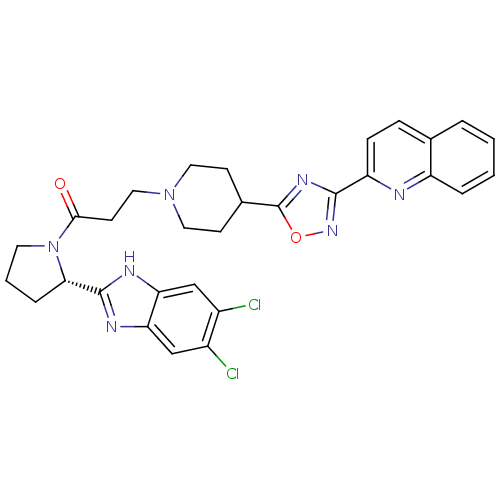
((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES Clc1cc2nc([nH]c2cc1Cl)[C@@H]1CCCN1C(=O)CCN1CCC(CC1)c1nc(no1)-c1ccc2ccccc2n1 |r| Show InChI InChI=1S/C30H29Cl2N7O2/c31-20-16-24-25(17-21(20)32)35-29(34-24)26-6-3-12-39(26)27(40)11-15-38-13-9-19(10-14-38)30-36-28(37-41-30)23-8-7-18-4-1-2-5-22(18)33-23/h1-2,4-5,7-8,16-17,19,26H,3,6,9-15H2,(H,34,35)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338035
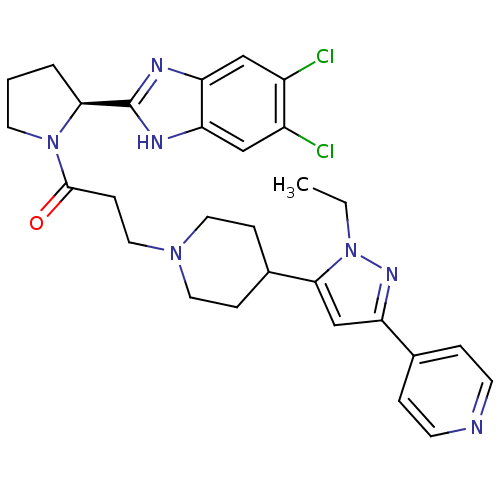
((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES CCn1nc(cc1C1CCN(CCC(=O)N2CCC[C@H]2c2nc3cc(Cl)c(Cl)cc3[nH]2)CC1)-c1ccncc1 |r| Show InChI InChI=1S/C29H33Cl2N7O/c1-2-38-27(18-23(35-38)19-5-10-32-11-6-19)20-7-13-36(14-8-20)15-9-28(39)37-12-3-4-26(37)29-33-24-16-21(30)22(31)17-25(24)34-29/h5-6,10-11,16-18,20,26H,2-4,7-9,12-15H2,1H3,(H,33,34)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50338007

((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES Cn1nc(cc1-c1ccncc1)C1CCN(CCC(=O)N2CCC[C@H]2c2nc3cc(Cl)c(Cl)cc3[nH]2)CC1 |r| Show InChI InChI=1S/C28H31Cl2N7O/c1-35-26(19-4-9-31-10-5-19)17-22(34-35)18-6-12-36(13-7-18)14-8-27(38)37-11-2-3-25(37)28-32-23-15-20(29)21(30)16-24(23)33-28/h4-5,9-10,15-18,25H,2-3,6-8,11-14H2,1H3,(H,32,33)/t25-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PrCP in mouse plasma assessed as angiotensin 3 cleavage |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338037

