 Found 45 hits Enz. Inhib. hit(s) with all data for entry = 50037786
Found 45 hits Enz. Inhib. hit(s) with all data for entry = 50037786 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50195108
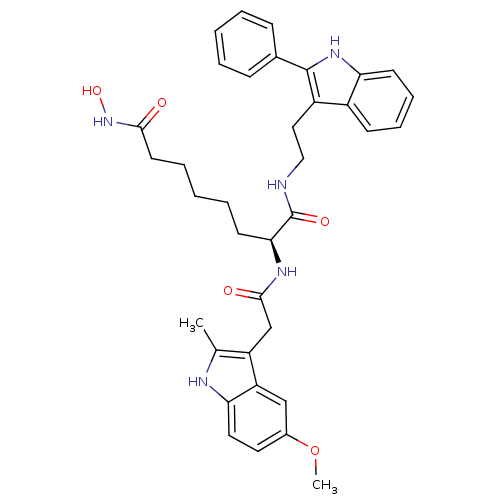
((S)-N8-hydroxy-2-(2-(5-methoxy-2-methyl-1H-indol-3...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CCCCCC(=O)NO)C(=O)NCCc3c([nH]c4ccccc34)-c3ccccc3)c2c1 Show InChI InChI=1S/C36H41N5O5/c1-23-28(29-21-25(46-2)17-18-31(29)38-23)22-34(43)39-32(15-7-4-8-16-33(42)41-45)36(44)37-20-19-27-26-13-9-10-14-30(26)40-35(27)24-11-5-3-6-12-24/h3,5-6,9-14,17-18,21,32,38,40,45H,4,7-8,15-16,19-20,22H2,1-2H3,(H,37,44)(H,39,43)(H,41,42)/t32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50195108

((S)-N8-hydroxy-2-(2-(5-methoxy-2-methyl-1H-indol-3...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CCCCCC(=O)NO)C(=O)NCCc3c([nH]c4ccccc34)-c3ccccc3)c2c1 Show InChI InChI=1S/C36H41N5O5/c1-23-28(29-21-25(46-2)17-18-31(29)38-23)22-34(43)39-32(15-7-4-8-16-33(42)41-45)36(44)37-20-19-27-26-13-9-10-14-30(26)40-35(27)24-11-5-3-6-12-24/h3,5-6,9-14,17-18,21,32,38,40,45H,4,7-8,15-16,19-20,22H2,1-2H3,(H,37,44)(H,39,43)(H,41,42)/t32-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50195108

((S)-N8-hydroxy-2-(2-(5-methoxy-2-methyl-1H-indol-3...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CCCCCC(=O)NO)C(=O)NCCc3c([nH]c4ccccc34)-c3ccccc3)c2c1 Show InChI InChI=1S/C36H41N5O5/c1-23-28(29-21-25(46-2)17-18-31(29)38-23)22-34(43)39-32(15-7-4-8-16-33(42)41-45)36(44)37-20-19-27-26-13-9-10-14-30(26)40-35(27)24-11-5-3-6-12-24/h3,5-6,9-14,17-18,21,32,38,40,45H,4,7-8,15-16,19-20,22H2,1-2H3,(H,37,44)(H,39,43)(H,41,42)/t32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM25144
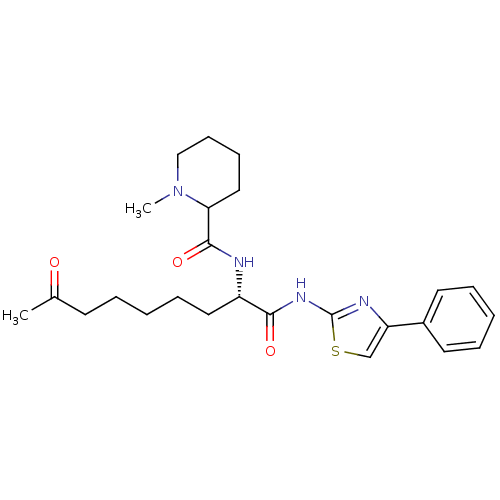
((2S)-2-[(1-methylpiperidin-2-yl)formamido]-8-oxo-N...)Show SMILES CN1CCCCC1C(=O)N[C@@H](CCCCCC(C)=O)C(=O)Nc1nc(cs1)-c1ccccc1 |r| Show InChI InChI=1S/C25H34N4O3S/c1-18(30)11-5-3-8-14-20(26-24(32)22-15-9-10-16-29(22)2)23(31)28-25-27-21(17-33-25)19-12-6-4-7-13-19/h4,6-7,12-13,17,20,22H,3,5,8-11,14-16H2,1-2H3,(H,26,32)(H,27,28,31)/t20-,22?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM25144

