 Found 383 hits Enz. Inhib. hit(s) with all data for entry = 50036752
Found 383 hits Enz. Inhib. hit(s) with all data for entry = 50036752 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080684
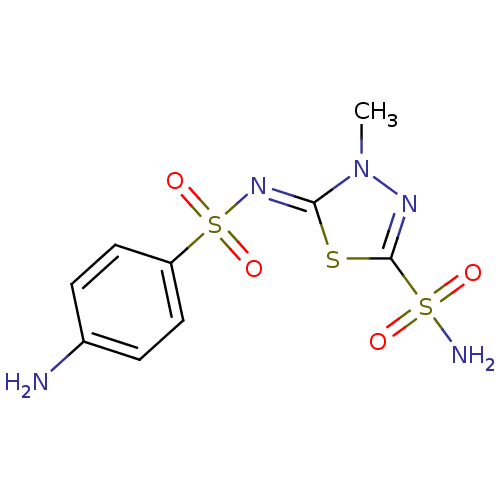
(5-(4-aminophenylsulfonylimino)-4-methyl-4,5-dihydr...)Show SMILES Cn1nc(s\c1=N/S(=O)(=O)c1ccc(N)cc1)S(N)(=O)=O Show InChI InChI=1S/C9H11N5O4S3/c1-14-8(19-9(12-14)20(11,15)16)13-21(17,18)7-4-2-6(10)3-5-7/h2-5H,10H2,1H3,(H2,11,15,16)/b13-8- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM50080684

(5-(4-aminophenylsulfonylimino)-4-methyl-4,5-dihydr...)Show SMILES Cn1nc(s\c1=N/S(=O)(=O)c1ccc(N)cc1)S(N)(=O)=O Show InChI InChI=1S/C9H11N5O4S3/c1-14-8(19-9(12-14)20(11,15)16)13-21(17,18)7-4-2-6(10)3-5-7/h2-5H,10H2,1H3,(H2,11,15,16)/b13-8- | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine carbonic anhydrase IV (CA4), isolated from bovine lung microsomes |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50080684

(5-(4-aminophenylsulfonylimino)-4-methyl-4,5-dihydr...)Show SMILES Cn1nc(s\c1=N/S(=O)(=O)c1ccc(N)cc1)S(N)(=O)=O Show InChI InChI=1S/C9H11N5O4S3/c1-14-8(19-9(12-14)20(11,15)16)13-21(17,18)7-4-2-6(10)3-5-7/h2-5H,10H2,1H3,(H2,11,15,16)/b13-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase I (CA1) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080658
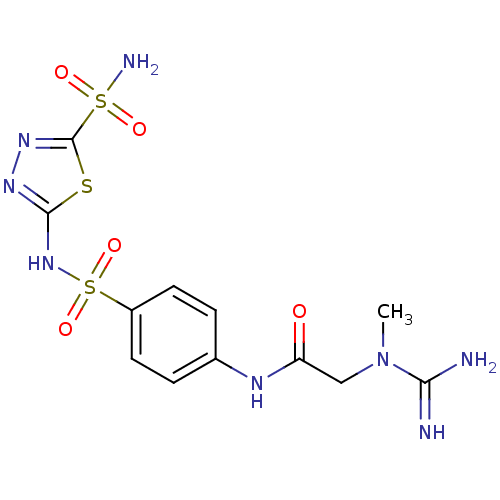
(2-(N-Methyl-guanidino)-N-[4-(5-sulfamoyl-[1,3,4]th...)Show SMILES CN(CC(=O)Nc1ccc(cc1)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O)C(N)=N Show InChI InChI=1S/C12H16N8O5S3/c1-20(10(13)14)6-9(21)16-7-2-4-8(5-3-7)28(24,25)19-11-17-18-12(26-11)27(15,22)23/h2-5H,6H2,1H3,(H3,13,14)(H,16,21)(H,17,19)(H2,15,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080647
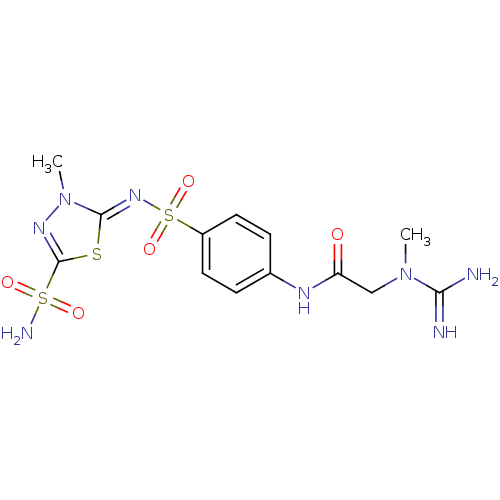
(2-(N-Methyl-guanidino)-N-{4-[3-methyl-5-sulfamoyl-...)Show SMILES CN(CC(=O)Nc1ccc(cc1)S(=O)(=O)\N=c1/sc(nn1C)S(N)(=O)=O)C(N)=N Show InChI InChI=1S/C13H18N8O5S3/c1-20(11(14)15)7-10(22)17-8-3-5-9(6-4-8)29(25,26)19-12-21(2)18-13(27-12)28(16,23)24/h3-6H,7H2,1-2H3,(H3,14,15)(H,17,22)(H2,16,23,24)/b19-12- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080640
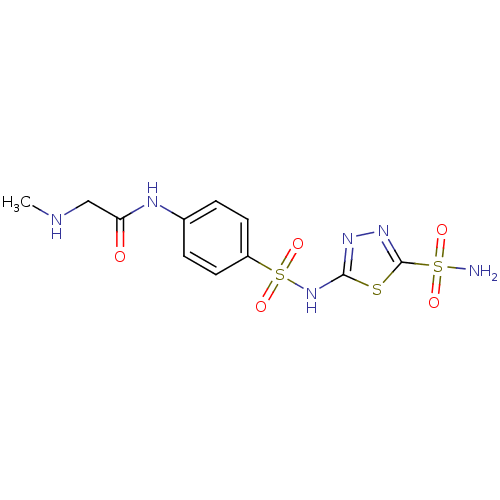
(2-Methylamino-N-[4-(5-sulfamoyl-[1,3,4]thiadiazol-...)Show SMILES CNCC(=O)Nc1ccc(cc1)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C11H14N6O5S3/c1-13-6-9(18)14-7-2-4-8(5-3-7)25(21,22)17-10-15-16-11(23-10)24(12,19)20/h2-5,13H,6H2,1H3,(H,14,18)(H,15,17)(H2,12,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080698
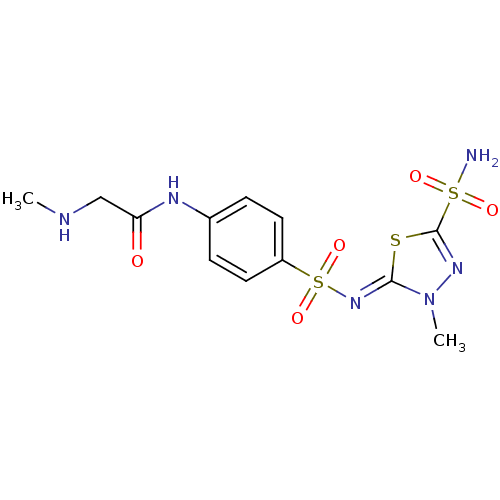
(2-Methylamino-N-{4-[3-methyl-5-sulfamoyl-3H-[1,3,4...)Show SMILES CNCC(=O)Nc1ccc(cc1)S(=O)(=O)\N=c1/sc(nn1C)S(N)(=O)=O Show InChI InChI=1S/C12H16N6O5S3/c1-14-7-10(19)15-8-3-5-9(6-4-8)26(22,23)17-11-18(2)16-12(24-11)25(13,20)21/h3-6,14H,7H2,1-2H3,(H,15,19)(H2,13,20,21)/b17-11- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080690
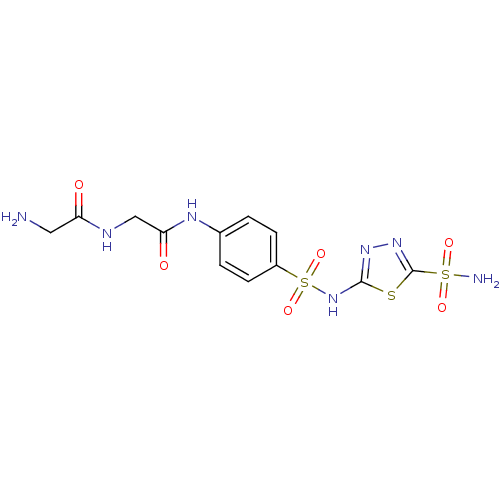
(2-Amino-N-{[4-(5-sulfamoyl-[1,3,4]thiadiazol-2-yls...)Show SMILES NCC(=O)NCC(=O)Nc1ccc(cc1)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C12H15N7O6S3/c13-5-9(20)15-6-10(21)16-7-1-3-8(4-2-7)28(24,25)19-11-17-18-12(26-11)27(14,22)23/h1-4H,5-6,13H2,(H,15,20)(H,16,21)(H,17,19)(H2,14,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080691

