

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50234245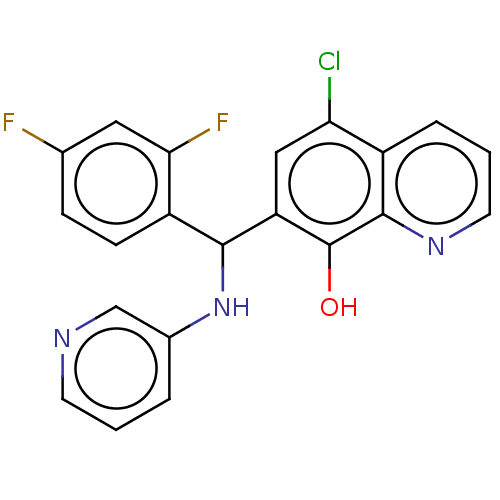 (CHEMBL4060854) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50234255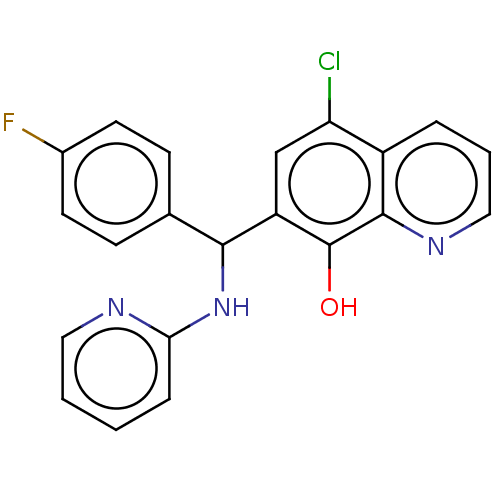 (CHEMBL4091467) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM52237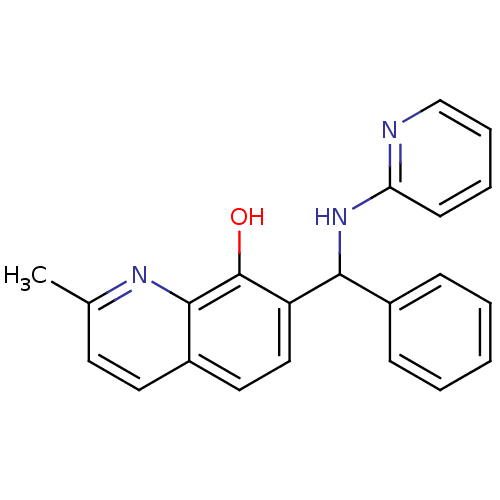 (2-methyl-7-[phenyl-(2-pyridinylamino)methyl]-8-qui...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50234257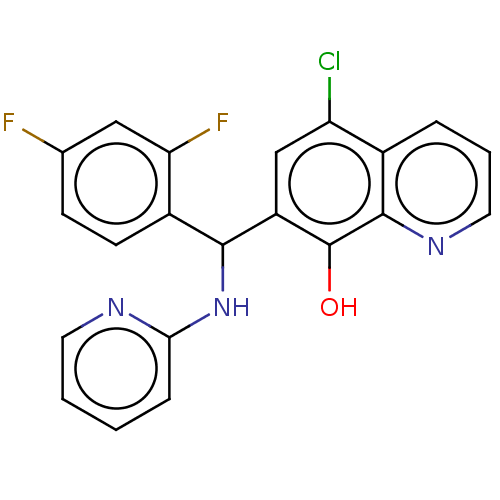 (CHEMBL4089872) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50234270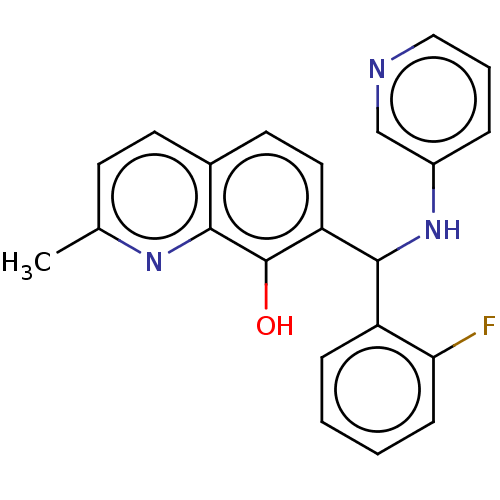 (CHEMBL4071334) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50234272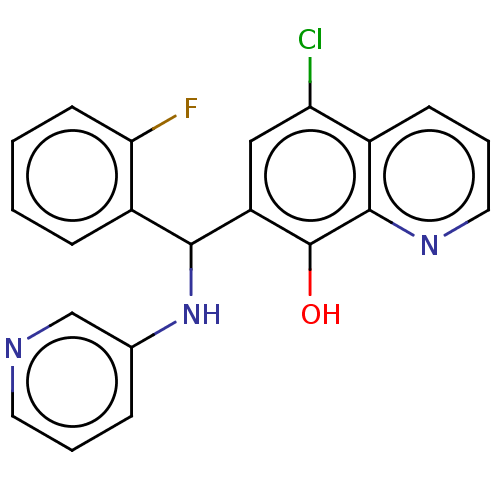 (CHEMBL4084638) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50234273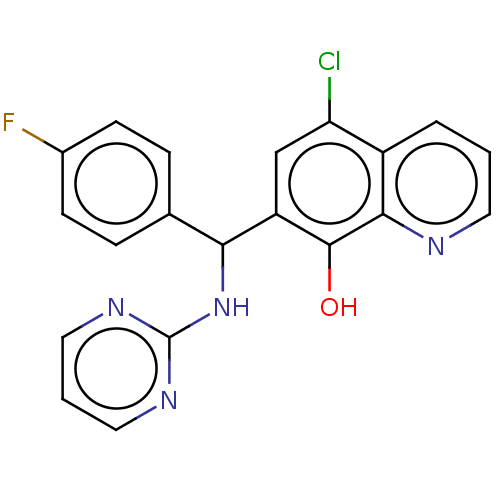 (CHEMBL4100165) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50234248 (CHEMBL4068440) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50234264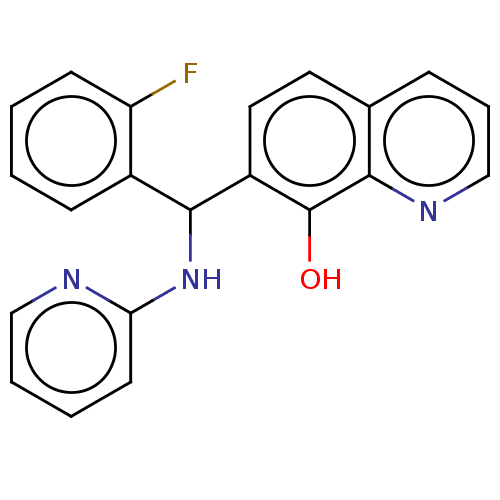 (CHEMBL1326401) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50234259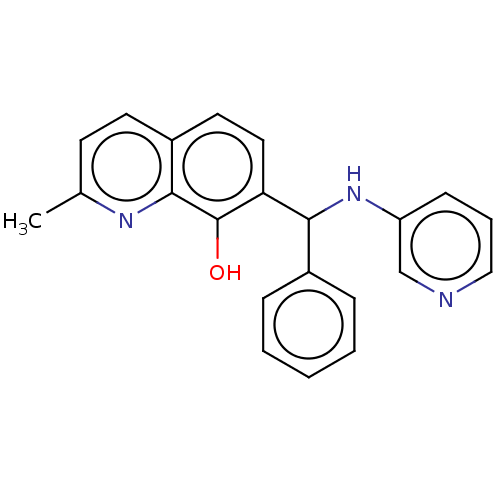 (CHEMBL4099530) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50442297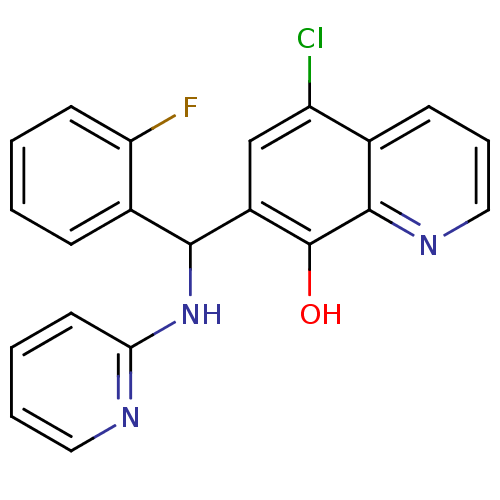 (CHEMBL2442244) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50234258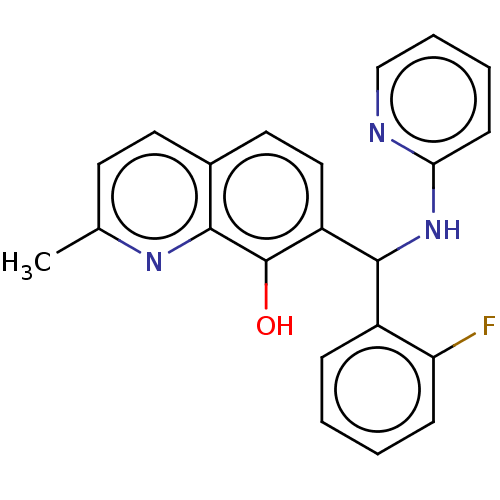 (CHEMBL4098372) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50362898 (CHEMBL1945166) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50339142 (CHEMBL1688558 | rac-7-(phenyl(pyridin-2-ylamino)me...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50447139 (CHEMBL3112900) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50234262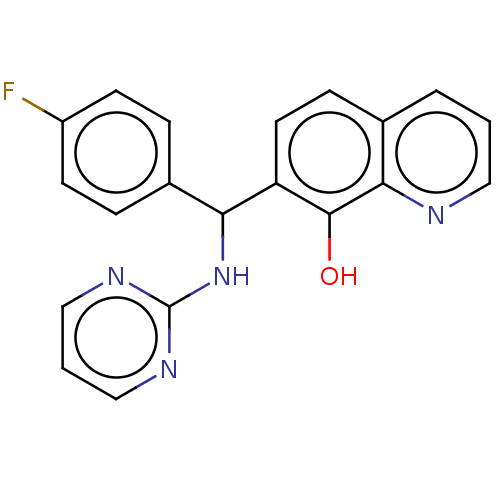 (CHEMBL4085460) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013712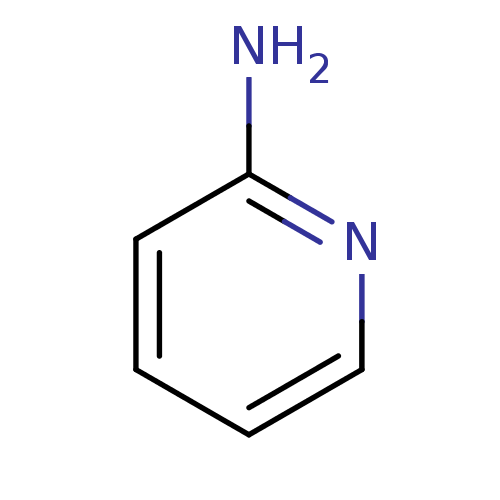 (2-aminopyridin | 2-aminopyridine | CHEMBL21619 | P...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50234276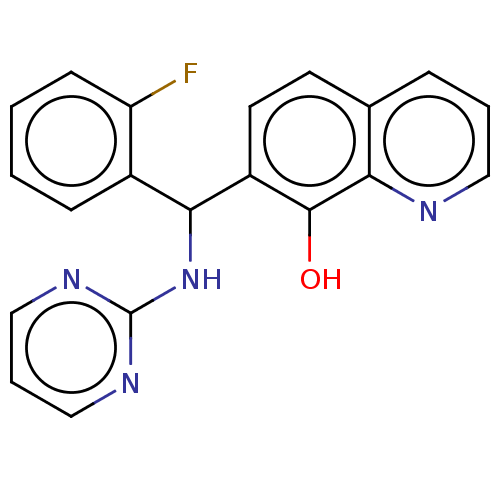 (CHEMBL4070394) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM76305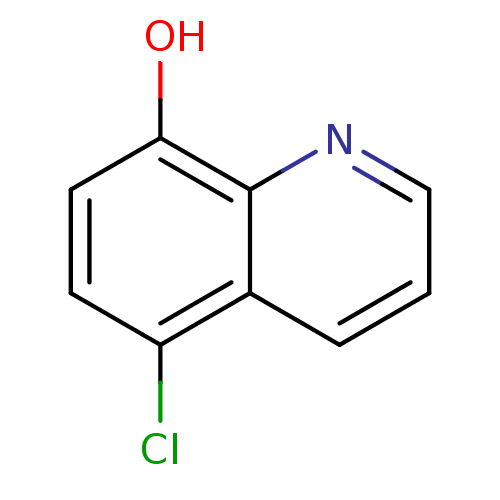 (5-chloranylquinolin-8-ol | 5-chloro-8-quinolinol |...