 Found 56 hits Enz. Inhib. hit(s) with all data for entry = 50035249
Found 56 hits Enz. Inhib. hit(s) with all data for entry = 50035249 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117612

(4-[3-(5,6-Dimethoxy-benzo[b]thiophen-2-yl)-3-oxo-p...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CCc1cc[n+](Cc2ccc(F)cc2)cc1 Show InChI InChI=1S/C25H23FNO3S/c1-29-22-13-19-14-25(31-24(19)15-23(22)30-2)21(28)8-5-17-9-11-27(12-10-17)16-18-3-6-20(26)7-4-18/h3-4,6-7,9-15H,5,8,16H2,1-2H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50366796

(CHEMBL609440)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CCc1cc[n+](Cc2ccc(o2)[N+]([O-])=O)cc1 Show InChI InChI=1S/C23H21N2O6S/c1-29-19-11-16-12-22(32-21(16)13-20(19)30-2)18(26)5-3-15-7-9-24(10-8-15)14-17-4-6-23(31-17)25(27)28/h4,6-13H,3,5,14H2,1-2H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117595
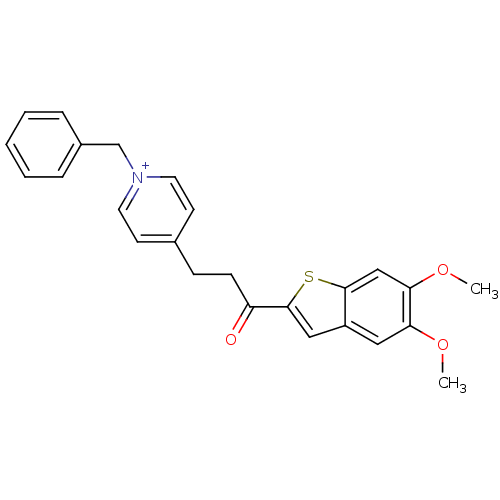
(1-Benzyl-4-[3-(5,6-dimethoxy-benzo[b]thiophen-2-yl...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CCc1cc[n+](Cc2ccccc2)cc1 Show InChI InChI=1S/C25H24NO3S/c1-28-22-14-20-15-25(30-24(20)16-23(22)29-2)21(27)9-8-18-10-12-26(13-11-18)17-19-6-4-3-5-7-19/h3-7,10-16H,8-9,17H2,1-2H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117614

(4-[3-(5,6-Dimethoxy-benzo[b]thiophen-2-yl)-3-oxo-p...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CCc1cc[n+](Cc2ccsc2)cc1 Show InChI InChI=1S/C23H22NO3S2/c1-26-20-11-18-12-23(29-22(18)13-21(20)27-2)19(25)4-3-16-5-8-24(9-6-16)14-17-7-10-28-15-17/h5-13,15H,3-4,14H2,1-2H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117618

(4-[3-(5,6-Dimethoxy-benzo[b]thiophen-2-yl)-3-oxo-p...)Show SMILES CCOCC[n+]1ccc(CCC(=O)c2cc3cc(OC)c(OC)cc3s2)cc1 Show InChI InChI=1S/C22H26NO4S/c1-4-27-12-11-23-9-7-16(8-10-23)5-6-18(24)22-14-17-13-19(25-2)20(26-3)15-21(17)28-22/h7-10,13-15H,4-6,11-12H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117594

(1-Benzyl-4-[3-(5,6-dimethoxy-benzo[b]thiophen-2-yl...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CCC1CC[N+](C)(Cc2ccccc2)CC1 |(-.09,-3.27,;1.22,-2.5,;2.55,-3.27,;3.88,-2.5,;5.21,-3.27,;6.68,-2.78,;7.59,-4.04,;6.68,-5.3,;5.21,-4.81,;3.88,-5.58,;2.55,-4.81,;1.22,-5.58,;-.09,-4.81,;9.13,-4.04,;9.9,-5.37,;9.9,-2.71,;11.44,-2.71,;12.21,-1.38,;13.74,-1.22,;14.37,.18,;13.47,1.42,;12.28,2.4,;14.1,2.84,;15.64,2.99,;16.27,4.39,;17.79,4.54,;18.7,3.29,;18.07,1.88,;16.53,1.74,;11.95,1.28,;11.3,-.12,)| Show InChI InChI=1S/C26H32NO3S/c1-27(18-20-7-5-4-6-8-20)13-11-19(12-14-27)9-10-22(28)26-16-21-15-23(29-2)24(30-3)17-25(21)31-26/h4-8,15-17,19H,9-14,18H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117609
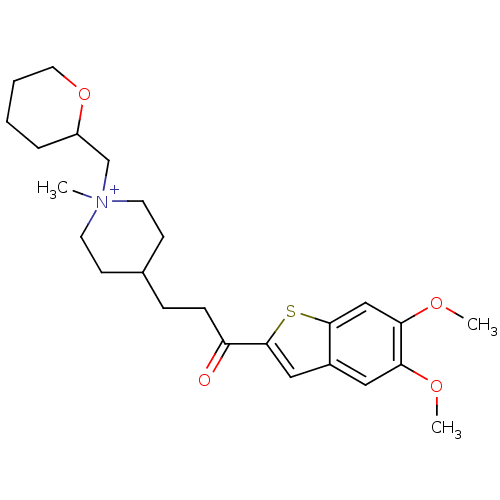
(4-[3-(5,6-Dimethoxy-benzo[b]thiophen-2-yl)-3-oxo-p...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CCC1CC[N+](C)(CC2CCCCO2)CC1 |(-1.63,-4.95,;-.3,-4.18,;1.03,-4.95,;2.36,-4.18,;3.69,-4.95,;5.16,-4.46,;6.07,-5.72,;5.16,-6.98,;3.69,-6.49,;2.36,-7.26,;1.03,-6.49,;-.3,-7.26,;-1.63,-6.49,;7.61,-5.72,;8.38,-7.05,;8.38,-4.37,;9.92,-4.37,;10.69,-3.04,;9.78,-1.8,;10.41,-.4,;11.95,-.24,;10.97,.95,;13.45,.11,;13.89,1.58,;12.84,2.7,;13.3,4.18,;14.8,4.53,;15.85,3.4,;15.41,1.93,;12.84,-1.49,;12.21,-2.89,)| Show InChI InChI=1S/C25H36NO4S/c1-26(17-20-6-4-5-13-30-20)11-9-18(10-12-26)7-8-21(27)25-15-19-14-22(28-2)23(29-3)16-24(19)31-25/h14-16,18,20H,4-13,17H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117620
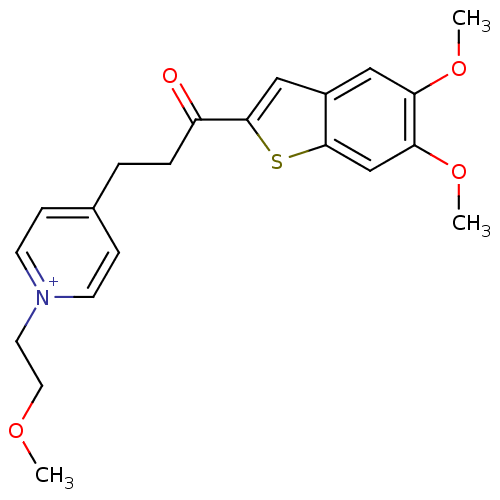
(4-[3-(5,6-Dimethoxy-benzo[b]thiophen-2-yl)-3-oxo-p...)Show SMILES COCC[n+]1ccc(CCC(=O)c2cc3cc(OC)c(OC)cc3s2)cc1 Show InChI InChI=1S/C21H24NO4S/c1-24-11-10-22-8-6-15(7-9-22)4-5-17(23)21-13-16-12-18(25-2)19(26-3)14-20(16)27-21/h6-9,12-14H,4-5,10-11H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117622
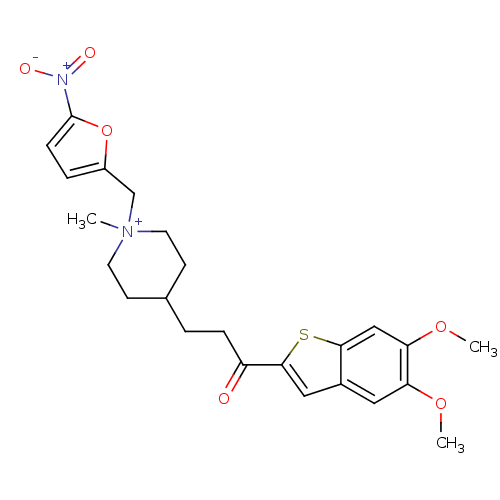
(4-[3-(5,6-Dimethoxy-benzo[b]thiophen-2-yl)-3-oxo-p...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CCC1CC[N+](C)(Cc2ccc(o2)[N+]([O-])=O)CC1 |(-1.63,-4.96,;-.3,-4.19,;1.04,-4.96,;2.36,-4.19,;3.69,-4.96,;5.18,-4.47,;6.09,-5.73,;5.18,-6.99,;3.69,-6.51,;2.37,-7.27,;1.04,-6.5,;-.3,-7.27,;-1.63,-6.5,;7.63,-5.73,;8.4,-7.06,;8.4,-4.38,;9.95,-4.38,;10.71,-3.05,;9.8,-1.81,;10.44,-.4,;11.98,-.24,;11,.95,;13.48,.11,;13.92,1.58,;12.98,2.81,;13.87,4.08,;15.35,3.64,;15.39,2.1,;16.56,4.58,;17.99,3.97,;16.37,6.09,;12.87,-1.5,;12.24,-2.9,)| Show InChI InChI=1S/C24H29N2O6S/c1-26(15-18-5-7-24(32-18)25(28)29)10-8-16(9-11-26)4-6-19(27)23-13-17-12-20(30-2)21(31-3)14-22(17)33-23/h5,7,12-14,16H,4,6,8-11,15H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117623