(3-[2-(N-Methyl-guanidino)-acetylamino]-N-(5-sulfam...)Show SMILES CN(CC(=O)NCCC(=O)Nc1nnc(s1)S(N)(=O)=O)C(N)=N Show InChI InChI=1S/C9H16N8O4S2/c1-17(7(10)11)4-6(19)13-3-2-5(18)14-8-15-16-9(22-8)23(12,20)21/h2-4H2,1H3,(H3,10,11)(H,13,19)(H2,12,20,21)(H,14,15,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10870

(2-N-(4-aminobenzene)-1,3,4-thiadiazole-2,5-disulfo...)Show InChI InChI=1S/C8H9N5O4S3/c9-5-1-3-6(4-2-5)20(16,17)13-7-11-12-8(18-7)19(10,14)15/h1-4H,9H2,(H,11,13)(H2,10,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080727
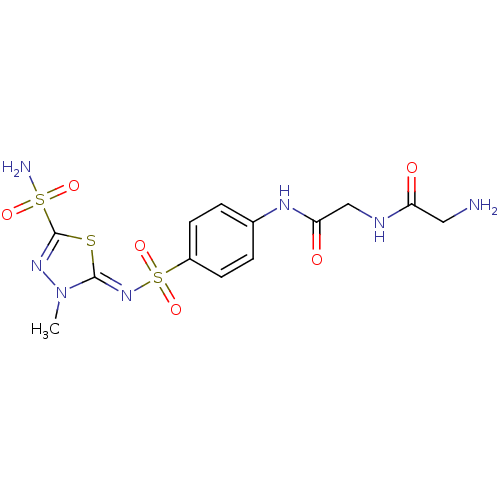
(2-Amino-N-({4-[3-methyl-5-sulfamoyl-3H-[1,3,4]thia...)Show SMILES Cn1nc(s\c1=N/S(=O)(=O)c1ccc(NC(=O)CNC(=O)CN)cc1)S(N)(=O)=O Show InChI InChI=1S/C13H17N7O6S3/c1-20-12(27-13(18-20)28(15,23)24)19-29(25,26)9-4-2-8(3-5-9)17-11(22)7-16-10(21)6-14/h2-5H,6-7,14H2,1H3,(H,16,21)(H,17,22)(H2,15,23,24)/b19-12- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080648