((S)-3-(4-(3-(benzo[d][1,3]dioxol-5-ylmethyl)-1-eth...)Show SMILES CCn1nc(Cc2ccc3OCOc3c2)cc1C1CCN(CCC(=O)N2CCC[C@H]2c2nc3cc(Cl)c(Cl)cc3[nH]2)CC1 |r| Show InChI InChI=1S/C32H36Cl2N6O3/c1-2-40-28(16-22(37-40)14-20-5-6-29-30(15-20)43-19-42-29)21-7-11-38(12-8-21)13-9-31(41)39-10-3-4-27(39)32-35-25-17-23(33)24(34)18-26(25)36-32/h5-6,15-18,21,27H,2-4,7-14,19H2,1H3,(H,35,36)/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338005
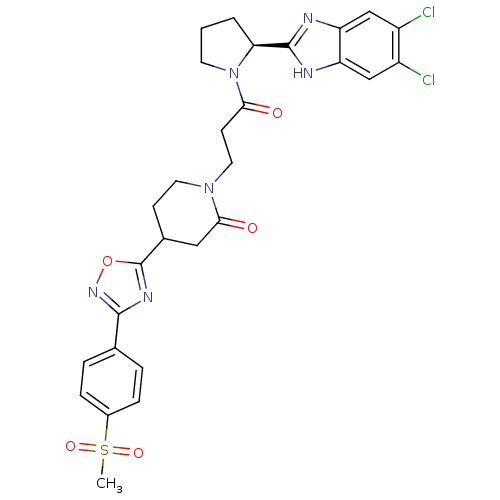
(1-(3-((S)-2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1noc(n1)C1CCN(CCC(=O)N2CCC[C@H]2c2nc3cc(Cl)c(Cl)cc3[nH]2)C(=O)C1 |r| Show InChI InChI=1S/C28H28Cl2N6O5S/c1-42(39,40)18-6-4-16(5-7-18)26-33-28(41-34-26)17-8-11-35(25(38)13-17)12-9-24(37)36-10-2-3-23(36)27-31-21-14-19(29)20(30)15-22(21)32-27/h4-7,14-15,17,23H,2-3,8-13H2,1H3,(H,31,32)/t17?,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338010
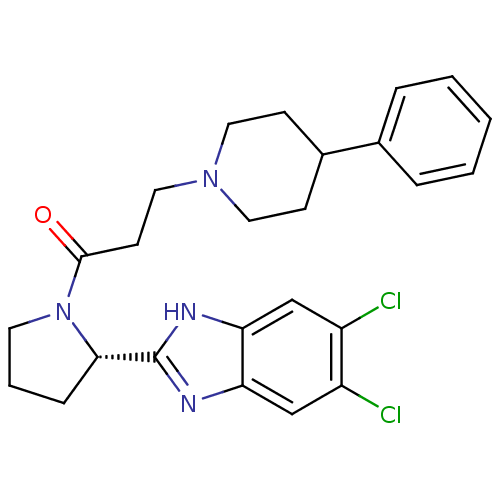
((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES Clc1cc2nc([nH]c2cc1Cl)[C@@H]1CCCN1C(=O)CCN1CCC(CC1)c1ccccc1 |r| Show InChI InChI=1S/C25H28Cl2N4O/c26-19-15-21-22(16-20(19)27)29-25(28-21)23-7-4-11-31(23)24(32)10-14-30-12-8-18(9-13-30)17-5-2-1-3-6-17/h1-3,5-6,15-16,18,23H,4,7-14H2,(H,28,29)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50338033

((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1noc(n1)C1CCN(CCC(=O)N2CCC[C@H]2c2nc3cc(Cl)c(Cl)cc3[nH]2)CC1 |r| Show InChI InChI=1S/C28H30Cl2N6O4S/c1-41(38,39)19-6-4-17(5-7-19)26-33-28(40-34-26)18-8-12-35(13-9-18)14-10-25(37)36-11-2-3-24(36)27-31-22-15-20(29)21(30)16-23(22)32-27/h4-7,15-16,18,24H,2-3,8-14H2,1H3,(H,31,32)/t24-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PrCP in mouse plasma assessed as angiotensin 3 cleavage |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338027

((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES Clc1cc2nc([nH]c2cc1Cl)[C@@H]1CCCN1C(=O)CCN1CCC(CC1)c1nc(no1)-c1ccccn1 |r| Show InChI InChI=1S/C26H27Cl2N7O2/c27-17-14-20-21(15-18(17)28)31-25(30-20)22-5-3-10-35(22)23(36)8-13-34-11-6-16(7-12-34)26-32-24(33-37-26)19-4-1-2-9-29-19/h1-2,4,9,14-16,22H,3,5-8,10-13H2,(H,30,31)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338030
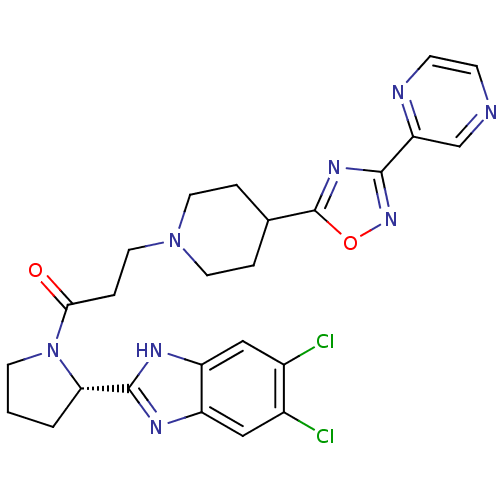
((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES Clc1cc2nc([nH]c2cc1Cl)[C@@H]1CCCN1C(=O)CCN1CCC(CC1)c1nc(no1)-c1cnccn1 |r| Show InChI InChI=1S/C25H26Cl2N8O2/c26-16-12-18-19(13-17(16)27)31-24(30-18)21-2-1-8-35(21)22(36)5-11-34-9-3-15(4-10-34)25-32-23(33-37-25)20-14-28-6-7-29-20/h6-7,12-15,21H,1-5,8-11H2,(H,30,31)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338006