(1-Cyclobutylmethyl-4-[3-(5,6-dimethoxy-benzo[b]thi...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CCc1cc[n+](CC2CCC2)cc1 Show InChI InChI=1S/C23H26NO3S/c1-26-20-12-18-13-23(28-22(18)14-21(20)27-2)19(25)7-6-16-8-10-24(11-9-16)15-17-4-3-5-17/h8-14,17H,3-7,15H2,1-2H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117599

(1-Benzyl-4-[3-(5,6-dimethoxy-benzo[b]thiophen-2-yl...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CC(O)C1CC[N+](C)(Cc2ccccc2)CC1 |(3.16,-1.52,;4.48,-.75,;5.81,-1.52,;7.12,-.75,;8.45,-1.52,;9.94,-1.03,;10.85,-2.29,;9.94,-3.55,;8.45,-3.06,;7.14,-3.83,;5.81,-3.06,;4.48,-3.83,;3.16,-3.06,;12.39,-2.29,;13.16,-3.62,;13.16,-.96,;14.7,-.96,;15.47,-2.29,;15.47,.37,;16.99,.27,;17.86,1.54,;17.18,2.93,;16.18,4.08,;18.04,4.2,;19.58,4.1,;20.25,2.71,;21.78,2.61,;22.64,3.89,;21.96,5.27,;20.42,5.37,;15.64,3.03,;14.78,1.75,)| Show InChI InChI=1S/C26H32NO4S/c1-27(17-18-7-5-4-6-8-18)11-9-19(10-12-27)21(28)15-22(29)26-14-20-13-23(30-2)24(31-3)16-25(20)32-26/h4-8,13-14,16,19,21,28H,9-12,15,17H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117602

