

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50134803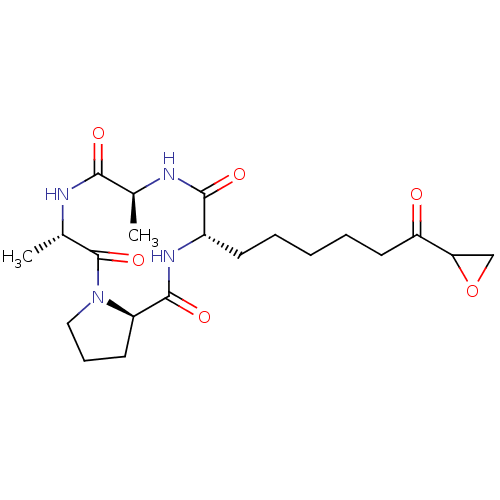 (5,8-Dimethyl-11-(6-oxiranyl-6-oxo-hexyl)-decahydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma"La Sapienza" Curated by ChEMBL | Assay Description Inhibition of Histone deacetylase 4 in mammalian cells. | J Med Chem 46: 4826-9 (2003) Article DOI: 10.1021/jm034167p BindingDB Entry DOI: 10.7270/Q28C9X0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50134799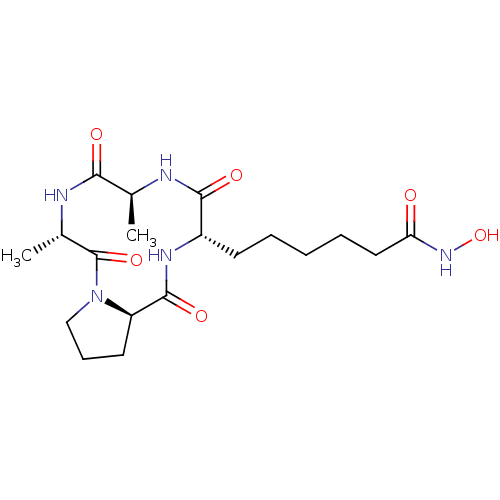 (6-((5S,8S,11S,13aR)-5,8-Dimethyl-4,7,10,13-tetraox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma"La Sapienza" Curated by ChEMBL | Assay Description Inhibition of Histone deacetylase 4 in mammalian cells. | J Med Chem 46: 4826-9 (2003) Article DOI: 10.1021/jm034167p BindingDB Entry DOI: 10.7270/Q28C9X0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM19130 ((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma"La Sapienza" Curated by ChEMBL | Assay Description Inhibitory activity against maize Histone deacetylase 2 at 1 mM | J Med Chem 46: 4826-9 (2003) Article DOI: 10.1021/jm034167p BindingDB Entry DOI: 10.7270/Q28C9X0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma"La Sapienza" Curated by ChEMBL | Assay Description Tested for maize Histone deacetylase 2 inhibitory activity | J Med Chem 46: 4826-9 (2003) Article DOI: 10.1021/jm034167p BindingDB Entry DOI: 10.7270/Q28C9X0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM50134800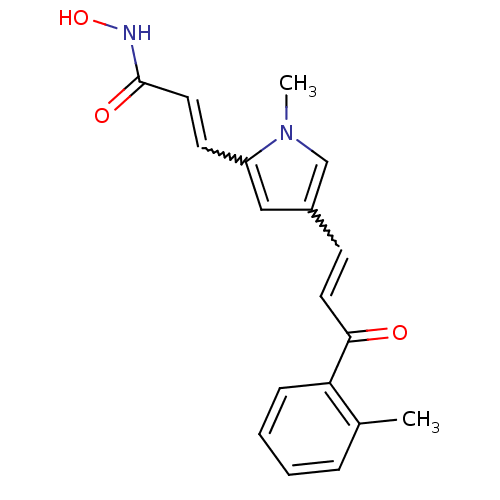 ((E)-N-Hydroxy-3-[1-methyl-4-((E)-3-oxo-3-o-tolyl-p...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma"La Sapienza" Curated by ChEMBL | Assay Description Tested for maize Histone deacetylase 2 inhibitory activity | J Med Chem 46: 4826-9 (2003) Article DOI: 10.1021/jm034167p BindingDB Entry DOI: 10.7270/Q28C9X0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM50134804 ((E)-N-Hydroxy-3-[1-methyl-4-((E)-3-oxo-3-phenyl-pr...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma"La Sapienza" Curated by ChEMBL | Assay Description Tested for maize Histone deacetylase 2 inhibitory activity | J Med Chem 46: 4826-9 (2003) Article DOI: 10.1021/jm034167p BindingDB Entry DOI: 10.7270/Q28C9X0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50354086 (FK-228 | Istodax | ROMIDEPSIN) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma"La Sapienza" Curated by ChEMBL | Assay Description Inhibition of Histone deacetylase 4 in mammalian cells. | J Med Chem 46: 4826-9 (2003) Article DOI: 10.1021/jm034167p BindingDB Entry DOI: 10.7270/Q28C9X0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM50134802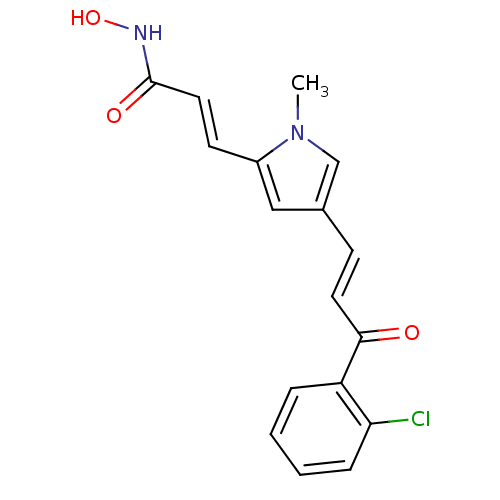 ((E)-3-{4-[(E)-3-(2-Chloro-phenyl)-3-oxo-propenyl]-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma"La Sapienza" Curated by ChEMBL | Assay Description Tested for maize Histone deacetylase 2 inhibitory activity | J Med Chem 46: 4826-9 (2003) Article DOI: 10.1021/jm034167p BindingDB Entry DOI: 10.7270/Q28C9X0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM50134795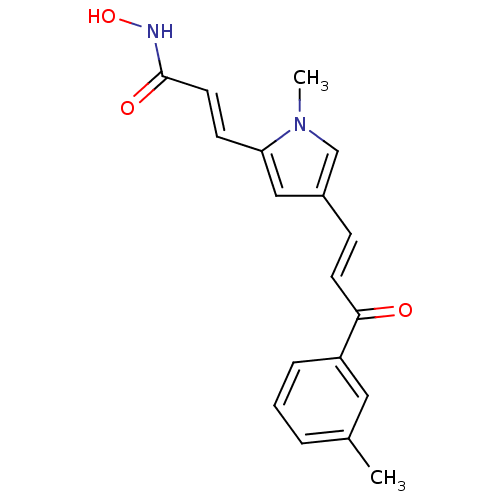 ((E)-N-Hydroxy-3-[1-methyl-4-((E)-3-oxo-3-m-tolyl-p...