((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES CC(C)(CCCC(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1)N1CCC(CC1)c1nc(no1)-c1ccccn1 |r| Show InChI InChI=1S/C30H35Cl2N7O2/c1-30(2,38-15-10-19(11-16-38)29-36-27(37-41-29)22-7-3-4-13-33-22)12-5-9-26(40)39-14-6-8-25(39)28-34-23-17-20(31)21(32)18-24(23)35-28/h3-4,7,13,17-19,25H,5-6,8-12,14-16H2,1-2H3,(H,34,35)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338013
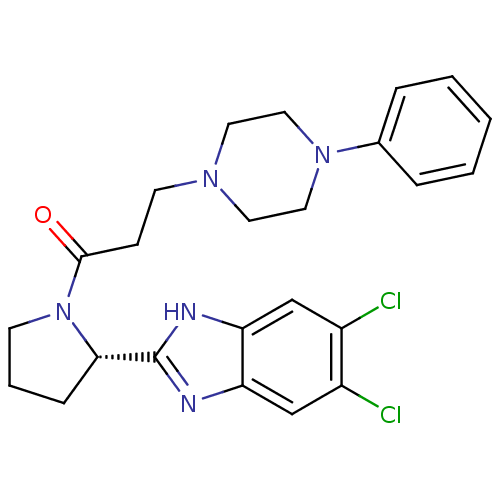
((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES Clc1cc2nc([nH]c2cc1Cl)[C@@H]1CCCN1C(=O)CCN1CCN(CC1)c1ccccc1 |r| Show InChI InChI=1S/C24H27Cl2N5O/c25-18-15-20-21(16-19(18)26)28-24(27-20)22-7-4-9-31(22)23(32)8-10-29-11-13-30(14-12-29)17-5-2-1-3-6-17/h1-3,5-6,15-16,22H,4,7-14H2,(H,27,28)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338034
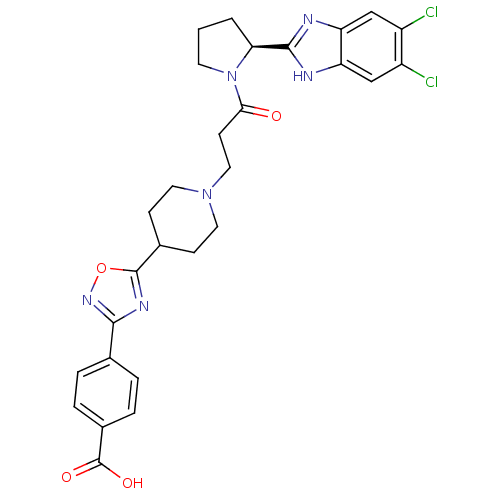
((S)-4-(5-(1-(3-(2-(5,6-dichloro-1H-benzo[d]imidazo...)Show SMILES OC(=O)c1ccc(cc1)-c1noc(n1)C1CCN(CCC(=O)N2CCC[C@H]2c2nc3cc(Cl)c(Cl)cc3[nH]2)CC1 |r| Show InChI InChI=1S/C28H28Cl2N6O4/c29-19-14-21-22(15-20(19)30)32-26(31-21)23-2-1-10-36(23)24(37)9-13-35-11-7-17(8-12-35)27-33-25(34-40-27)16-3-5-18(6-4-16)28(38)39/h3-6,14-15,17,23H,1-2,7-13H2,(H,31,32)(H,38,39)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338014
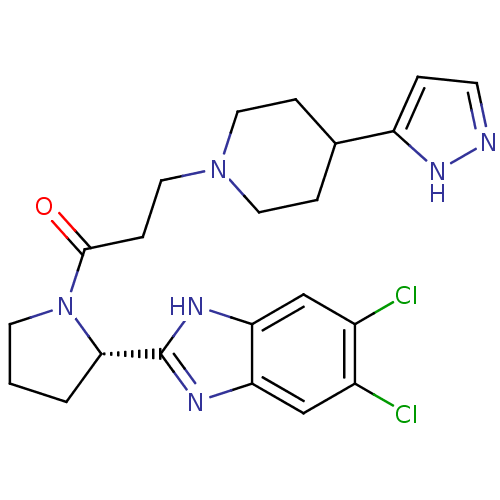
((S)-3-(4-(1H-pyrazol-3-yl)piperidin-1-yl)-1-(2-(5,...)Show SMILES Clc1cc2nc([nH]c2cc1Cl)[C@@H]1CCCN1C(=O)CCN1CCC(CC1)c1ccn[nH]1 |r| Show InChI InChI=1S/C22H26Cl2N6O/c23-15-12-18-19(13-16(15)24)27-22(26-18)20-2-1-8-30(20)21(31)6-11-29-9-4-14(5-10-29)17-3-7-25-28-17/h3,7,12-14,20H,1-2,4-6,8-11H2,(H,25,28)(H,26,27)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338028

((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES Clc1cc2nc([nH]c2cc1Cl)[C@@H]1CCCN1C(=O)CCN1CCC(CC1)c1nc(no1)-c1cccnc1 |r| Show InChI InChI=1S/C26H27Cl2N7O2/c27-18-13-20-21(14-19(18)28)31-25(30-20)22-4-2-9-35(22)23(36)7-12-34-10-5-16(6-11-34)26-32-24(33-37-26)17-3-1-8-29-15-17/h1,3,8,13-16,22H,2,4-7,9-12H2,(H,30,31)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50338006