(4-[3-(5,6-Dimethoxy-benzo[b]thiophen-2-yl)-3-oxo-p...)Show SMILES COCC[N+]1(C)CCC(CCC(=O)c2cc3cc(OC)c(OC)cc3s2)CC1 |(15.88,3.41,;15.42,1.94,;13.92,1.58,;13.48,.11,;11.98,-.24,;10.79,.74,;10.44,-.4,;9.8,-1.81,;10.71,-3.05,;9.95,-4.38,;8.4,-4.38,;7.63,-5.73,;8.4,-7.06,;6.09,-5.73,;5.18,-4.47,;3.69,-4.96,;2.36,-4.19,;1.04,-4.96,;-.3,-4.19,;-1.63,-4.96,;1.04,-6.5,;-.3,-7.27,;-1.63,-6.5,;2.37,-7.27,;3.69,-6.51,;5.18,-6.99,;12.24,-2.9,;12.87,-1.5,)| Show InChI InChI=1S/C22H32NO4S/c1-23(11-12-25-2)9-7-16(8-10-23)5-6-18(24)22-14-17-13-19(26-3)20(27-4)15-21(17)28-22/h13-16H,5-12H2,1-4H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117597
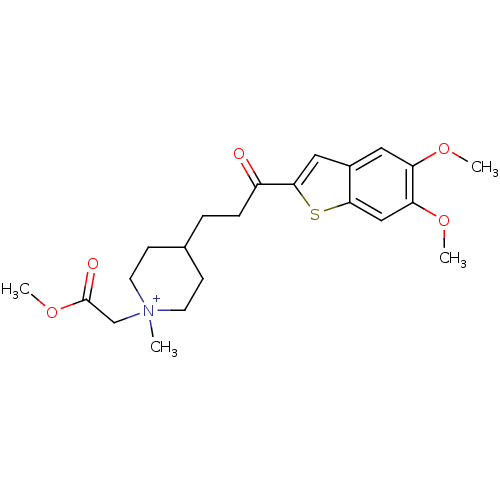
(4-[3-(5,6-Dimethoxy-benzo[b]thiophen-2-yl)-3-oxo-p...)Show SMILES COC(=O)C[N+]1(C)CCC(CCC(=O)c2cc3cc(OC)c(OC)cc3s2)CC1 |(15.88,3.41,;15.42,1.94,;13.92,1.58,;12.87,2.71,;13.48,.11,;11.98,-.24,;11,.95,;12.87,-1.5,;12.24,-2.9,;10.71,-3.05,;9.95,-4.38,;8.4,-4.38,;7.63,-5.73,;8.4,-7.06,;6.09,-5.73,;5.18,-4.47,;3.69,-4.96,;2.36,-4.19,;1.04,-4.96,;-.3,-4.19,;-1.63,-4.96,;1.04,-6.5,;-.3,-7.27,;-1.63,-6.5,;2.37,-7.27,;3.69,-6.51,;5.18,-6.99,;9.8,-1.81,;10.44,-.4,)| Show InChI InChI=1S/C22H30NO5S/c1-23(14-22(25)28-4)9-7-15(8-10-23)5-6-17(24)21-12-16-11-18(26-2)19(27-3)13-20(16)29-21/h11-13,15H,5-10,14H2,1-4H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117611
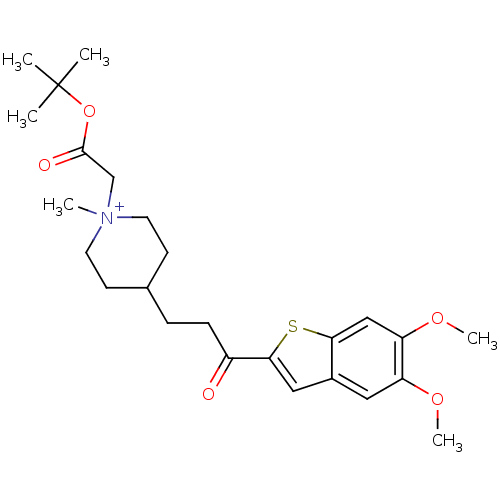
(1-tert-Butoxycarbonylmethyl-4-[3-(5,6-dimethoxy-be...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CCC1CC[N+](C)(CC(=O)OC(C)(C)C)CC1 |(-4.12,-6.7,;-2.8,-5.93,;-1.47,-6.7,;-.16,-5.93,;1.17,-6.7,;2.66,-6.21,;3.57,-7.47,;2.66,-8.73,;1.17,-8.24,;-.14,-9.01,;-1.47,-8.24,;-2.8,-9.01,;-4.12,-8.24,;5.11,-7.47,;5.88,-8.8,;5.88,-6.14,;7.42,-6.14,;8.19,-4.81,;7.28,-3.55,;7.92,-2.15,;9.45,-2.01,;8.47,-.82,;10.95,-1.64,;11.39,-.17,;10.34,.95,;12.89,.18,;13.33,1.65,;11.84,2.05,;13.73,3.14,;14.82,1.25,;10.34,-3.25,;9.71,-4.65,)| Show InChI InChI=1S/C25H36NO5S/c1-25(2,3)31-24(28)16-26(4)11-9-17(10-12-26)7-8-19(27)23-14-18-13-20(29-5)21(30-6)15-22(18)32-23/h13-15,17H,7-12,16H2,1-6H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117608

(1-Benzyl-4-[2-(5,6-dimethoxy-benzo[b]thiophen-2-yl...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CC1(O)CC[N+](C)(Cc2ccccc2)CC1 Show InChI InChI=1S/C25H30NO4S.BrH/c1-26(17-18-7-5-4-6-8-18)11-9-25(28,10-12-26)16-20(27)24-14-19-13-21(29-2)22(30-3)15-23(19)31-24;/h4-8,13-15,28H,9-12,16-17H2,1-3H3;1H/q+1;/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117596

(1-Cyclopropylmethyl-4-[3-(5,6-dimethoxy-benzo[b]th...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CCc1cc[n+](CC2CC2)cc1 Show InChI InChI=1S/C22H24NO3S/c1-25-19-11-17-12-22(27-21(17)13-20(19)26-2)18(24)6-5-15-7-9-23(10-8-15)14-16-3-4-16/h7-13,16H,3-6,14H2,1-2H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117606

(1-Benzyl-4-[5-(5,6-dimethoxy-benzo[b]thiophen-2-yl...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CCCCC1CC[N+](C)(Cc2ccccc2)CC1 |(-.74,-2.58,;.58,-1.81,;1.92,-2.58,;3.23,-1.81,;4.56,-2.58,;6.05,-2.09,;6.96,-3.35,;6.05,-4.61,;4.56,-4.12,;3.25,-4.89,;1.92,-4.12,;.58,-4.89,;-.74,-4.12,;8.5,-3.35,;9.27,-4.68,;9.27,-2.01,;10.82,-2.01,;11.59,-.68,;13.13,-.68,;13.88,.67,;15.4,.9,;15.95,2.34,;14.98,3.54,;13.74,4.44,;15.54,4.98,;17.06,5.22,;17.6,6.65,;19.12,6.91,;20.1,5.7,;19.54,4.27,;18.02,4.02,;13.46,3.29,;12.9,1.85,)| Show InChI InChI=1S/C28H36NO3S/c1-29(20-22-10-5-4-6-11-22)15-13-21(14-16-29)9-7-8-12-24(30)28-18-23-17-25(31-2)26(32-3)19-27(23)33-28/h4-6,10-11,17-19,21H,7-9,12-16,20H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117615
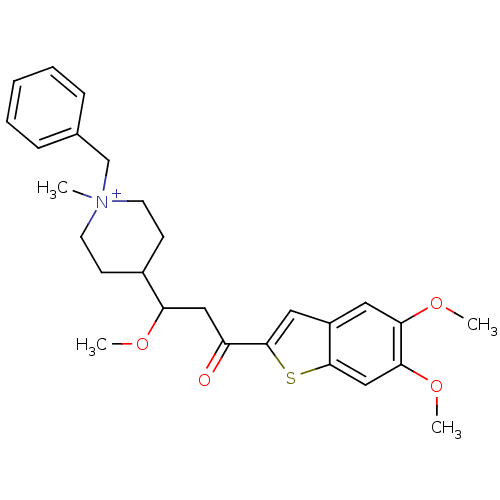
(1-Benzyl-4-[3-(5,6-dimethoxy-benzo[b]thiophen-2-yl...)Show SMILES COC(CC(=O)c1cc2cc(OC)c(OC)cc2s1)C1CC[N+](C)(Cc2ccccc2)CC1 |(11.32,-7.89,;12.1,-6.55,;11.32,-5.22,;9.78,-5.22,;9.01,-6.55,;9.78,-7.89,;7.47,-6.55,;6.55,-5.29,;5.07,-5.78,;3.74,-5.01,;2.42,-5.78,;1.08,-5.01,;-.23,-5.78,;2.42,-7.33,;1.08,-8.1,;-.23,-7.33,;3.75,-8.1,;5.07,-7.33,;6.55,-7.82,;12.1,-3.89,;13.63,-3.98,;14.5,-2.72,;13.82,-1.32,;12.81,-.17,;14.68,-.05,;16.23,-.15,;17.07,1.11,;18.61,1.02,;19.29,-.38,;18.42,-1.64,;16.89,-1.55,;12.28,-1.22,;11.41,-2.51,)| Show InChI InChI=1S/C27H34NO4S/c1-28(18-19-8-6-5-7-9-19)12-10-20(11-13-28)23(30-2)16-22(29)27-15-21-14-24(31-3)25(32-4)17-26(21)33-27/h5-9,14-15,17,20,23H,10-13,16,18H2,1-4H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117599