((2S)-2-[(1-methylpiperidin-2-yl)formamido]-8-oxo-N...)Show SMILES CN1CCCCC1C(=O)N[C@@H](CCCCCC(C)=O)C(=O)Nc1nc(cs1)-c1ccccc1 |r| Show InChI InChI=1S/C25H34N4O3S/c1-18(30)11-5-3-8-14-20(26-24(32)22-15-9-10-16-29(22)2)23(31)28-25-27-21(17-33-25)19-12-6-4-7-13-19/h4,6-7,12-13,17,20,22H,3,5,8-11,14-16H2,1-2H3,(H,26,32)(H,27,28,31)/t20-,22?/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50195119
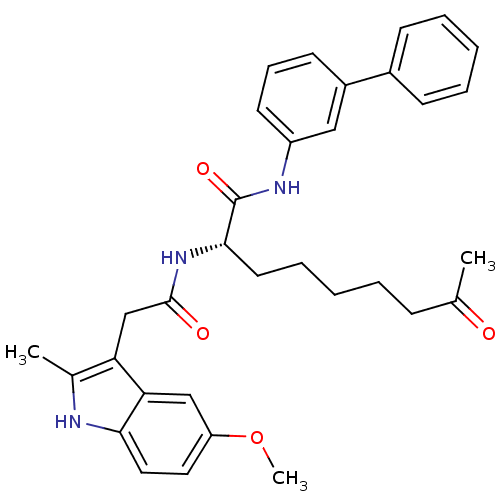
((S)-N-(biphenyl-3-yl)-2-(2-(5-methoxy-2-methyl-1H-...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CCCCCC(C)=O)C(=O)Nc3cccc(c3)-c3ccccc3)c2c1 Show InChI InChI=1S/C33H37N3O4/c1-22(37)11-6-4-9-16-31(33(39)35-26-15-10-14-25(19-26)24-12-7-5-8-13-24)36-32(38)21-28-23(2)34-30-18-17-27(40-3)20-29(28)30/h5,7-8,10,12-15,17-20,31,34H,4,6,9,11,16,21H2,1-3H3,(H,35,39)(H,36,38)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50195129
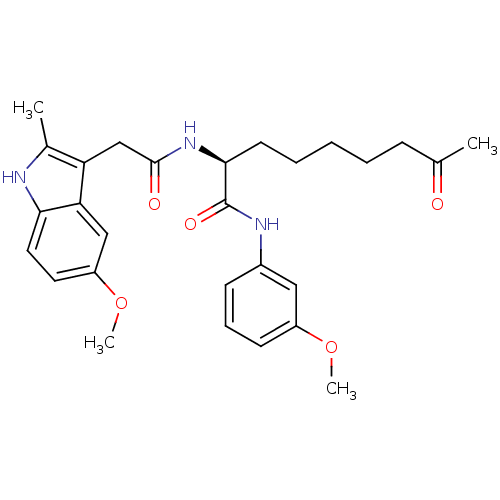
((S)-2-(2-(5-methoxy-2-methyl-1H-indol-3-yl)acetami...)Show SMILES COc1cccc(NC(=O)[C@H](CCCCCC(C)=O)NC(=O)Cc2c(C)[nH]c3ccc(OC)cc23)c1 Show InChI InChI=1S/C28H35N3O5/c1-18(32)9-6-5-7-12-26(28(34)30-20-10-8-11-21(15-20)35-3)31-27(33)17-23-19(2)29-25-14-13-22(36-4)16-24(23)25/h8,10-11,13-16,26,29H,5-7,9,12,17H2,1-4H3,(H,30,34)(H,31,33)/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50195118