((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES CC(C)(CCCC(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1)N1CCC(CC1)c1nc(no1)-c1ccccn1 |r| Show InChI InChI=1S/C30H35Cl2N7O2/c1-30(2,38-15-10-19(11-16-38)29-36-27(37-41-29)22-7-3-4-13-33-22)12-5-9-26(40)39-14-6-8-25(39)28-34-23-17-20(31)21(32)18-24(23)35-28/h3-4,7,13,17-19,25H,5-6,8-12,14-16H2,1-2H3,(H,34,35)/t25-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338031

((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES Clc1cc2nc([nH]c2cc1Cl)[C@@H]1CCCN1C(=O)CCN1CCC(CC1)c1nc(no1)-c1ncccn1 |r| Show InChI InChI=1S/C25H26Cl2N8O2/c26-16-13-18-19(14-17(16)27)31-22(30-18)20-3-1-9-35(20)21(36)6-12-34-10-4-15(5-11-34)25-32-24(33-37-25)23-28-7-2-8-29-23/h2,7-8,13-15,20H,1,3-6,9-12H2,(H,30,31)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338023

((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES Clc1cc2nc([nH]c2cc1Cl)[C@@H]1CCCN1C(=O)CCN1CCC(CC1)c1ccncc1 |r| Show InChI InChI=1S/C24H27Cl2N5O/c25-18-14-20-21(15-19(18)26)29-24(28-20)22-2-1-10-31(22)23(32)7-13-30-11-5-17(6-12-30)16-3-8-27-9-4-16/h3-4,8-9,14-15,17,22H,1-2,5-7,10-13H2,(H,28,29)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338016

((S)-3-(4-(1H-pyrazol-4-yl)piperidin-1-yl)-1-(2-(5,...)Show SMILES Clc1cc2nc([nH]c2cc1Cl)[C@@H]1CCCN1C(=O)CCN1CCC(CC1)c1cn[nH]c1 |r| Show InChI InChI=1S/C22H26Cl2N6O/c23-16-10-18-19(11-17(16)24)28-22(27-18)20-2-1-6-30(20)21(31)5-9-29-7-3-14(4-8-29)15-12-25-26-13-15/h10-14,20H,1-9H2,(H,25,26)(H,27,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338026
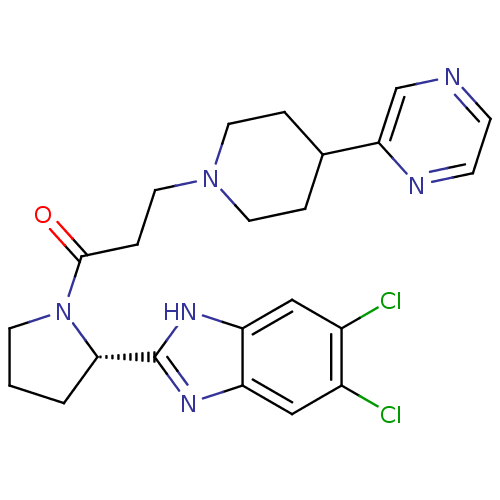
((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES Clc1cc2nc([nH]c2cc1Cl)[C@@H]1CCCN1C(=O)CCN1CCC(CC1)c1cnccn1 |r| Show InChI InChI=1S/C23H26Cl2N6O/c24-16-12-18-19(13-17(16)25)29-23(28-18)21-2-1-8-31(21)22(32)5-11-30-9-3-15(4-10-30)20-14-26-6-7-27-20/h6-7,12-15,21H,1-5,8-11H2,(H,28,29)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338036
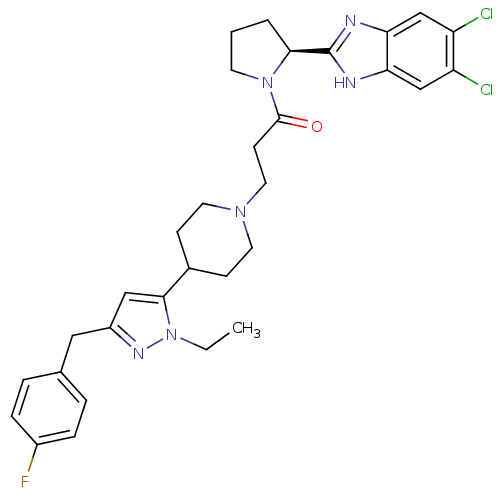
((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES CCn1nc(Cc2ccc(F)cc2)cc1C1CCN(CCC(=O)N2CCC[C@H]2c2nc3cc(Cl)c(Cl)cc3[nH]2)CC1 |r| Show InChI InChI=1S/C31H35Cl2FN6O/c1-2-40-29(17-23(37-40)16-20-5-7-22(34)8-6-20)21-9-13-38(14-10-21)15-11-30(41)39-12-3-4-28(39)31-35-26-18-24(32)25(33)19-27(26)36-31/h5-8,17-19,21,28H,2-4,9-16H2,1H3,(H,35,36)/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338011
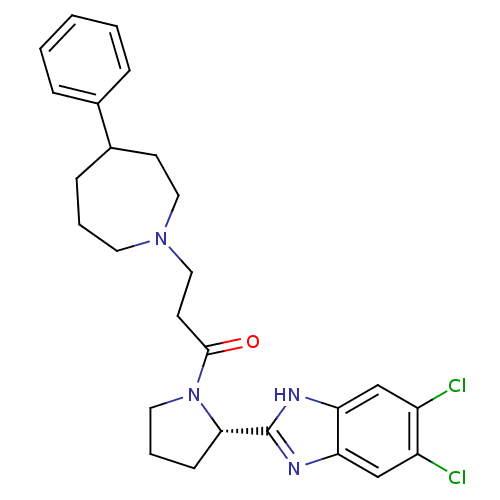
(1-((S)-2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES Clc1cc2nc([nH]c2cc1Cl)[C@@H]1CCCN1C(=O)CCN1CCCC(CC1)c1ccccc1 |r| Show InChI InChI=1S/C26H30Cl2N4O/c27-20-16-22-23(17-21(20)28)30-26(29-22)24-9-5-13-32(24)25(33)11-15-31-12-4-8-19(10-14-31)18-6-2-1-3-7-18/h1-3,6-7,16-17,19,24H,4-5,8-15H2,(H,29,30)/t19?,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338009
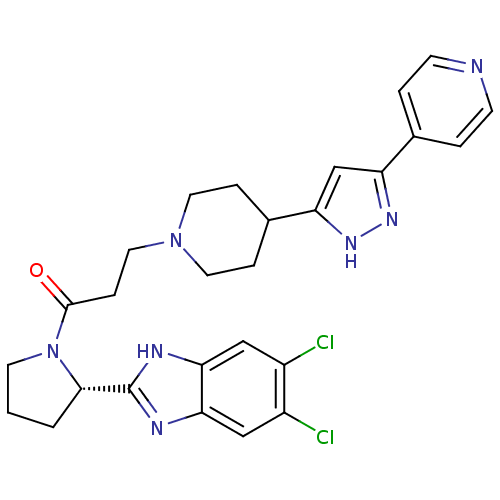
((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES Clc1cc2nc([nH]c2cc1Cl)[C@@H]1CCCN1C(=O)CCN1CCC(CC1)c1cc(n[nH]1)-c1ccncc1 |r| Show InChI InChI=1S/C27H29Cl2N7O/c28-19-14-23-24(15-20(19)29)32-27(31-23)25-2-1-10-36(25)26(37)7-13-35-11-5-18(6-12-35)22-16-21(33-34-22)17-3-8-30-9-4-17/h3-4,8-9,14-16,18,25H,1-2,5-7,10-13H2,(H,31,32)(H,33,34)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50338029