(1-Benzyl-4-[3-(5,6-dimethoxy-benzo[b]thiophen-2-yl...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CC(O)C1CC[N+](C)(Cc2ccccc2)CC1 |(3.16,-1.52,;4.48,-.75,;5.81,-1.52,;7.12,-.75,;8.45,-1.52,;9.94,-1.03,;10.85,-2.29,;9.94,-3.55,;8.45,-3.06,;7.14,-3.83,;5.81,-3.06,;4.48,-3.83,;3.16,-3.06,;12.39,-2.29,;13.16,-3.62,;13.16,-.96,;14.7,-.96,;15.47,-2.29,;15.47,.37,;16.99,.27,;17.86,1.54,;17.18,2.93,;16.18,4.08,;18.04,4.2,;19.58,4.1,;20.25,2.71,;21.78,2.61,;22.64,3.89,;21.96,5.27,;20.42,5.37,;15.64,3.03,;14.78,1.75,)| Show InChI InChI=1S/C26H32NO4S/c1-27(17-18-7-5-4-6-8-18)11-9-19(10-12-27)21(28)15-22(29)26-14-20-13-23(30-2)24(31-3)16-25(20)32-26/h4-8,13-14,16,19,21,28H,9-12,15,17H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 219 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117599

(1-Benzyl-4-[3-(5,6-dimethoxy-benzo[b]thiophen-2-yl...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CC(O)C1CC[N+](C)(Cc2ccccc2)CC1 |(3.16,-1.52,;4.48,-.75,;5.81,-1.52,;7.12,-.75,;8.45,-1.52,;9.94,-1.03,;10.85,-2.29,;9.94,-3.55,;8.45,-3.06,;7.14,-3.83,;5.81,-3.06,;4.48,-3.83,;3.16,-3.06,;12.39,-2.29,;13.16,-3.62,;13.16,-.96,;14.7,-.96,;15.47,-2.29,;15.47,.37,;16.99,.27,;17.86,1.54,;17.18,2.93,;16.18,4.08,;18.04,4.2,;19.58,4.1,;20.25,2.71,;21.78,2.61,;22.64,3.89,;21.96,5.27,;20.42,5.37,;15.64,3.03,;14.78,1.75,)| Show InChI InChI=1S/C26H32NO4S/c1-27(17-18-7-5-4-6-8-18)11-9-19(10-12-27)21(28)15-22(29)26-14-20-13-23(30-2)24(31-3)16-25(20)32-26/h4-8,13-14,16,19,21,28H,9-12,15,17H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 219 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117605

(4-[3-(5,6-Dimethoxy-benzo[b]thiophen-2-yl)-3-oxo-p...)Show SMILES CC[n+]1ccc(CCC(=O)c2cc3cc(OC)c(OC)cc3s2)cc1 Show InChI InChI=1S/C20H22NO3S/c1-4-21-9-7-14(8-10-21)5-6-16(22)20-12-15-11-17(23-2)18(24-3)13-19(15)25-20/h7-13H,4-6H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117600

(1-Benzyl-4-[5-(5,6-dimethoxy-benzo[b]thiophen-2-yl...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CC(O)CCC1CC[N+](C)(Cc2ccccc2)CC1 |(-.74,-2.58,;.58,-1.81,;1.92,-2.58,;3.23,-1.81,;4.56,-2.58,;6.05,-2.09,;6.96,-3.35,;6.05,-4.61,;4.56,-4.12,;3.25,-4.89,;1.92,-4.12,;.58,-4.89,;-.74,-4.12,;8.5,-3.35,;9.27,-4.68,;9.27,-2.01,;10.82,-2.01,;11.59,-3.35,;11.59,-.68,;13.13,-.68,;13.88,.67,;15.4,.9,;15.95,2.34,;14.98,3.54,;13.74,4.44,;15.54,4.98,;17.06,5.22,;18.02,4.02,;19.54,4.27,;20.1,5.7,;19.12,6.91,;17.6,6.65,;13.46,3.29,;12.9,1.85,)| Show InChI InChI=1S/C28H36NO4S/c1-29(19-21-7-5-4-6-8-21)13-11-20(12-14-29)9-10-23(30)17-24(31)28-16-22-15-25(32-2)26(33-3)18-27(22)34-28/h4-8,15-16,18,20,23,30H,9-14,17,19H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117613
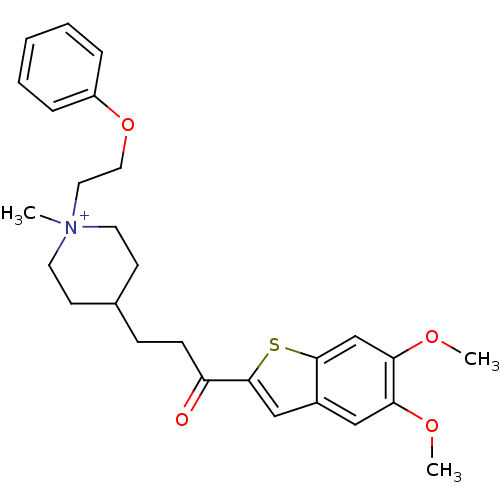
(4-[3-(5,6-Dimethoxy-benzo[b]thiophen-2-yl)-3-oxo-p...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CCC1CC[N+](C)(CCOc2ccccc2)CC1 |(-4.12,-6.71,;-2.8,-5.94,;-1.47,-6.71,;-.16,-5.94,;1.18,-6.71,;2.66,-6.22,;3.57,-7.48,;2.66,-8.74,;1.18,-8.25,;-.14,-9.02,;-1.47,-8.25,;-2.8,-9.02,;-4.12,-8.25,;5.11,-7.48,;5.88,-8.81,;5.88,-6.14,;7.42,-6.14,;8.19,-4.81,;7.28,-3.56,;7.92,-2.16,;9.45,-2.01,;8.47,-.82,;10.96,-1.64,;11.4,-.17,;12.89,.18,;13.34,1.65,;12.29,2.77,;12.72,4.24,;14.22,4.6,;15.29,3.48,;14.85,2,;10.35,-3.25,;9.72,-4.65,)| Show InChI InChI=1S/C27H34NO4S/c1-28(15-16-32-22-7-5-4-6-8-22)13-11-20(12-14-28)9-10-23(29)27-18-21-17-24(30-2)25(31-3)19-26(21)33-27/h4-8,17-20H,9-16H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117616