(2-Amino-N-[4-(5-sulfamoyl-[1,3,4]thiadiazol-2-ylsu...)Show SMILES NCC(=O)Nc1ccc(cc1)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C10H12N6O5S3/c11-5-8(17)13-6-1-3-7(4-2-6)24(20,21)16-9-14-15-10(22-9)23(12,18)19/h1-4H,5,11H2,(H,13,17)(H,14,16)(H2,12,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080726
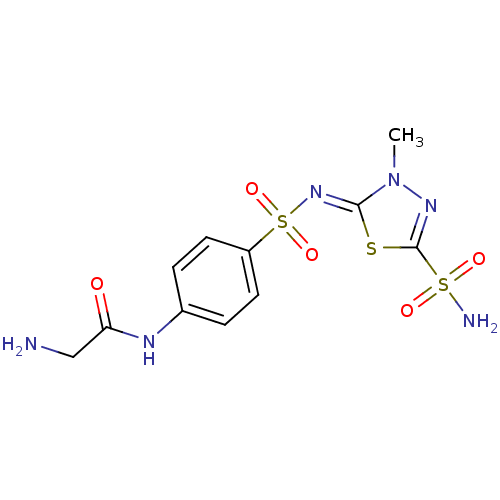
(2-Amino-N-{4-[3-methyl-5-sulfamoyl-3H-[1,3,4]thiad...)Show SMILES Cn1nc(s\c1=N/S(=O)(=O)c1ccc(NC(=O)CN)cc1)S(N)(=O)=O Show InChI InChI=1S/C11H14N6O5S3/c1-17-10(23-11(15-17)24(13,19)20)16-25(21,22)8-4-2-7(3-5-8)14-9(18)6-12/h2-5H,6,12H2,1H3,(H,14,18)(H2,13,19,20)/b16-10- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080737
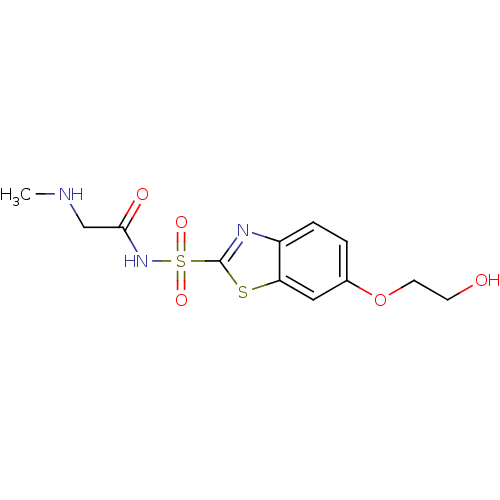
(6-(2-Hydroxy-ethoxy)-benzothiazole-2-sulfonic acid...)Show InChI InChI=1S/C12H15N3O5S2/c1-13-7-11(17)15-22(18,19)12-14-9-3-2-8(20-5-4-16)6-10(9)21-12/h2-3,6,13,16H,4-5,7H2,1H3,(H,15,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50079068
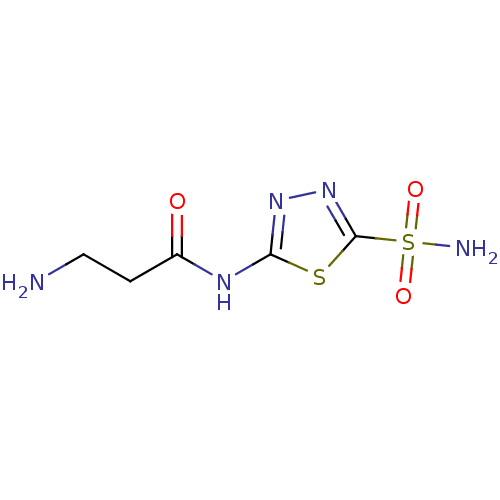
(3-amino-N-(5-sulfamoyl-[1,3,4]thiadiazol-2-yl)-pro...)Show InChI InChI=1S/C5H9N5O3S2/c6-2-1-3(11)8-4-9-10-5(14-4)15(7,12)13/h1-2,6H2,(H2,7,12,13)(H,8,9,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080735
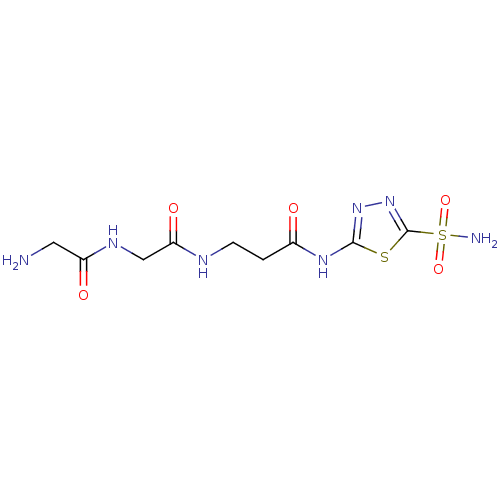
(3-[2-(2-Amino-acetylamino)-acetylamino]-N-(5-sulfa...)Show InChI InChI=1S/C9H15N7O5S2/c10-3-6(18)13-4-7(19)12-2-1-5(17)14-8-15-16-9(22-8)23(11,20)21/h1-4,10H2,(H,12,19)(H,13,18)(H2,11,20,21)(H,14,15,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080655