((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES Clc1cc2nc([nH]c2cc1Cl)[C@@H]1CCCN1C(=O)CCN1CCC(CC1)c1nc(no1)-c1ccncc1 |r| Show InChI InChI=1S/C26H27Cl2N7O2/c27-18-14-20-21(15-19(18)28)31-25(30-20)22-2-1-10-35(22)23(36)7-13-34-11-5-17(6-12-34)26-32-24(33-37-26)16-3-8-29-9-4-16/h3-4,8-9,14-15,17,22H,1-2,5-7,10-13H2,(H,30,31)/t22-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PrCP in mouse plasma assessed as angiotensin 3 cleavage |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338021

((S)-3-(4-(2H-tetrazol-2-yl)piperidin-1-yl)-1-(2-(5...)Show SMILES Clc1cc2nc([nH]c2cc1Cl)[C@@H]1CCCN1C(=O)CCN1CCC(CC1)n1ncnn1 |r| Show InChI InChI=1S/C20H24Cl2N8O/c21-14-10-16-17(11-15(14)22)26-20(25-16)18-2-1-6-29(18)19(31)5-9-28-7-3-13(4-8-28)30-24-12-23-27-30/h10-13,18H,1-9H2,(H,25,26)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338008
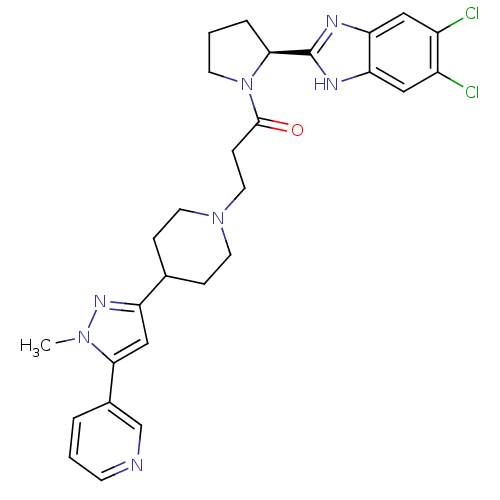
((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES Cn1nc(cc1-c1cccnc1)C1CCN(CCC(=O)N2CCC[C@H]2c2nc3cc(Cl)c(Cl)cc3[nH]2)CC1 |r| Show InChI InChI=1S/C28H31Cl2N7O/c1-35-26(19-4-2-9-31-17-19)16-22(34-35)18-6-11-36(12-7-18)13-8-27(38)37-10-3-5-25(37)28-32-23-14-20(29)21(30)15-24(23)33-28/h2,4,9,14-18,25H,3,5-8,10-13H2,1H3,(H,32,33)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338015