(1-Benzyl-4-[2-(5,6-dimethoxy-benzo[b]thiophen-2-yl...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)C=C1CC[N+](C)(Cc2ccccc2)CC1 |(3.16,-1.52,;4.48,-.75,;5.81,-1.52,;7.12,-.75,;8.45,-1.52,;9.94,-1.03,;10.85,-2.29,;9.94,-3.55,;8.45,-3.06,;7.14,-3.83,;5.81,-3.06,;4.48,-3.83,;3.16,-3.06,;12.39,-2.29,;13.16,-3.62,;13.16,-.96,;14.7,-.96,;15.1,-2.43,;16.59,-2.82,;17.67,-1.73,;18.43,-.38,;19.16,-2.11,;19.58,-3.59,;21.05,-3.97,;21.47,-5.44,;20.4,-6.56,;18.91,-6.17,;18.49,-4.69,;17.25,-.24,;15.76,.14,)| Show InChI InChI=1S/C25H28NO3S/c1-26(17-19-7-5-4-6-8-19)11-9-18(10-12-26)13-21(27)25-15-20-14-22(28-2)23(29-3)16-24(20)30-25/h4-8,13-16H,9-12,17H2,1-3H3/q+1/b18-13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117603
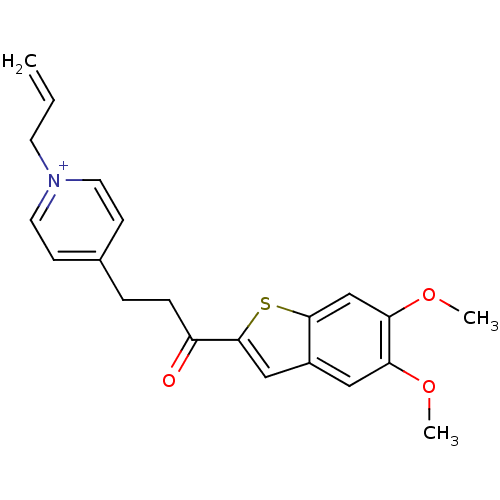
(1-Allyl-4-[3-(5,6-dimethoxy-benzo[b]thiophen-2-yl)...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CCc1cc[n+](CC=C)cc1 Show InChI InChI=1S/C21H22NO3S/c1-4-9-22-10-7-15(8-11-22)5-6-17(23)21-13-16-12-18(24-2)19(25-3)14-20(16)26-21/h4,7-8,10-14H,1,5-6,9H2,2-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117599

(1-Benzyl-4-[3-(5,6-dimethoxy-benzo[b]thiophen-2-yl...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CC(O)C1CC[N+](C)(Cc2ccccc2)CC1 |(3.16,-1.52,;4.48,-.75,;5.81,-1.52,;7.12,-.75,;8.45,-1.52,;9.94,-1.03,;10.85,-2.29,;9.94,-3.55,;8.45,-3.06,;7.14,-3.83,;5.81,-3.06,;4.48,-3.83,;3.16,-3.06,;12.39,-2.29,;13.16,-3.62,;13.16,-.96,;14.7,-.96,;15.47,-2.29,;15.47,.37,;16.99,.27,;17.86,1.54,;17.18,2.93,;16.18,4.08,;18.04,4.2,;19.58,4.1,;20.25,2.71,;21.78,2.61,;22.64,3.89,;21.96,5.27,;20.42,5.37,;15.64,3.03,;14.78,1.75,)| Show InChI InChI=1S/C26H32NO4S/c1-27(17-18-7-5-4-6-8-18)11-9-19(10-12-27)21(28)15-22(29)26-14-20-13-23(30-2)24(31-3)16-25(20)32-26/h4-8,13-14,16,19,21,28H,9-12,15,17H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 665 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117607

(1-Benzyl-4-[2-(5,6-dimethoxy-benzo[b]thiophen-2-yl...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CC1=CC[N+](C)(Cc2ccccc2)CC1 |t:18| Show InChI InChI=1S/C25H28NO3S/c1-26(17-19-7-5-4-6-8-19)11-9-18(10-12-26)13-21(27)25-15-20-14-22(28-2)23(29-3)16-24(20)30-25/h4-9,14-16H,10-13,17H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117604

(4-[3-(5,6-Dimethoxy-benzo[b]thiophen-2-yl)-3-oxo-p...)Show InChI InChI=1S/C19H20NO3S/c1-20-8-6-13(7-9-20)4-5-15(21)19-11-14-10-16(22-2)17(23-3)12-18(14)24-19/h6-12H,4-5H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117617

(4-[3-(5,6-Dimethoxy-benzo[b]thiophen-2-yl)-3-oxo-p...)Show SMILES COC(=O)c1ccc(C[n+]2ccc(CCC(=O)c3cc4cc(OC)c(OC)cc4s3)cc2)cc1 Show InChI InChI=1S/C27H26NO5S/c1-31-23-14-21-15-26(34-25(21)16-24(23)32-2)22(29)9-6-18-10-12-28(13-11-18)17-19-4-7-20(8-5-19)27(30)33-3/h4-5,7-8,10-16H,6,9,17H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117619