(3-(2-Methylamino-acetylamino)-N-(5-sulfamoyl-[1,3,...)Show InChI InChI=1S/C8H14N6O4S2/c1-10-4-6(16)11-3-2-5(15)12-7-13-14-8(19-7)20(9,17)18/h10H,2-4H2,1H3,(H,11,16)(H2,9,17,18)(H,12,13,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080694
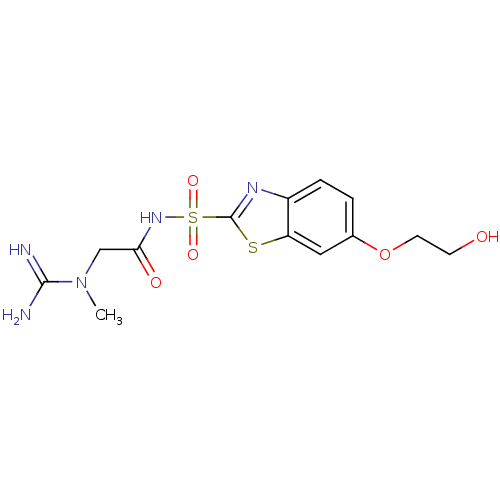
(6-(2-Hydroxy-ethoxy)-benzothiazole-2-sulfonic acid...)Show SMILES CN(CC(=O)NS(=O)(=O)c1nc2ccc(OCCO)cc2s1)C(N)=N Show InChI InChI=1S/C13H17N5O5S2/c1-18(12(14)15)7-11(20)17-25(21,22)13-16-9-3-2-8(23-5-4-19)6-10(9)24-13/h2-3,6,19H,4-5,7H2,1H3,(H3,14,15)(H,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080670
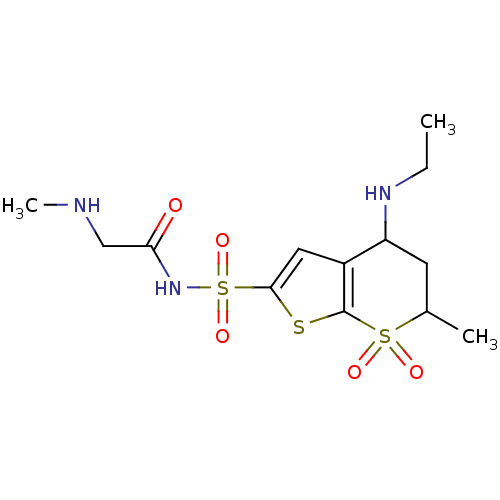
(4-Ethylamino-6-methyl-7,7-dioxo-4,5,6,7-tetrahydro...)Show SMILES CCNC1CC(C)S(=O)(=O)c2sc(cc12)S(=O)(=O)NC(=O)CNC Show InChI InChI=1S/C13H21N3O5S3/c1-4-15-10-5-8(2)23(18,19)13-9(10)6-12(22-13)24(20,21)16-11(17)7-14-3/h6,8,10,14-15H,4-5,7H2,1-3H3,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080716
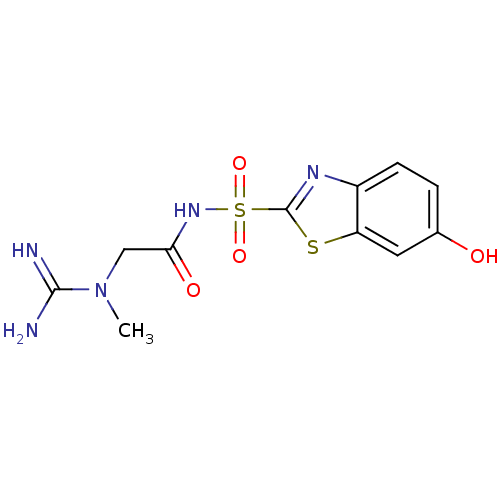
(6-Hydroxy-benzothiazole-2-sulfonic acid [2-(N-meth...)Show InChI InChI=1S/C11H13N5O4S2/c1-16(10(12)13)5-9(18)15-22(19,20)11-14-7-3-2-6(17)4-8(7)21-11/h2-4,17H,5H2,1H3,(H3,12,13)(H,15,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10885

((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...)Show SMILES CCN[C@H]1CN(CCCOC)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C12H21N3O5S3/c1-3-14-10-8-15(5-4-6-20-2)23(18,19)12-9(10)7-11(21-12)22(13,16)17/h7,10,14H,3-6,8H2,1-2H3,(H2,13,16,17)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase I (CA1) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080707
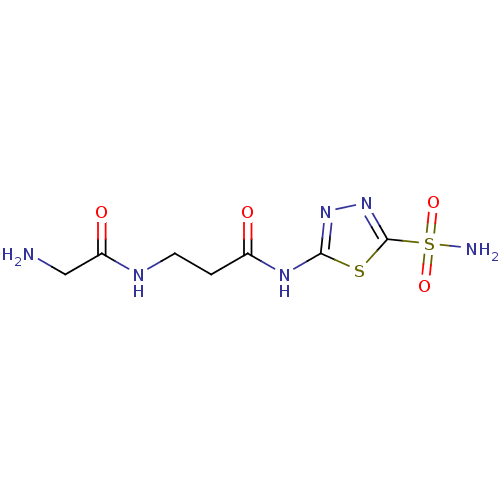
(3-(2-Amino-acetylamino)-N-(5-sulfamoyl-[1,3,4]thia...)Show InChI InChI=1S/C7H12N6O4S2/c8-3-5(15)10-2-1-4(14)11-6-12-13-7(18-6)19(9,16)17/h1-3,8H2,(H,10,15)(H2,9,16,17)(H,11,12,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080717
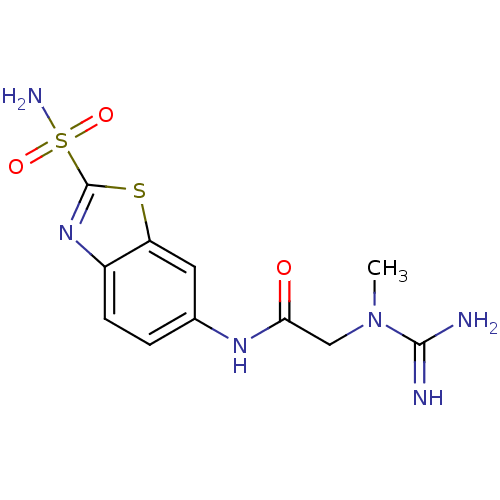
(2-(N-Methyl-guanidino)-N-(2-sulfamoyl-benzothiazol...)Show InChI InChI=1S/C11H14N6O3S2/c1-17(10(12)13)5-9(18)15-6-2-3-7-8(4-6)21-11(16-7)22(14,19)20/h2-4H,5H2,1H3,(H3,12,13)(H,15,18)(H2,14,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080705