((S)-3-(4-(1H-pyrazol-1-yl)piperidin-1-yl)-1-(2-(5,...)Show SMILES Clc1cc2nc([nH]c2cc1Cl)[C@@H]1CCCN1C(=O)CCN1CCC(CC1)n1cccn1 |r| Show InChI InChI=1S/C22H26Cl2N6O/c23-16-13-18-19(14-17(16)24)27-22(26-18)20-3-1-8-29(20)21(31)6-12-28-10-4-15(5-11-28)30-9-2-7-25-30/h2,7,9,13-15,20H,1,3-6,8,10-12H2,(H,26,27)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50338008

((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES Cn1nc(cc1-c1cccnc1)C1CCN(CCC(=O)N2CCC[C@H]2c2nc3cc(Cl)c(Cl)cc3[nH]2)CC1 |r| Show InChI InChI=1S/C28H31Cl2N7O/c1-35-26(19-4-2-9-31-17-19)16-22(34-35)18-6-11-36(12-7-18)13-8-27(38)37-10-3-5-25(37)28-32-23-14-20(29)21(30)15-24(23)33-28/h2,4,9,14-18,25H,3,5-8,10-13H2,1H3,(H,32,33)/t25-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PrCP in mouse plasma assessed as angiotensin 3 cleavage |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338019
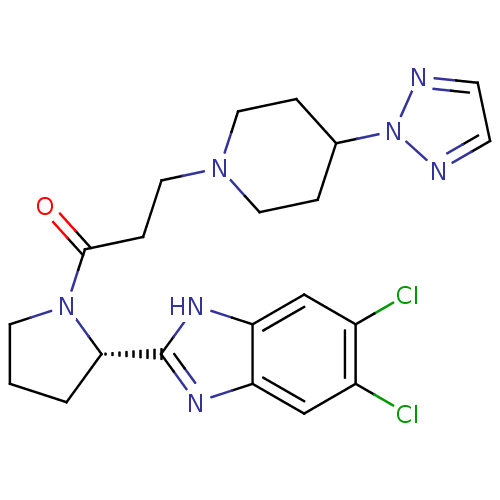
((S)-3-(4-(2H-1,2,3-triazol-2-yl)piperidin-1-yl)-1-...)Show SMILES Clc1cc2nc([nH]c2cc1Cl)[C@@H]1CCCN1C(=O)CCN1CCC(CC1)n1nccn1 |r| Show InChI InChI=1S/C21H25Cl2N7O/c22-15-12-17-18(13-16(15)23)27-21(26-17)19-2-1-8-29(19)20(31)5-11-28-9-3-14(4-10-28)30-24-6-7-25-30/h6-7,12-14,19H,1-5,8-11H2,(H,26,27)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338025

((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES Clc1cc2nc([nH]c2cc1Cl)[C@@H]1CCCN1C(=O)CCN1CCC(CC1)c1ncccn1 |r| Show InChI InChI=1S/C23H26Cl2N6O/c24-16-13-18-19(14-17(16)25)29-23(28-18)20-3-1-9-31(20)21(32)6-12-30-10-4-15(5-11-30)22-26-7-2-8-27-22/h2,7-8,13-15,20H,1,3-6,9-12H2,(H,28,29)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338022

((S)-3-(4-(1,2,4-oxadiazol-3-yl)piperidin-1-yl)-1-(...)Show SMILES Clc1cc2nc([nH]c2cc1Cl)[C@@H]1CCCN1C(=O)CCN1CCC(CC1)c1ncon1 |r| Show InChI InChI=1S/C21H24Cl2N6O2/c22-14-10-16-17(11-15(14)23)26-21(25-16)18-2-1-6-29(18)19(30)5-9-28-7-3-13(4-8-28)20-24-12-31-27-20/h10-13,18H,1-9H2,(H,25,26)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338020

((S)-3-(4-(1H-1,2,4-triazol-1-yl)piperidin-1-yl)-1-...)Show SMILES Clc1cc2nc([nH]c2cc1Cl)[C@@H]1CCCN1C(=O)CCN1CCC(CC1)n1cncn1 |r| Show InChI InChI=1S/C21H25Cl2N7O/c22-15-10-17-18(11-16(15)23)27-21(26-17)19-2-1-6-29(19)20(31)5-9-28-7-3-14(4-8-28)30-13-24-12-25-30/h10-14,19H,1-9H2,(H,26,27)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338024
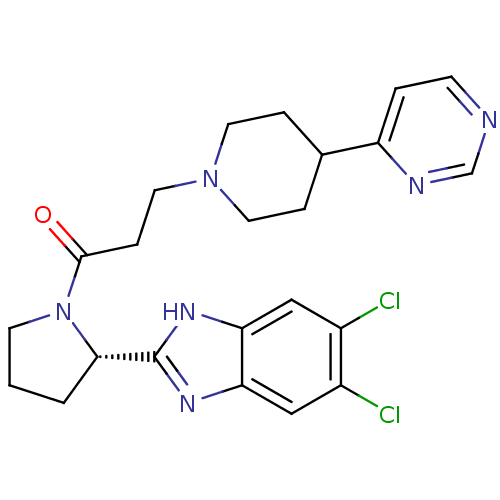
((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES Clc1cc2nc([nH]c2cc1Cl)[C@@H]1CCCN1C(=O)CCN1CCC(CC1)c1ccncn1 |r| Show InChI InChI=1S/C23H26Cl2N6O/c24-16-12-19-20(13-17(16)25)29-23(28-19)21-2-1-8-31(21)22(32)6-11-30-9-4-15(5-10-30)18-3-7-26-14-27-18/h3,7,12-15,21H,1-2,4-6,8-11H2,(H,28,29)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50338016