(1-Cyanomethyl-4-[3-(5,6-dimethoxy-benzo[b]thiophen...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CCC1CC[N+](C)(CC#N)CC1 |(-4.13,-6.71,;-2.8,-5.94,;-1.47,-6.71,;-.16,-5.94,;1.18,-6.71,;2.66,-6.22,;3.57,-7.48,;2.66,-8.74,;1.18,-8.25,;-.14,-9.02,;-1.47,-8.25,;-2.8,-9.02,;-4.13,-8.25,;5.12,-7.48,;5.89,-8.81,;5.89,-6.14,;7.43,-6.14,;8.2,-4.81,;7.29,-3.56,;7.93,-2.16,;9.46,-2.01,;8.48,-.82,;10.96,-1.64,;11.41,-.17,;11.85,1.3,;10.35,-3.25,;9.72,-4.66,)| Show InChI InChI=1S/C21H27N2O3S/c1-23(11-8-22)9-6-15(7-10-23)4-5-17(24)21-13-16-12-18(25-2)19(26-3)14-20(16)27-21/h12-15H,4-7,9-11H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117624
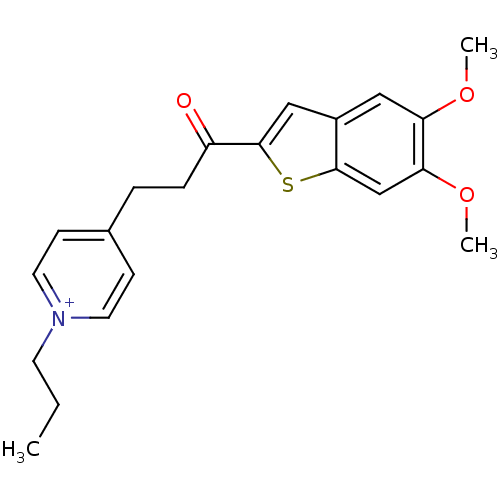
(4-[3-(5,6-Dimethoxy-benzo[b]thiophen-2-yl)-3-oxo-p...)Show SMILES CCC[n+]1ccc(CCC(=O)c2cc3cc(OC)c(OC)cc3s2)cc1 Show InChI InChI=1S/C21H24NO3S/c1-4-9-22-10-7-15(8-11-22)5-6-17(23)21-13-16-12-18(24-2)19(25-3)14-20(16)26-21/h7-8,10-14H,4-6,9H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117601
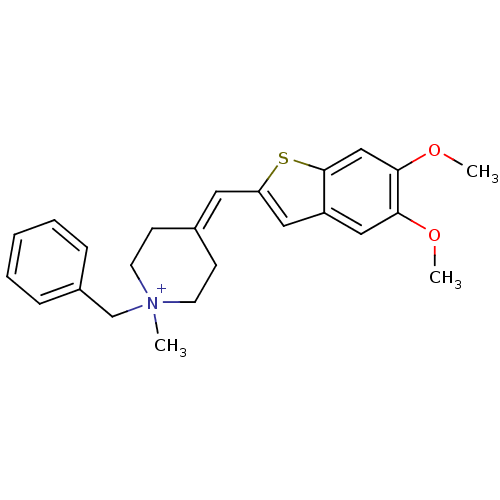
(1-Benzyl-4-(5,6-dimethoxy-benzo[b]thiophen-2-ylmet...)Show SMILES COc1cc2cc(C=C3CC[N+](C)(Cc4ccccc4)CC3)sc2cc1OC |(3.16,-1.52,;4.48,-.75,;5.81,-1.52,;7.12,-.75,;8.45,-1.52,;9.94,-1.03,;10.85,-2.29,;12.39,-2.29,;13.14,-.96,;14.68,-.79,;15.29,.62,;14.38,1.86,;13.17,2.82,;14.99,3.26,;16.53,3.43,;17.13,4.85,;18.65,5.02,;19.58,3.78,;18.97,2.37,;17.44,2.19,;12.84,1.68,;12.23,.27,;9.94,-3.55,;8.45,-3.06,;7.14,-3.83,;5.81,-3.06,;4.48,-3.83,;3.16,-3.06,)| Show InChI InChI=1S/C24H28NO2S/c1-25(17-19-7-5-4-6-8-19)11-9-18(10-12-25)13-21-14-20-15-22(26-2)23(27-3)16-24(20)28-21/h4-8,13-16H,9-12,17H2,1-3H3/q+1/b18-13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117621
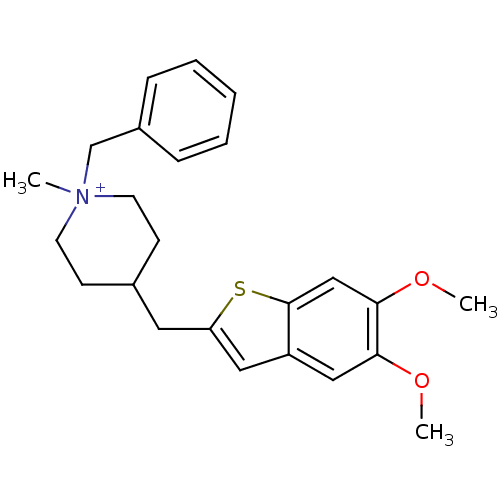
(1-Benzyl-4-(5,6-dimethoxy-benzo[b]thiophen-2-ylmet...)Show SMILES COc1cc2cc(CC3CC[N+](C)(Cc4ccccc4)CC3)sc2cc1OC |(3.16,-1.52,;4.48,-.75,;5.81,-1.52,;7.12,-.75,;8.45,-1.52,;9.94,-1.03,;10.85,-2.29,;12.39,-2.29,;13.14,-.96,;14.68,-.79,;15.29,.62,;14.38,1.86,;13.17,2.82,;14.99,3.26,;16.53,3.43,;17.13,4.85,;18.65,5.02,;19.58,3.78,;18.97,2.37,;17.44,2.19,;12.84,1.68,;12.23,.27,;9.94,-3.55,;8.45,-3.06,;7.14,-3.83,;5.81,-3.06,;4.48,-3.83,;3.16,-3.06,)| Show InChI InChI=1S/C24H30NO2S/c1-25(17-19-7-5-4-6-8-19)11-9-18(10-12-25)13-21-14-20-15-22(26-2)23(27-3)16-24(20)28-21/h4-8,14-16,18H,9-13,17H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117610

(1-Benzyl-4-[(5,6-dimethoxy-benzo[b]thiophen-2-yl)-...)Show SMILES COc1cc2cc(sc2cc1OC)C(O)C1CC[N+](C)(Cc2ccccc2)CC1 |(3.16,-1.52,;4.48,-.75,;5.81,-1.52,;7.12,-.75,;8.45,-1.52,;9.94,-1.03,;10.85,-2.29,;9.94,-3.55,;8.45,-3.06,;7.14,-3.83,;5.81,-3.06,;4.48,-3.83,;3.16,-3.06,;12.39,-2.29,;13.16,-3.62,;13.14,-.96,;14.68,-.79,;15.29,.62,;14.38,1.86,;13.17,2.82,;14.99,3.26,;16.53,3.43,;17.44,2.19,;18.97,2.37,;19.58,3.78,;18.65,5.02,;17.13,4.85,;12.84,1.68,;12.23,.27,)| Show InChI InChI=1S/C24H30NO3S/c1-25(16-17-7-5-4-6-8-17)11-9-18(10-12-25)24(26)23-14-19-13-20(27-2)21(28-3)15-22(19)29-23/h4-8,13-15,18,24,26H,9-12,16H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
Concentration required for the inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117601