(6-Hydroxy-benzothiazole-2-sulfonic acid (2-methyla...)Show InChI InChI=1S/C10H11N3O4S2/c1-11-5-9(15)13-19(16,17)10-12-7-3-2-6(14)4-8(7)18-10/h2-4,11,14H,5H2,1H3,(H,13,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080740
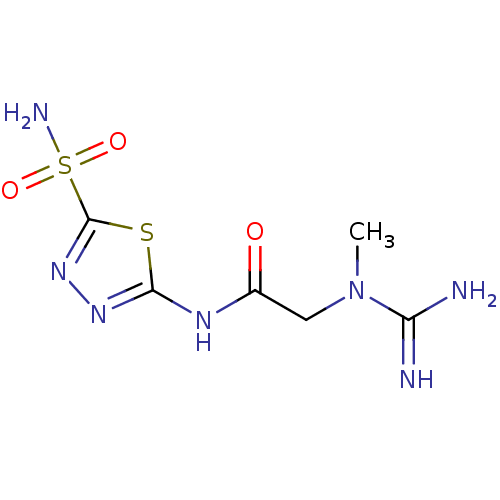
(2-(N-Methyl-guanidino)-N-(5-sulfamoyl-[1,3,4]thiad...)Show InChI InChI=1S/C6H11N7O3S2/c1-13(4(7)8)2-3(14)10-5-11-12-6(17-5)18(9,15)16/h2H2,1H3,(H3,7,8)(H2,9,15,16)(H,10,11,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080680
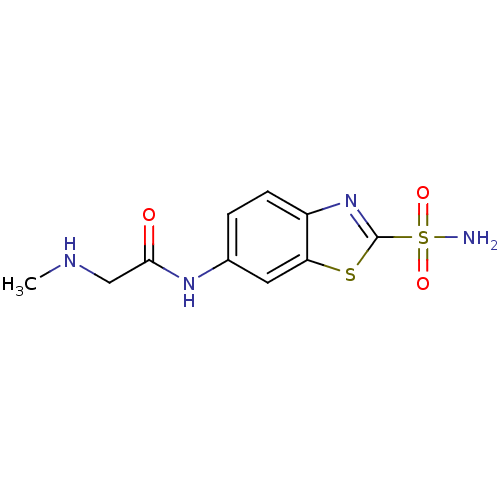
(2-Methylamino-N-(2-sulfamoyl-benzothiazol-6-yl)-ac...)Show InChI InChI=1S/C10H12N4O3S2/c1-12-5-9(15)13-6-2-3-7-8(4-6)18-10(14-7)19(11,16)17/h2-4,12H,5H2,1H3,(H,13,15)(H2,11,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM50080658

(2-(N-Methyl-guanidino)-N-[4-(5-sulfamoyl-[1,3,4]th...)Show SMILES CN(CC(=O)Nc1ccc(cc1)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O)C(N)=N Show InChI InChI=1S/C12H16N8O5S3/c1-20(10(13)14)6-9(21)16-7-2-4-8(5-3-7)28(24,25)19-11-17-18-12(26-11)27(15,22)23/h2-5H,6H2,1H3,(H3,13,14)(H,16,21)(H,17,19)(H2,15,22,23) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine carbonic anhydrase IV (CA4), isolated from bovine lung microsomes |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM10870

(2-N-(4-aminobenzene)-1,3,4-thiadiazole-2,5-disulfo...)Show InChI InChI=1S/C8H9N5O4S3/c9-5-1-3-6(4-2-5)20(16,17)13-7-11-12-8(18-7)19(10,14)15/h1-4H,9H2,(H,11,13)(H2,10,14,15) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine carbonic anhydrase IV (CA4), isolated from bovine lung microsomes |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080649
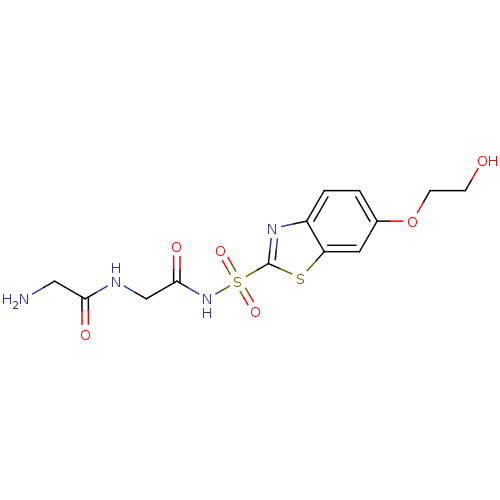
(2-Amino-N-{2-[6-(2-hydroxy-ethoxy)-benzothiazole-2...)Show SMILES NCC(=O)NCC(=O)NS(=O)(=O)c1nc2ccc(OCCO)cc2s1 Show InChI InChI=1S/C13H16N4O6S2/c14-6-11(19)15-7-12(20)17-25(21,22)13-16-9-2-1-8(23-4-3-18)5-10(9)24-13/h1-2,5,18H,3-4,6-7,14H2,(H,15,19)(H,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080635