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma"La Sapienza" Curated by ChEMBL | Assay Description Tested for maize Histone deacetylase 2 inhibitory activity | J Med Chem 46: 4826-9 (2003) Article DOI: 10.1021/jm034167p BindingDB Entry DOI: 10.7270/Q28C9X0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM50134797 ((E)-N-Hydroxy-3-[1-methyl-4-((E)-3-oxo-3-p-tolyl-p...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma"La Sapienza" Curated by ChEMBL | Assay Description Tested for maize Histone deacetylase 2 inhibitory activity | J Med Chem 46: 4826-9 (2003) Article DOI: 10.1021/jm034167p BindingDB Entry DOI: 10.7270/Q28C9X0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM50134796 ((E)-3-{4-[(E)-3-(3-Chloro-phenyl)-3-oxo-propenyl]-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma"La Sapienza" Curated by ChEMBL | Assay Description Tested for maize Histone deacetylase 2 inhibitory activity | J Med Chem 46: 4826-9 (2003) Article DOI: 10.1021/jm034167p BindingDB Entry DOI: 10.7270/Q28C9X0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM50134801 ((E)-3-{4-[(E)-3-(4-Chloro-phenyl)-3-oxo-propenyl]-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma"La Sapienza" Curated by ChEMBL | Assay Description Tested for maize Histone deacetylase 2 inhibitory activity | J Med Chem 46: 4826-9 (2003) Article DOI: 10.1021/jm034167p BindingDB Entry DOI: 10.7270/Q28C9X0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50134805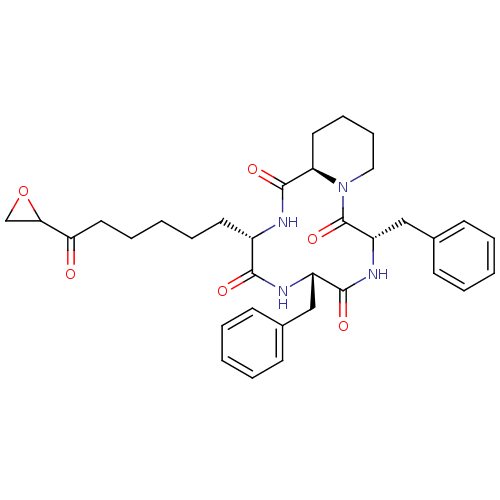 (6,9-Dibenzyl-12-(6-oxiranyl-6-oxo-hexyl)-decahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.24E+5 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma"La Sapienza" Curated by ChEMBL | Assay Description Tested for inhibition of Histone deacetylase 6 induced acetylated tubulin in mammalian cells. | J Med Chem 46: 4826-9 (2003) Article DOI: 10.1021/jm034167p BindingDB Entry DOI: 10.7270/Q28C9X0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50134805 (6,9-Dibenzyl-12-(6-oxiranyl-6-oxo-hexyl)-decahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 820 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma"La Sapienza" Curated by ChEMBL | Assay Description Inhibition of Histone deacetylase 1 induced acetylated histone in mammalian cells | J Med Chem 46: 4826-9 (2003) Article DOI: 10.1021/jm034167p BindingDB Entry DOI: 10.7270/Q28C9X0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50354086 (FK-228 | Istodax | ROMIDEPSIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma"La Sapienza" Curated by ChEMBL | Assay Description Inhibition of Histone deacetylase 1 induced acetylated histone in mammalian cells. | J Med Chem 46: 4826-9 (2003) Article DOI: 10.1021/jm034167p BindingDB Entry DOI: 10.7270/Q28C9X0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50134803 (5,8-Dimethyl-11-(6-oxiranyl-6-oxo-hexyl)-decahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.60E+5 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma"La Sapienza" Curated by ChEMBL | Assay Description Tested for inhibition of Histone deacetylase 6 