((S)-3-(4-(1H-pyrazol-4-yl)piperidin-1-yl)-1-(2-(5,...)Show SMILES Clc1cc2nc([nH]c2cc1Cl)[C@@H]1CCCN1C(=O)CCN1CCC(CC1)c1cn[nH]c1 |r| Show InChI InChI=1S/C22H26Cl2N6O/c23-16-10-18-19(11-17(16)24)28-22(27-18)20-2-1-6-30(20)21(31)5-9-29-7-3-14(4-8-29)15-12-25-26-13-15/h10-14,20H,1-9H2,(H,25,26)(H,27,28)/t20-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50338006

((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES CC(C)(CCCC(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1)N1CCC(CC1)c1nc(no1)-c1ccccn1 |r| Show InChI InChI=1S/C30H35Cl2N7O2/c1-30(2,38-15-10-19(11-16-38)29-36-27(37-41-29)22-7-3-4-13-33-22)12-5-9-26(40)39-14-6-8-25(39)28-34-23-17-20(31)21(32)18-24(23)35-28/h3-4,7,13,17-19,25H,5-6,8-12,14-16H2,1-2H3,(H,34,35)/t25-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PrCP in mouse plasma assessed as angiotensin 3 cleavage |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338017
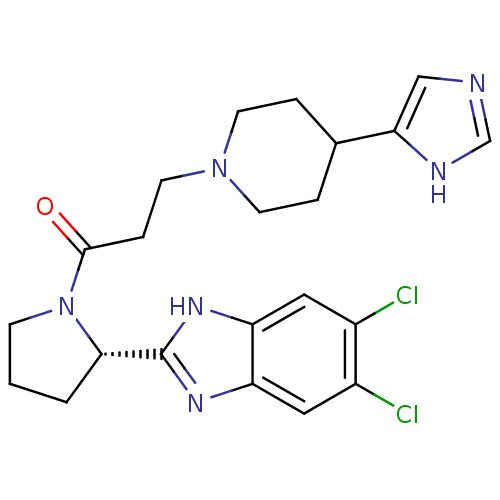
((S)-3-(4-(1H-imidazol-4-yl)piperidin-1-yl)-1-(2-(5...)Show SMILES Clc1cc2nc([nH]c2cc1Cl)[C@@H]1CCCN1C(=O)CCN1CCC(CC1)c1cnc[nH]1 |r| Show InChI InChI=1S/C22H26Cl2N6O/c23-15-10-17-18(11-16(15)24)28-22(27-17)20-2-1-6-30(20)21(31)5-9-29-7-3-14(4-8-29)19-12-25-13-26-19/h10-14,20H,1-9H2,(H,25,26)(H,27,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338012
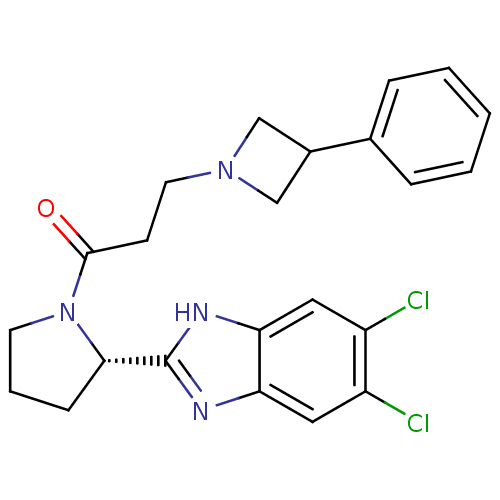
((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES Clc1cc2nc([nH]c2cc1Cl)[C@@H]1CCCN1C(=O)CCN1CC(C1)c1ccccc1 |r| Show InChI InChI=1S/C23H24Cl2N4O/c24-17-11-19-20(12-18(17)25)27-23(26-19)21-7-4-9-29(21)22(30)8-10-28-13-16(14-28)15-5-2-1-3-6-15/h1-3,5-6,11-12,16,21H,4,7-10,13-14H2,(H,26,27)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338018
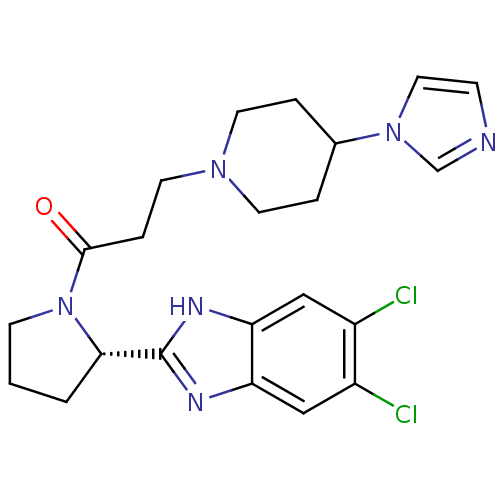
((S)-3-(4-(1H-imidazol-1-yl)piperidin-1-yl)-1-(2-(5...)Show SMILES Clc1cc2nc([nH]c2cc1Cl)[C@@H]1CCCN1C(=O)CCN1CCC(CC1)n1ccnc1 |r| Show InChI InChI=1S/C22H26Cl2N6O/c23-16-12-18-19(13-17(16)24)27-22(26-18)20-2-1-7-30(20)21(31)5-10-28-8-3-15(4-9-28)29-11-6-25-14-29/h6,11-15,20H,1-5,7-10H2,(H,26,27)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 155 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50338028