(1-Benzyl-4-(5,6-dimethoxy-benzo[b]thiophen-2-ylmet...)Show SMILES COc1cc2cc(C=C3CC[N+](C)(Cc4ccccc4)CC3)sc2cc1OC |(3.16,-1.52,;4.48,-.75,;5.81,-1.52,;7.12,-.75,;8.45,-1.52,;9.94,-1.03,;10.85,-2.29,;12.39,-2.29,;13.14,-.96,;14.68,-.79,;15.29,.62,;14.38,1.86,;13.17,2.82,;14.99,3.26,;16.53,3.43,;17.13,4.85,;18.65,5.02,;19.58,3.78,;18.97,2.37,;17.44,2.19,;12.84,1.68,;12.23,.27,;9.94,-3.55,;8.45,-3.06,;7.14,-3.83,;5.81,-3.06,;4.48,-3.83,;3.16,-3.06,)| Show InChI InChI=1S/C24H28NO2S/c1-25(17-19-7-5-4-6-8-19)11-9-18(10-12-25)13-21-14-20-15-22(26-2)23(27-3)16-24(20)28-21/h4-8,13-16H,9-12,17H2,1-3H3/q+1/b18-13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117614

(4-[3-(5,6-Dimethoxy-benzo[b]thiophen-2-yl)-3-oxo-p...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CCc1cc[n+](Cc2ccsc2)cc1 Show InChI InChI=1S/C23H22NO3S2/c1-26-20-11-18-12-23(29-22(18)13-21(20)27-2)19(25)4-3-16-5-8-24(9-6-16)14-17-7-10-28-15-17/h5-13,15H,3-4,14H2,1-2H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117616

(1-Benzyl-4-[2-(5,6-dimethoxy-benzo[b]thiophen-2-yl...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)C=C1CC[N+](C)(Cc2ccccc2)CC1 |(3.16,-1.52,;4.48,-.75,;5.81,-1.52,;7.12,-.75,;8.45,-1.52,;9.94,-1.03,;10.85,-2.29,;9.94,-3.55,;8.45,-3.06,;7.14,-3.83,;5.81,-3.06,;4.48,-3.83,;3.16,-3.06,;12.39,-2.29,;13.16,-3.62,;13.16,-.96,;14.7,-.96,;15.1,-2.43,;16.59,-2.82,;17.67,-1.73,;18.43,-.38,;19.16,-2.11,;19.58,-3.59,;21.05,-3.97,;21.47,-5.44,;20.4,-6.56,;18.91,-6.17,;18.49,-4.69,;17.25,-.24,;15.76,.14,)| Show InChI InChI=1S/C25H28NO3S/c1-26(17-19-7-5-4-6-8-19)11-9-18(10-12-26)13-21(27)25-15-20-14-22(28-2)23(29-3)16-24(20)30-25/h4-8,13-16H,9-12,17H2,1-3H3/q+1/b18-13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 7.67E+3 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117599

(1-Benzyl-4-[3-(5,6-dimethoxy-benzo[b]thiophen-2-yl...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CC(O)C1CC[N+](C)(Cc2ccccc2)CC1 |(3.16,-1.52,;4.48,-.75,;5.81,-1.52,;7.12,-.75,;8.45,-1.52,;9.94,-1.03,;10.85,-2.29,;9.94,-3.55,;8.45,-3.06,;7.14,-3.83,;5.81,-3.06,;4.48,-3.83,;3.16,-3.06,;12.39,-2.29,;13.16,-3.62,;13.16,-.96,;14.7,-.96,;15.47,-2.29,;15.47,.37,;16.99,.27,;17.86,1.54,;17.18,2.93,;16.18,4.08,;18.04,4.2,;19.58,4.1,;20.25,2.71,;21.78,2.61,;22.64,3.89,;21.96,5.27,;20.42,5.37,;15.64,3.03,;14.78,1.75,)| Show InChI InChI=1S/C26H32NO4S/c1-27(17-18-7-5-4-6-8-18)11-9-19(10-12-27)21(28)15-22(29)26-14-20-13-23(30-2)24(31-3)16-25(20)32-26/h4-8,13-14,16,19,21,28H,9-12,15,17H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 170 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117596

(1-Cyclopropylmethyl-4-[3-(5,6-dimethoxy-benzo[b]th...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CCc1cc[n+](CC2CC2)cc1 Show InChI InChI=1S/C22H24NO3S/c1-25-19-11-17-12-22(27-21(17)13-20(19)26-2)18(24)6-5-15-7-9-23(10-8-15)14-16-3-4-16/h7-13,16H,3-6,14H2,1-2H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117615

(1-Benzyl-4-[3-(5,6-dimethoxy-benzo[b]thiophen-2-yl...)Show SMILES COC(CC(=O)c1cc2cc(OC)c(OC)cc2s1)C1CC[N+](C)(Cc2ccccc2)CC1 |(11.32,-7.89,;12.1,-6.55,;11.32,-5.22,;9.78,-5.22,;9.01,-6.55,;9.78,-7.89,;7.47,-6.55,;6.55,-5.29,;5.07,-5.78,;3.74,-5.01,;2.42,-5.78,;1.08,-5.01,;-.23,-5.78,;2.42,-7.33,;1.08,-8.1,;-.23,-7.33,;3.75,-8.1,;5.07,-7.33,;6.55,-7.82,;12.1,-3.89,;13.63,-3.98,;14.5,-2.72,;13.82,-1.32,;12.81,-.17,;14.68,-.05,;16.23,-.15,;17.07,1.11,;18.61,1.02,;19.29,-.38,;18.42,-1.64,;16.89,-1.55,;12.28,-1.22,;11.41,-2.51,)| Show InChI InChI=1S/C27H34NO4S/c1-28(18-19-8-6-5-7-9-19)12-10-20(11-13-28)23(30-2)16-22(29)27-15-21-14-24(31-3)25(32-4)17-26(21)33-27/h5-9,14-15,17,20,23H,10-13,16,18H2,1-4H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 650 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117604

(4-[3-(5,6-Dimethoxy-benzo[b]thiophen-2-yl)-3-oxo-p...)Show InChI InChI=1S/C19H20NO3S/c1-20-8-6-13(7-9-20)4-5-15(21)19-11-14-10-16(22-2)17(23-3)12-18(14)24-19/h6-12H,4-5H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117620

(4-[3-(5,6-Dimethoxy-benzo[b]thiophen-2-yl)-3-oxo-p...)Show SMILES COCC[n+]1ccc(CCC(=O)c2cc3cc(OC)c(OC)cc3s2)cc1 Show InChI InChI=1S/C21H24NO4S/c1-24-11-10-22-8-6-15(7-9-22)4-5-17(23)21-13-16-12-18(25-2)19(26-3)14-20(16)27-21/h6-9,12-14H,4-5,10-11H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 290 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117605