(2-(N-Methyl-guanidino)-N-[3-methyl-5-sulfamoyl-3H-...)Show SMILES CN(CC(=O)N=c1sc(nn1C)S(N)(=O)=O)C(N)=N |w:5.4| Show InChI InChI=1S/C7H13N7O3S2/c1-13(5(8)9)3-4(15)11-6-14(2)12-7(18-6)19(10,16)17/h3H2,1-2H3,(H3,8,9)(H2,10,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080733
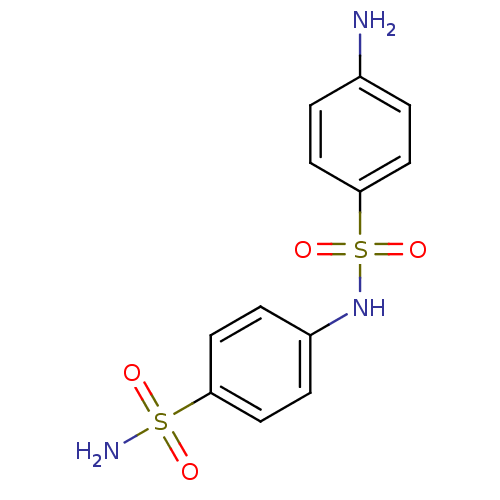
(4-(4-aminophenylsulfonamido)-1-benzenesulfonamide ...)Show InChI InChI=1S/C12H13N3O4S2/c13-9-1-5-12(6-2-9)21(18,19)15-10-3-7-11(8-4-10)20(14,16)17/h1-8,15H,13H2,(H2,14,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080730
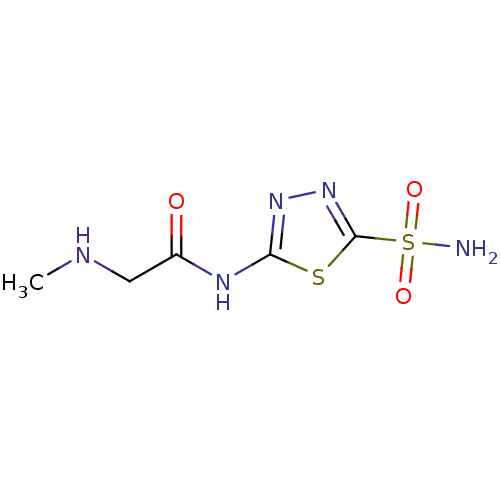
(2-Methylamino-N-(5-sulfamoyl-[1,3,4]thiadiazol-2-y...)Show InChI InChI=1S/C5H9N5O3S2/c1-7-2-3(11)8-4-9-10-5(14-4)15(6,12)13/h7H,2H2,1H3,(H2,6,12,13)(H,8,9,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10870

(2-N-(4-aminobenzene)-1,3,4-thiadiazole-2,5-disulfo...)Show InChI InChI=1S/C8H9N5O4S3/c9-5-1-3-6(4-2-5)20(16,17)13-7-11-12-8(18-7)19(10,14)15/h1-4H,9H2,(H,11,13)(H2,10,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase I (CA1) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080724
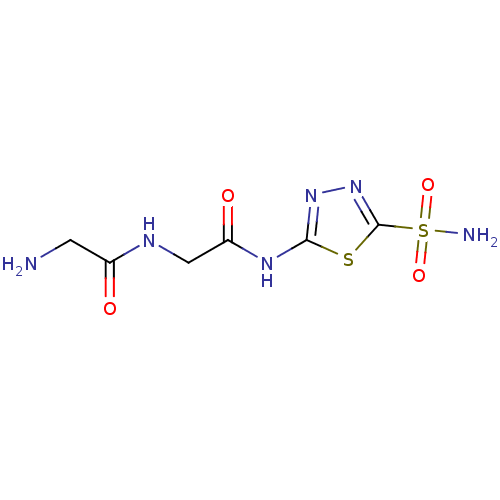
(2-Amino-N-[(5-sulfamoyl-[1,3,4]thiadiazol-2-ylcarb...)Show InChI InChI=1S/C6H10N6O4S2/c7-1-3(13)9-2-4(14)10-5-11-12-6(17-5)18(8,15)16/h1-2,7H2,(H,9,13)(H2,8,15,16)(H,10,11,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080741
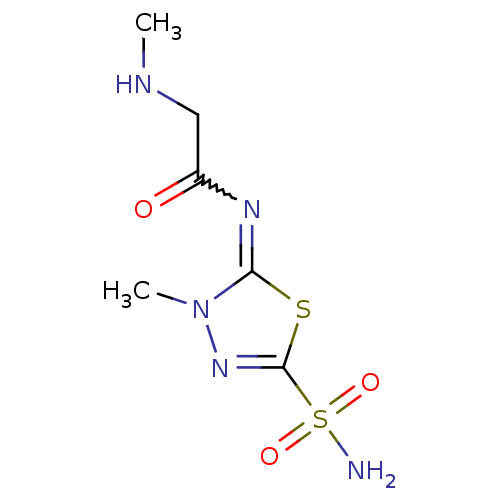
(2-Methylamino-N-[3-methyl-5-sulfamoyl-3H-[1,3,4]th...)Show InChI InChI=1S/C6H11N5O3S2/c1-8-3-4(12)9-5-11(2)10-6(15-5)16(7,13)14/h8H,3H2,1-2H3,(H2,7,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM50080647

(2-(N-Methyl-guanidino)-N-{4-[3-methyl-5-sulfamoyl-...)Show SMILES CN(CC(=O)Nc1ccc(cc1)S(=O)(=O)\N=c1/sc(nn1C)S(N)(=O)=O)C(N)=N Show InChI InChI=1S/C13H18N8O5S3/c1-20(11(14)15)7-10(22)17-8-3-5-9(6-4-8)29(25,26)19-12-21(2)18-13(27-12)28(16,23)24/h3-6H,7H2,1-2H3,(H3,14,15)(H,17,22)(H2,16,23,24)/b19-12- | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine carbonic anhydrase IV (CA4), isolated from bovine lung microsomes |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM50080690