induced acetylated tubulin in mammalian cells. | J Med Chem 46: 4826-9 (2003) Article DOI: 10.1021/jm034167p BindingDB Entry DOI: 10.7270/Q28C9X0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM22449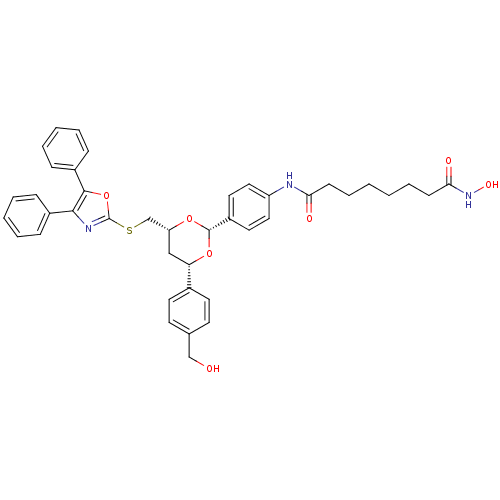 (CHEMBL356769 | N-(4-{(2R,4R,6S)-4-{[(4,5-diphenyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma"La Sapienza" Curated by ChEMBL | Assay Description Tested for inhibition of Histone deacetylase 6 induced acetylated tubulin in mammalian cells. | J Med Chem 46: 4826-9 (2003) Article DOI: 10.1021/jm034167p BindingDB Entry DOI: 10.7270/Q28C9X0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50134803 (5,8-Dimethyl-11-(6-oxiranyl-6-oxo-hexyl)-decahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma"La Sapienza" Curated by ChEMBL | Assay Description Inhibition of Histone deacetylase 1 induced acetylated histone in mammalian cells. | J Med Chem 46: 4826-9 (2003) Article DOI: 10.1021/jm034167p BindingDB Entry DOI: 10.7270/Q28C9X0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50134799 (6-((5S,8S,11S,13aR)-5,8-Dimethyl-4,7,10,13-tetraox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma"La Sapienza" Curated by ChEMBL | Assay Description Inhibition of Histone deacetylase 1 induced acetylated histone in mammalian cells. | J Med Chem 46: 4826-9 (2003) Article DOI: 10.1021/jm034167p BindingDB Entry DOI: 10.7270/Q28C9X0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50134799 (6-((5S,8S,11S,13aR)-5,8-Dimethyl-4,7,10,13-tetraox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma"La Sapienza" Curated by ChEMBL | Assay Description Tested for inhibition of Histone deacetylase 6 induced acetylated tubulin in mammalian cells. | J Med Chem 46: 4826-9 (2003) Article DOI: 10.1021/jm034167p BindingDB Entry DOI: 10.7270/Q28C9X0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50354086 (FK-228 | Istodax | ROMIDEPSIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.40E+7 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma"La Sapienza" Curated by ChEMBL | Assay Description Tested for inhibition of Histone deacetylase 6 induced acetylated tubulin in mammalian cells. | J Med Chem 46: 4826-9 (2003) Article DOI: 10.1021/jm034167p BindingDB Entry DOI: 10.7270/Q28C9X0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM22449 (CHEMBL356769 | N-(4-{(2R,4R,6S)-4-{[(4,5-diphenyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.17E+5 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma"La Sapienza" Curated by ChEMBL | Assay Description Inhibition of Histone deacetylase 1 induced acetylated histone in mammalian cells. | J Med Chem 46: 4826-9 (2003) Article DOI: 10.1021/jm034167p BindingDB Entry DOI: 10.7270/Q28C9X0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19130 ((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma"La Sapienza" Curated by ChEMBL | Assay Description Inhibition of Histone deacetylase 1 induced acetylated histone in mammalian cells. | J Med Chem 46: 4826-9 (2003) Article DOI: 10.1021/jm034167p BindingDB Entry DOI: 10.7270/Q28C9X0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||