(4-[3-(5,6-Dimethoxy-benzo[b]thiophen-2-yl)-3-oxo-p...)Show SMILES CC[n+]1ccc(CCC(=O)c2cc3cc(OC)c(OC)cc3s2)cc1 Show InChI InChI=1S/C20H22NO3S/c1-4-21-9-7-14(8-10-21)5-6-16(22)20-12-15-11-17(23-2)18(24-3)13-19(15)25-20/h7-13H,4-6H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117623

(1-Cyclobutylmethyl-4-[3-(5,6-dimethoxy-benzo[b]thi...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CCc1cc[n+](CC2CCC2)cc1 Show InChI InChI=1S/C23H26NO3S/c1-26-20-12-18-13-23(28-22(18)14-21(20)27-2)19(25)7-6-16-8-10-24(11-9-16)15-17-4-3-5-17/h8-14,17H,3-7,15H2,1-2H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.72E+3 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117594

(1-Benzyl-4-[3-(5,6-dimethoxy-benzo[b]thiophen-2-yl...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CCC1CC[N+](C)(Cc2ccccc2)CC1 |(-.09,-3.27,;1.22,-2.5,;2.55,-3.27,;3.88,-2.5,;5.21,-3.27,;6.68,-2.78,;7.59,-4.04,;6.68,-5.3,;5.21,-4.81,;3.88,-5.58,;2.55,-4.81,;1.22,-5.58,;-.09,-4.81,;9.13,-4.04,;9.9,-5.37,;9.9,-2.71,;11.44,-2.71,;12.21,-1.38,;13.74,-1.22,;14.37,.18,;13.47,1.42,;12.28,2.4,;14.1,2.84,;15.64,2.99,;16.27,4.39,;17.79,4.54,;18.7,3.29,;18.07,1.88,;16.53,1.74,;11.95,1.28,;11.3,-.12,)| Show InChI InChI=1S/C26H32NO3S/c1-27(18-20-7-5-4-6-8-20)13-11-19(12-14-27)9-10-22(28)26-16-21-15-23(29-2)24(30-3)17-25(21)31-26/h4-8,15-17,19H,9-14,18H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117595

(1-Benzyl-4-[3-(5,6-dimethoxy-benzo[b]thiophen-2-yl...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CCc1cc[n+](Cc2ccccc2)cc1 Show InChI InChI=1S/C25H24NO3S/c1-28-22-14-20-15-25(30-24(20)16-23(22)29-2)21(27)9-8-18-10-12-26(13-11-18)17-19-6-4-3-5-7-19/h3-7,10-16H,8-9,17H2,1-2H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 99 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117600

(1-Benzyl-4-[5-(5,6-dimethoxy-benzo[b]thiophen-2-yl...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CC(O)CCC1CC[N+](C)(Cc2ccccc2)CC1 |(-.74,-2.58,;.58,-1.81,;1.92,-2.58,;3.23,-1.81,;4.56,-2.58,;6.05,-2.09,;6.96,-3.35,;6.05,-4.61,;4.56,-4.12,;3.25,-4.89,;1.92,-4.12,;.58,-4.89,;-.74,-4.12,;8.5,-3.35,;9.27,-4.68,;9.27,-2.01,;10.82,-2.01,;11.59,-3.35,;11.59,-.68,;13.13,-.68,;13.88,.67,;15.4,.9,;15.95,2.34,;14.98,3.54,;13.74,4.44,;15.54,4.98,;17.06,5.22,;18.02,4.02,;19.54,4.27,;20.1,5.7,;19.12,6.91,;17.6,6.65,;13.46,3.29,;12.9,1.85,)| Show InChI InChI=1S/C28H36NO4S/c1-29(19-21-7-5-4-6-8-21)13-11-20(12-14-29)9-10-23(30)17-24(31)28-16-22-15-25(32-2)26(33-3)18-27(22)34-28/h4-8,15-16,18,20,23,30H,9-14,17,19H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117612

(4-[3-(5,6-Dimethoxy-benzo[b]thiophen-2-yl)-3-oxo-p...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CCc1cc[n+](Cc2ccc(F)cc2)cc1 Show InChI InChI=1S/C25H23FNO3S/c1-29-22-13-19-14-25(31-24(19)15-23(22)30-2)21(28)8-5-17-9-11-27(12-10-17)16-18-3-6-20(26)7-4-18/h3-4,6-7,9-15H,5,8,16H2,1-2H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.65E+3 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117606

(1-Benzyl-4-[5-(5,6-dimethoxy-benzo[b]thiophen-2-yl...)Show SMILES COc1cc2cc(sc2cc1OC)C(=O)CCCCC1CC[N+](C)(Cc2ccccc2)CC1 |(-.74,-2.58,;.58,-1.81,;1.92,-2.58,;3.23,-1.81,;4.56,-2.58,;6.05,-2.09,;6.96,-3.35,;6.05,-4.61,;4.56,-4.12,;3.25,-4.89,;1.92,-4.12,;.58,-4.89,;-.74,-4.12,;8.5,-3.35,;9.27,-4.68,;9.27,-2.01,;10.82,-2.01,;11.59,-.68,;13.13,-.68,;13.88,.67,;15.4,.9,;15.95,2.34,;14.98,3.54,;13.74,4.44,;15.54,4.98,;17.06,5.22,;17.6,6.65,;19.12,6.91,;20.1,5.7,;19.54,4.27,;18.02,4.02,;13.46,3.29,;12.9,1.85,)| Show InChI InChI=1S/C28H36NO3S/c1-29(20-22-10-5-4-6-11-22)15-13-21(14-16-29)9-7-8-12-24(30)28-18-23-17-25(31-2)26(32-3)19-27(23)33-28/h4-6,10-11,17-19,21H,7-9,12-16,20H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50117617

(4-[3-(5,6-Dimethoxy-benzo[b]thiophen-2-yl)-3-oxo-p...)Show SMILES COC(=O)c1ccc(C[n+]2ccc(CCC(=O)c3cc4cc(OC)c(OC)cc4s3)cc2)cc1 Show InChI InChI=1S/C27H26NO5S/c1-31-23-14-21-15-26(34-25(21)16-24(23)32-2)22(29)9-6-18-10-12-28(13-11-18)17-19-4-7-20(8-5-19)27(30)33-3/h4-5,7-8,10-16H,6,9,17H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.67E+3 | n/a | n/a | n/a | n/a |
Organon Laboratories Ltd.
Curated by ChEMBL
| Assay Description
In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. |
Bioorg Med Chem Lett 12: 2569-72 (2002)
BindingDB Entry DOI: 10.7270/Q28916C0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data