(2-Amino-N-{[4-(5-sulfamoyl-[1,3,4]thiadiazol-2-yls...)Show SMILES NCC(=O)NCC(=O)Nc1ccc(cc1)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C12H15N7O6S3/c13-5-9(20)15-6-10(21)16-7-1-3-8(4-2-7)28(24,25)19-11-17-18-12(26-11)27(14,22)23/h1-4H,5-6,13H2,(H,15,20)(H,16,21)(H,17,19)(H2,14,22,23) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine carbonic anhydrase IV (CA4), isolated from bovine lung microsomes |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM50080640

(2-Methylamino-N-[4-(5-sulfamoyl-[1,3,4]thiadiazol-...)Show SMILES CNCC(=O)Nc1ccc(cc1)S(=O)(=O)Nc1nnc(s1)S(N)(=O)=O Show InChI InChI=1S/C11H14N6O5S3/c1-13-6-9(18)14-7-2-4-8(5-3-7)25(21,22)17-10-15-16-11(23-10)24(12,19)20/h2-5,13H,6H2,1H3,(H,14,18)(H,15,17)(H2,12,19,20) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine carbonic anhydrase IV (CA4), isolated from bovine lung microsomes |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50079049
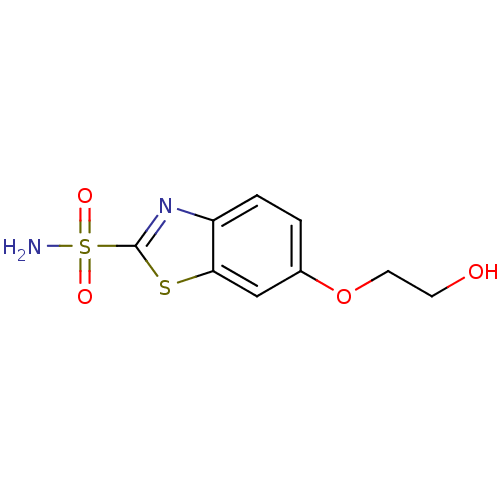
(6-(2-Hydroxy-ethoxy)-benzothiazole-2-sulfonic acid...)Show InChI InChI=1S/C9H10N2O4S2/c10-17(13,14)9-11-7-2-1-6(15-4-3-12)5-8(7)16-9/h1-2,5,12H,3-4H2,(H2,10,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080715

(2-Amino-N-{[3-methyl-5-sulfamoyl-3H-[1,3,4]thiadia...)Show SMILES Cn1nc(sc1=NC(=O)CNC(=O)CN)S(N)(=O)=O |w:6.7| Show InChI InChI=1S/C7H12N6O4S2/c1-13-6(18-7(12-13)19(9,16)17)11-5(15)3-10-4(14)2-8/h2-3,8H2,1H3,(H,10,14)(H2,9,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM50080698

(2-Methylamino-N-{4-[3-methyl-5-sulfamoyl-3H-[1,3,4...)Show SMILES CNCC(=O)Nc1ccc(cc1)S(=O)(=O)\N=c1/sc(nn1C)S(N)(=O)=O Show InChI InChI=1S/C12H16N6O5S3/c1-14-7-10(19)15-8-3-5-9(6-4-8)26(22,23)17-11-18(2)16-12(24-11)25(13,20)21/h3-6,14H,7H2,1-2H3,(H,15,19)(H2,13,20,21)/b17-11- | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine carbonic anhydrase IV (CA4), isolated from bovine lung microsomes |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080720

(CHEMBL123065 | N-(5-Chloro-2,4-disulfamoyl-phenyl)...)Show SMILES CN(CC(=O)Nc1cc(Cl)c(cc1S(N)(=O)=O)S(N)(=O)=O)C(N)=N Show InChI InChI=1S/C10H15ClN6O5S2/c1-17(10(12)13)4-9(18)16-6-2-5(11)7(23(14,19)20)3-8(6)24(15,21)22/h2-3H,4H2,1H3,(H3,12,13)(H,16,18)(H2,14,19,20)(H2,15,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10874
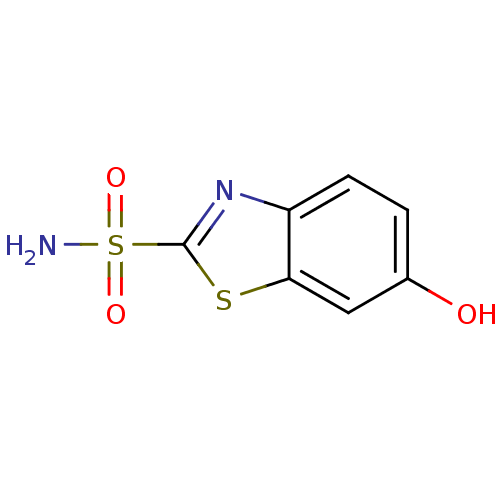
(6-hydroxy-1,3-benzothiazole-2-sulfonamide | CHEMBL...)Show InChI InChI=1S/C7H6N2O3S2/c8-14(11,12)7-9-5-2-1-4(10)3-6(5)13-7/h1-3,10H,(H2,8,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080703
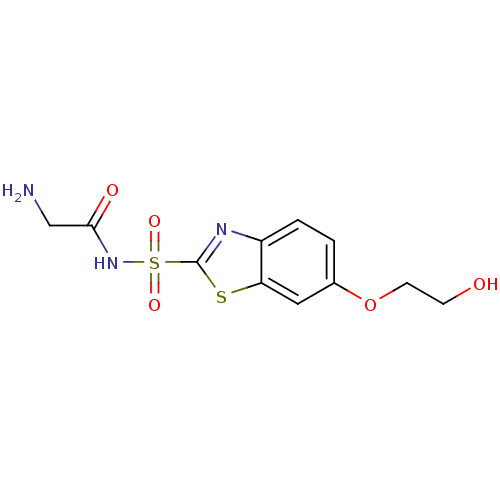
(6-(2-Hydroxy-ethoxy)-benzothiazole-2-sulfonic acid...)Show InChI InChI=1S/C11H13N3O5S2/c12-6-10(16)14-21(17,18)11-13-8-2-1-7(19-4-3-15)5-9(8)20-11/h1-2,5,15H,3-4,6,12H2,(H,14,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080732