((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES Clc1cc2nc([nH]c2cc1Cl)[C@@H]1CCCN1C(=O)CCN1CCC(CC1)c1nc(no1)-c1cccnc1 |r| Show InChI InChI=1S/C26H27Cl2N7O2/c27-18-13-20-21(14-19(18)28)31-25(30-20)22-4-2-9-35(22)23(36)7-12-34-10-5-16(6-11-34)26-32-24(33-37-26)17-3-1-8-29-15-17/h1,3,8,13-16,22H,2,4-7,9-12H2,(H,30,31)/t22-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 155 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PrCP in mouse plasma assessed as angiotensin 3 cleavage |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50338027

((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES Clc1cc2nc([nH]c2cc1Cl)[C@@H]1CCCN1C(=O)CCN1CCC(CC1)c1nc(no1)-c1ccccn1 |r| Show InChI InChI=1S/C26H27Cl2N7O2/c27-17-14-20-21(15-18(17)28)31-25(30-20)22-5-3-10-35(22)23(36)8-13-34-11-6-16(7-12-34)26-32-24(33-37-26)19-4-1-2-9-29-19/h1-2,4,9,14-16,22H,3,5-8,10-13H2,(H,30,31)/t22-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 173 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PrCP in mouse plasma assessed as angiotensin 3 cleavage |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50338034

((S)-4-(5-(1-(3-(2-(5,6-dichloro-1H-benzo[d]imidazo...)Show SMILES OC(=O)c1ccc(cc1)-c1noc(n1)C1CCN(CCC(=O)N2CCC[C@H]2c2nc3cc(Cl)c(Cl)cc3[nH]2)CC1 |r| Show InChI InChI=1S/C28H28Cl2N6O4/c29-19-14-21-22(15-20(19)30)32-26(31-21)23-2-1-10-36(23)24(37)9-13-35-11-7-17(8-12-35)27-33-25(34-40-27)16-3-5-18(6-4-16)28(38)39/h3-6,14-15,17,23H,1-2,7-13H2,(H,31,32)(H,38,39)/t23-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 241 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PrCP in mouse plasma assessed as angiotensin 3 cleavage |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50338014

((S)-3-(4-(1H-pyrazol-3-yl)piperidin-1-yl)-1-(2-(5,...)Show SMILES Clc1cc2nc([nH]c2cc1Cl)[C@@H]1CCCN1C(=O)CCN1CCC(CC1)c1ccn[nH]1 |r| Show InChI InChI=1S/C22H26Cl2N6O/c23-15-12-18-19(13-16(15)24)27-22(26-18)20-2-1-8-30(20)21(31)6-11-29-9-4-14(5-10-29)17-3-7-25-28-17/h3,7,12-14,20H,1-2,4-6,8-11H2,(H,25,28)(H,26,27)/t20-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PrCP in mouse plasma assessed as angiotensin 3 cleavage |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50338005

(1-(3-((S)-2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1noc(n1)C1CCN(CCC(=O)N2CCC[C@H]2c2nc3cc(Cl)c(Cl)cc3[nH]2)C(=O)C1 |r| Show InChI InChI=1S/C28H28Cl2N6O5S/c1-42(39,40)18-6-4-16(5-7-18)26-33-28(41-34-26)17-8-11-35(25(38)13-17)12-9-24(37)36-10-2-3-23(36)27-31-21-14-19(29)20(30)15-22(21)32-27/h4-7,14-15,17,23H,2-3,8-13H2,1H3,(H,31,32)/t17?,23-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 425 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PrCP in mouse plasma assessed as angiotensin 3 cleavage |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50338023

((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES Clc1cc2nc([nH]c2cc1Cl)[C@@H]1CCCN1C(=O)CCN1CCC(CC1)c1ccncc1 |r| Show InChI InChI=1S/C24H27Cl2N5O/c25-18-14-20-21(15-19(18)26)29-24(28-20)22-2-1-10-31(22)23(32)7-13-30-11-5-17(6-12-30)16-3-8-27-9-4-16/h3-4,8-9,14-15,17,22H,1-2,5-7,10-13H2,(H,28,29)/t22-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PrCP in mouse plasma assessed as angiotensin 3 cleavage |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data