((S)-2-(2-(5-methoxy-2-methyl-1H-indol-3-yl)acetami...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CCCCCC(C)=O)C(=O)Nc3nc(cs3)-c3ccccc3)c2c1 Show InChI InChI=1S/C30H34N4O4S/c1-19(35)10-6-4-9-13-26(29(37)34-30-33-27(18-39-30)21-11-7-5-8-12-21)32-28(36)17-23-20(2)31-25-15-14-22(38-3)16-24(23)25/h5,7-8,11-12,14-16,18,26,31H,4,6,9-10,13,17H2,1-3H3,(H,32,36)(H,33,34,37)/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50195111
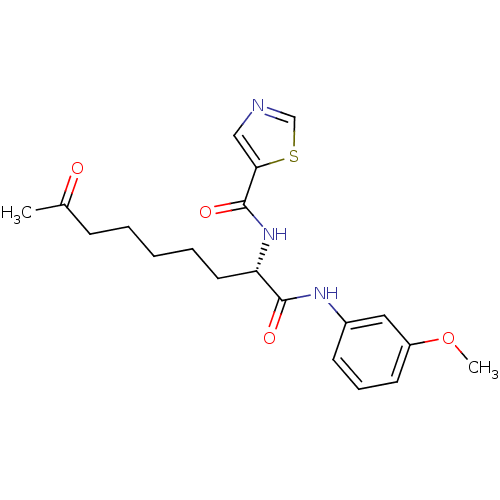
((S)-N-(1-(3-methoxyphenylamino)-1,8-dioxononan-2-y...)Show SMILES COc1cccc(NC(=O)[C@H](CCCCCC(C)=O)NC(=O)c2cncs2)c1 Show InChI InChI=1S/C20H25N3O4S/c1-14(24)7-4-3-5-10-17(23-20(26)18-12-21-13-28-18)19(25)22-15-8-6-9-16(11-15)27-2/h6,8-9,11-13,17H,3-5,7,10H2,1-2H3,(H,22,25)(H,23,26)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50238632
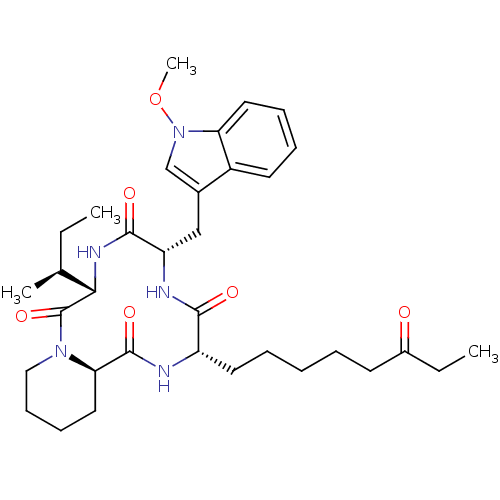
((6S,9S,12S,14aR)-6-((S)-sec-Butyl)-9-(1-methoxy-1H...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2cn(OC)c3ccccc23)NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@H]2CCCCN2C1=O |r| Show InChI InChI=1S/C34H49N5O6/c1-5-22(3)30-34(44)38-19-13-12-18-29(38)33(43)35-26(16-9-7-8-14-24(40)6-2)31(41)36-27(32(42)37-30)20-23-21-39(45-4)28-17-11-10-15-25(23)28/h10-11,15,17,21-22,26-27,29-30H,5-9,12-14,16,18-20H2,1-4H3,(H,35,43)(H,36,41)(H,37,42)/t22-,26-,27-,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50195135
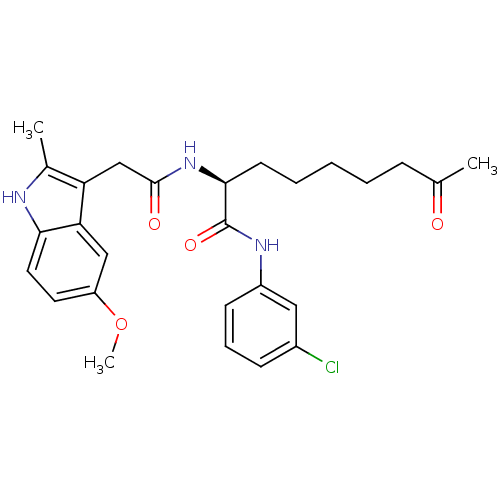
((S)-N-(3-chlorophenyl)-2-(2-(5-methoxy-2-methyl-1H...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CCCCCC(C)=O)C(=O)Nc3cccc(Cl)c3)c2c1 Show InChI InChI=1S/C27H32ClN3O4/c1-17(32)8-5-4-6-11-25(27(34)30-20-10-7-9-19(28)14-20)31-26(33)16-22-18(2)29-24-13-12-21(35-3)15-23(22)24/h7,9-10,12-15,25,29H,4-6,8,11,16H2,1-3H3,(H,30,34)(H,31,33)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50195108