(2-(N-Methyl-guanidino)-N-[4-(3-sulfamoyl-phenylsul...)Show SMILES CN(CC(=O)Nc1ccc(cc1)S(=O)(=O)Nc1cccc(c1)S(N)(=O)=O)C(N)=N Show InChI InChI=1S/C16H20N6O5S2/c1-22(16(17)18)10-15(23)20-11-5-7-13(8-6-11)29(26,27)21-12-3-2-4-14(9-12)28(19,24)25/h2-9,21H,10H2,1H3,(H3,17,18)(H,20,23)(H2,19,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080643
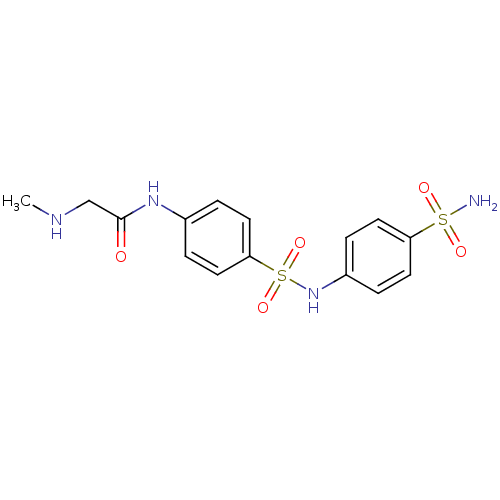
(2-Methylamino-N-[4-(4-sulfamoyl-phenylsulfamoyl)-p...)Show SMILES CNCC(=O)Nc1ccc(cc1)S(=O)(=O)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C15H18N4O5S2/c1-17-10-15(20)18-11-2-8-14(9-3-11)26(23,24)19-12-4-6-13(7-5-12)25(16,21)22/h2-9,17,19H,10H2,1H3,(H,18,20)(H2,16,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080700

(2-(N-Methyl-guanidino)-N-[4-(4-sulfamoyl-phenylsul...)Show SMILES CN(CC(=O)Nc1ccc(cc1)S(=O)(=O)Nc1ccc(cc1)S(N)(=O)=O)C(N)=N Show InChI InChI=1S/C16H20N6O5S2/c1-22(16(17)18)10-15(23)20-11-2-8-14(9-3-11)29(26,27)21-12-4-6-13(7-5-12)28(19,24)25/h2-9,21H,10H2,1H3,(H3,17,18)(H,20,23)(H2,19,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50080721
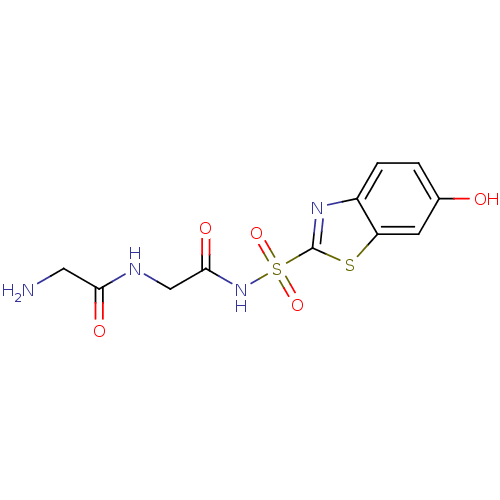
(2-Amino-N-[2-(6-hydroxy-benzothiazole-2-sulfonylam...)Show InChI InChI=1S/C11H12N4O5S2/c12-4-9(17)13-5-10(18)15-22(19,20)11-14-7-2-1-6(16)3-8(7)21-11/h1-3,16H,4-5,12H2,(H,13,17)(H,15,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant carbonic anhydrase II (CA2) |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM50080691

(3-[2-(N-Methyl-guanidino)-acetylamino]-N-(5-sulfam...)Show SMILES CN(CC(=O)NCCC(=O)Nc1nnc(s1)S(N)(=O)=O)C(N)=N Show InChI InChI=1S/C9H16N8O4S2/c1-17(7(10)11)4-6(19)13-3-2-5(18)14-8-15-16-9(22-8)23(12,20)21/h2-4H2,1H3,(H3,10,11)(H,13,19)(H2,12,20,21)(H,14,15,18) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine carbonic anhydrase IV (CA4), isolated from bovine lung microsomes |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 4
(Bos taurus (bovine)) | BDBM50080655

(3-(2-Methylamino-acetylamino)-N-(5-sulfamoyl-[1,3,...)Show InChI InChI=1S/C8H14N6O4S2/c1-10-4-6(16)11-3-2-5(15)12-7-13-14-8(19-7)20(9,17)18/h10H,2-4H2,1H3,(H,11,16)(H2,9,17,18)(H,12,13,15) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine carbonic anhydrase IV (CA4), isolated from bovine lung microsomes |
J Med Chem 42: 3690-700 (1999)
Article DOI: 10.1021/jm9901879
BindingDB Entry DOI: 10.7270/Q26Q1XXC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data