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50234246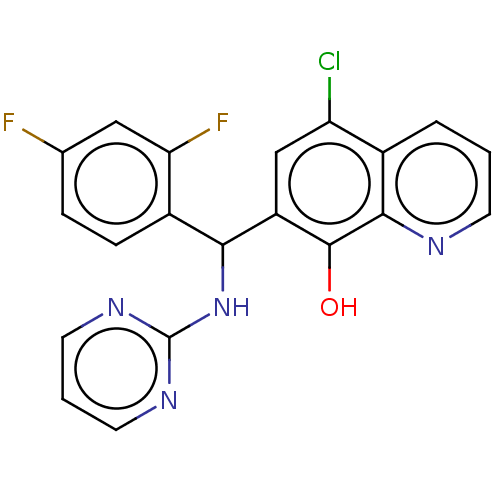 (CHEMBL4085501) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50234267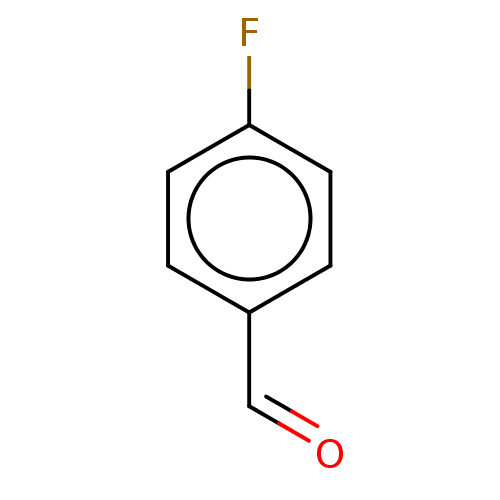 (CHEMBL3183207) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50234256 (CHEMBL4061926) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50339144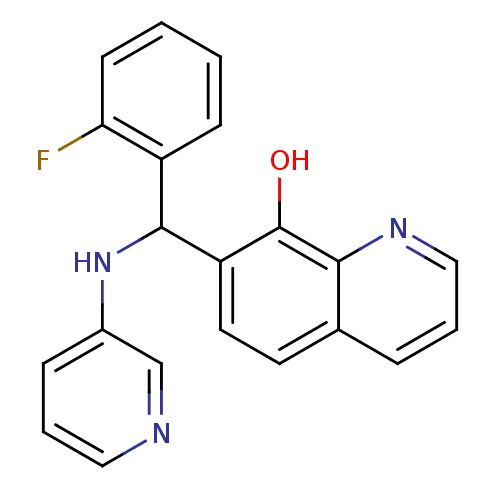 (CHEMBL1688560 | rac-7-((2-fluorophenyl)(pyridin-3-...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50234271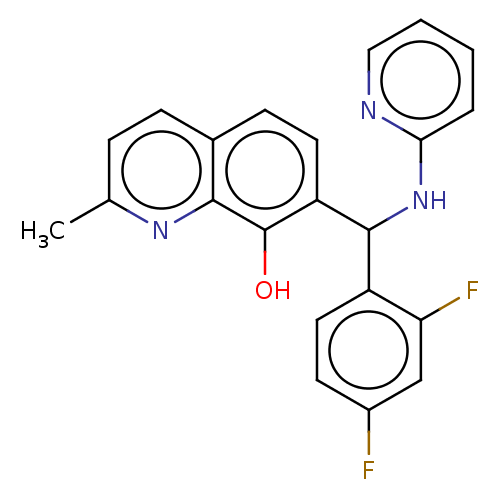 (CHEMBL4059783) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50234277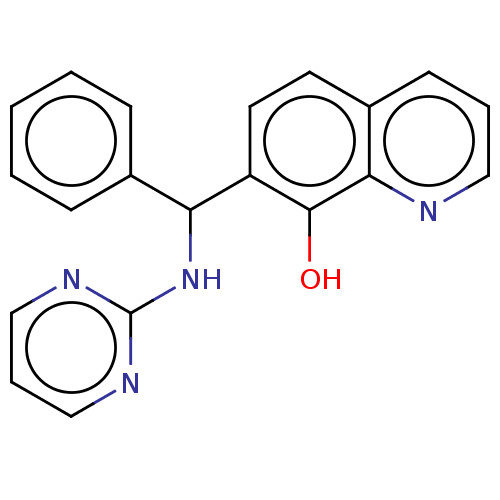 (CHEMBL4081983) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50234269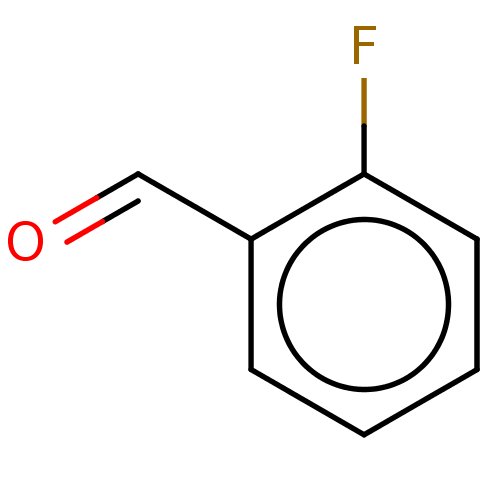 (CHEMBL4084647) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM60953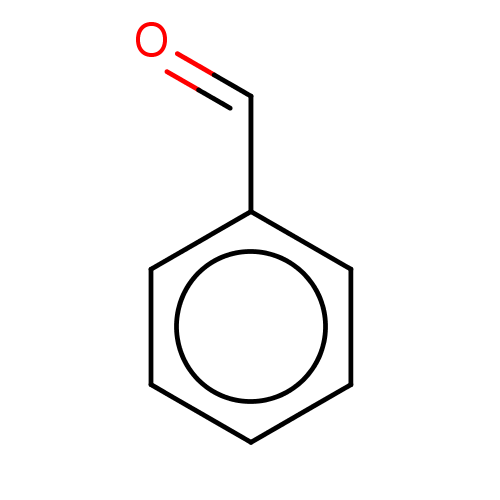 (BDBM50139371 | benzaldehyde) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50122005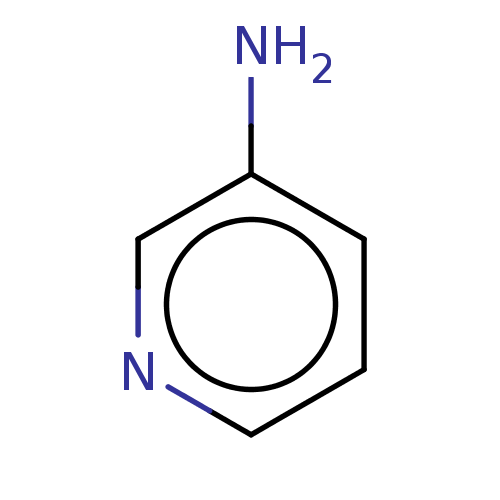 (CHEMBL25541 | Pyridin-3-ylamine) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50234252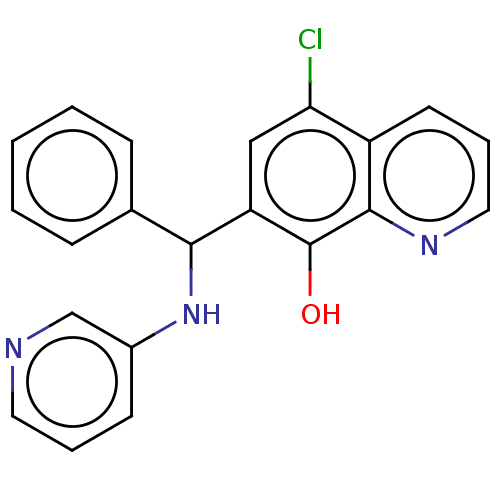 (CHEMBL4079277) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50234261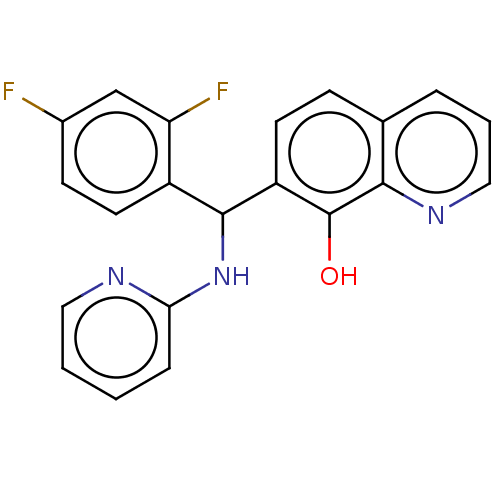 (CHEMBL4072352) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM32203 (8-quinolinol | CHEMBL310555 | US10005735, Table 1....) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50065785 (2-Methyl-quinolin-8-ol | CHEMBL316892) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50234254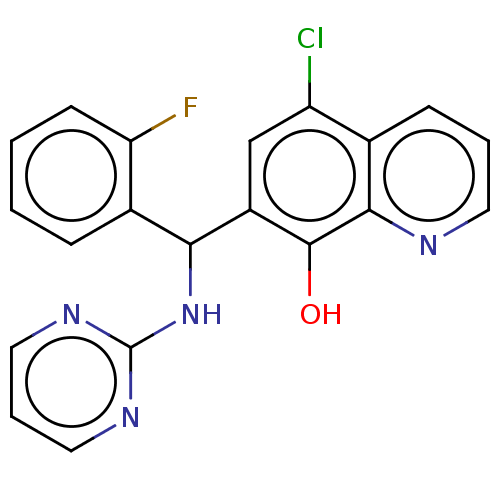 (CHEMBL4076803) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50447140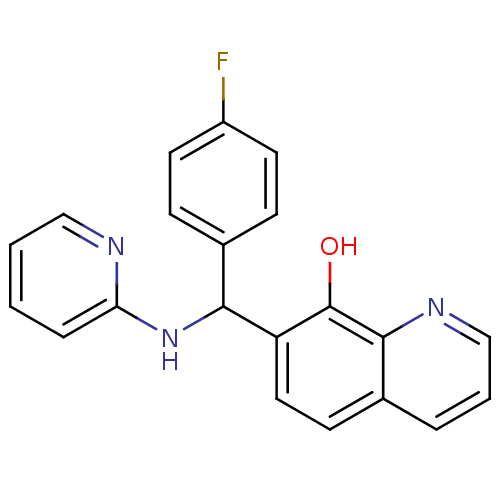 (CHEMBL3112899) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50234249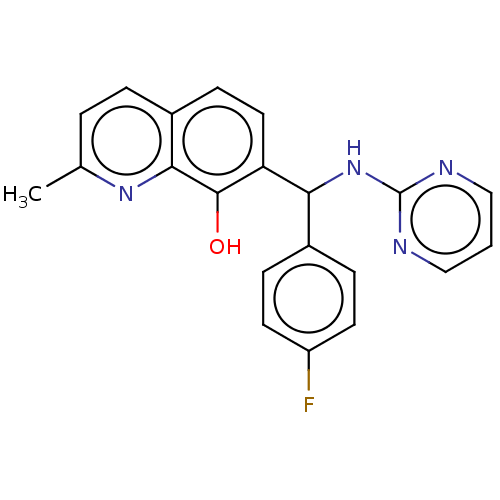 (CHEMBL4095785) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50234251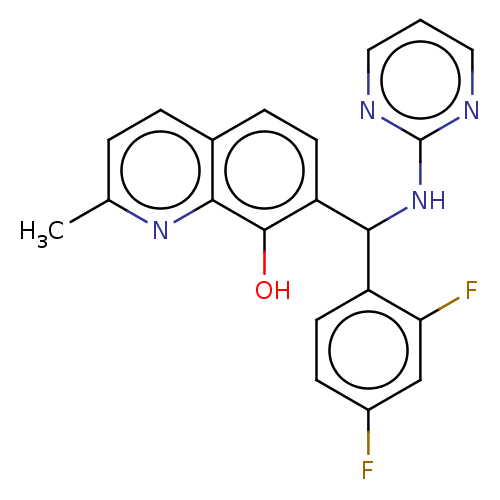 (CHEMBL4087160) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50234275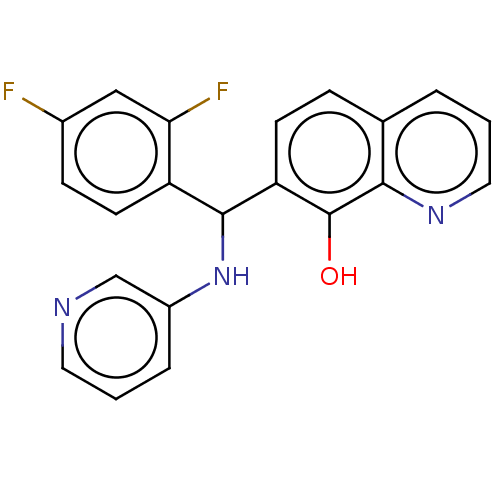 (CHEMBL4093118) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50234253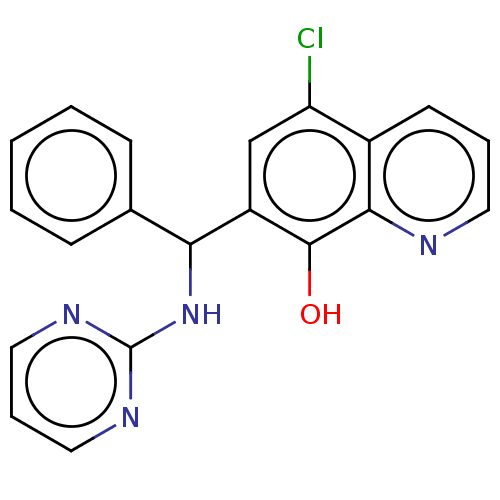 (CHEMBL4090577) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50234263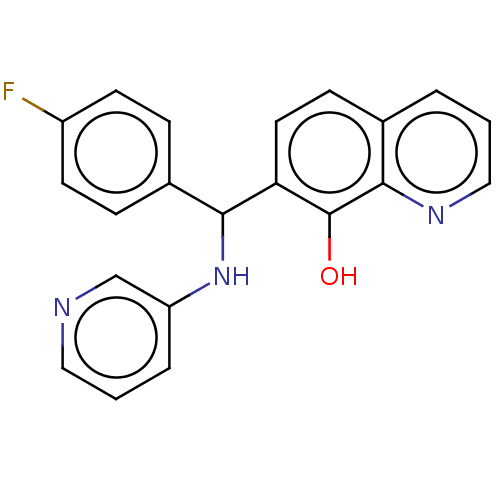 (CHEMBL4103385) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50354823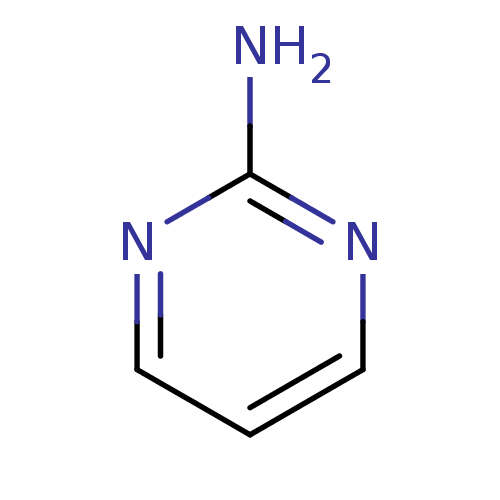 (CHEMBL88580) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50234274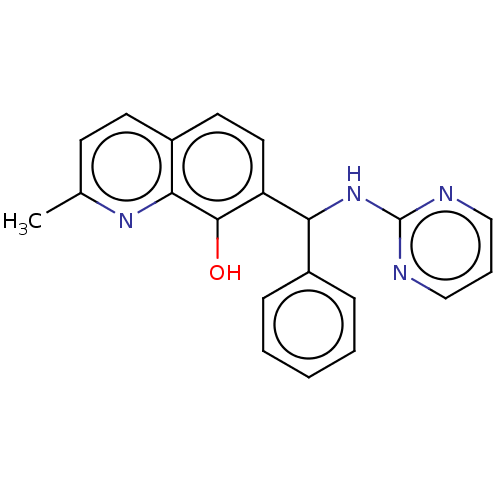 (CHEMBL4062693) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50234268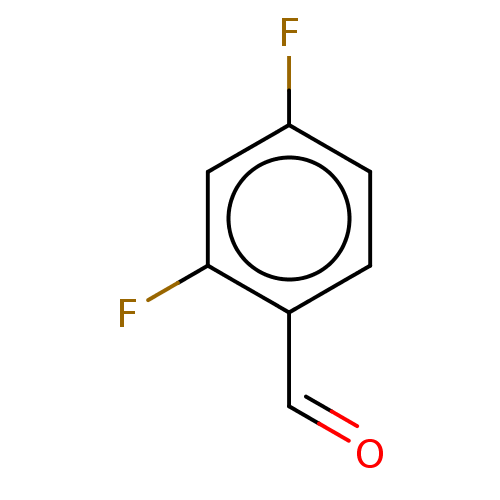 (CHEMBL4092237) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50234266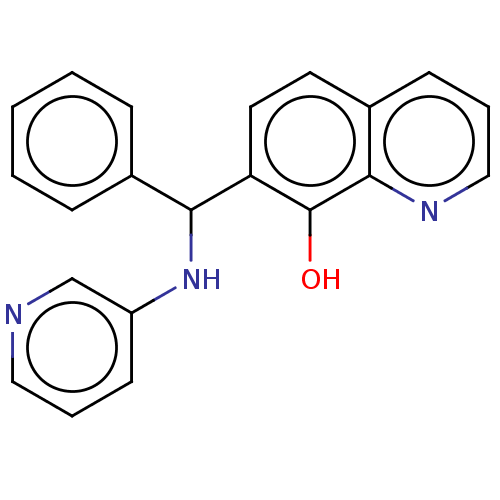 (CHEMBL4099990) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50234247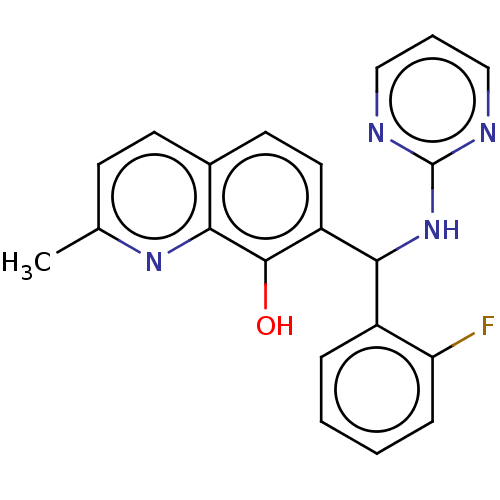 (CHEMBL4059959) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50234250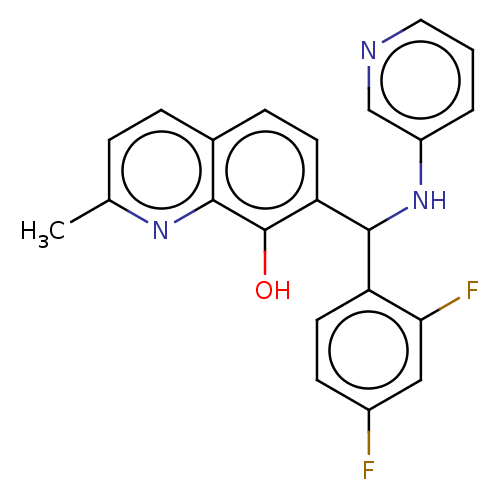 (CHEMBL4088059) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50234260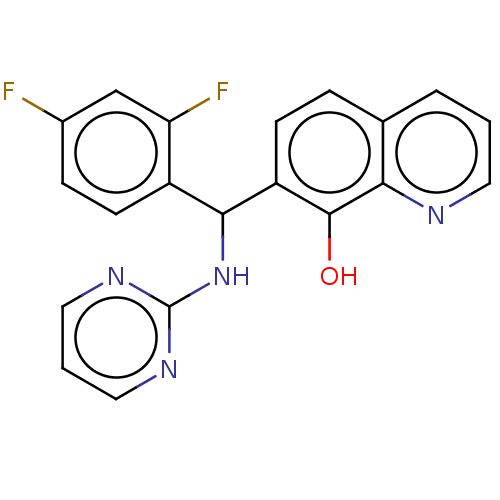 (CHEMBL4095202) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute of Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of protease activity of recombinant full length Clostridium botulinum Hall BoNT/A light chain using SNAP-25 peptide (187 to 203 residues) ... | Bioorg Med Chem Lett 27: 675-678 (2017) Article DOI: 10.1016/j.bmcl.2016.11.019 BindingDB Entry DOI: 10.7270/Q2NP26PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||