((S)-N8-hydroxy-2-(2-(5-methoxy-2-methyl-1H-indol-3...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CCCCCC(=O)NO)C(=O)NCCc3c([nH]c4ccccc34)-c3ccccc3)c2c1 Show InChI InChI=1S/C36H41N5O5/c1-23-28(29-21-25(46-2)17-18-31(29)38-23)22-34(43)39-32(15-7-4-8-16-33(42)41-45)36(44)37-20-19-27-26-13-9-10-14-30(26)40-35(27)24-11-5-3-6-12-24/h3,5-6,9-14,17-18,21,32,38,40,45H,4,7-8,15-16,19-20,22H2,1-2H3,(H,37,44)(H,39,43)(H,41,42)/t32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50195130
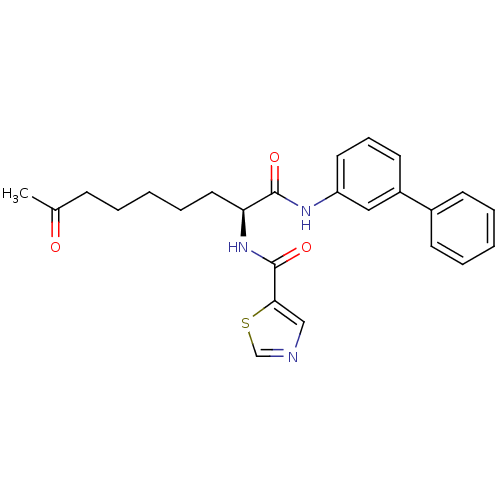
((S)-N-(1-(biphenyl-3-ylamino)-1,8-dioxononan-2-yl)...)Show SMILES CC(=O)CCCCC[C@H](NC(=O)c1cncs1)C(=O)Nc1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C25H27N3O3S/c1-18(29)9-4-2-7-14-22(28-25(31)23-16-26-17-32-23)24(30)27-21-13-8-12-20(15-21)19-10-5-3-6-11-19/h3,5-6,8,10-13,15-17,22H,2,4,7,9,14H2,1H3,(H,27,30)(H,28,31)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM25144

((2S)-2-[(1-methylpiperidin-2-yl)formamido]-8-oxo-N...)Show SMILES CN1CCCCC1C(=O)N[C@@H](CCCCCC(C)=O)C(=O)Nc1nc(cs1)-c1ccccc1 |r| Show InChI InChI=1S/C25H34N4O3S/c1-18(30)11-5-3-8-14-20(26-24(32)22-15-9-10-16-29(22)2)23(31)28-25-27-21(17-33-25)19-12-6-4-7-13-19/h4,6-7,12-13,17,20,22H,3,5,8-11,14-16H2,1-2H3,(H,26,32)(H,27,28,31)/t20-,22?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50195116

(CHEMBL217314 | N-((S)-1-(biphenyl-3-ylamino)-1,8-d...)Show SMILES CN1CCCCC1C(=O)N[C@@H](CCCCCC(C)=O)C(=O)Nc1cccc(c1)-c1ccccc1 Show InChI InChI=1S/C28H37N3O3/c1-21(32)12-5-3-8-17-25(30-28(34)26-18-9-10-19-31(26)2)27(33)29-24-16-11-15-23(20-24)22-13-6-4-7-14-22/h4,6-7,11,13-16,20,25-26H,3,5,8-10,12,17-19H2,1-2H3,(H,29,33)(H,30,34)/t25-,26?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50195120
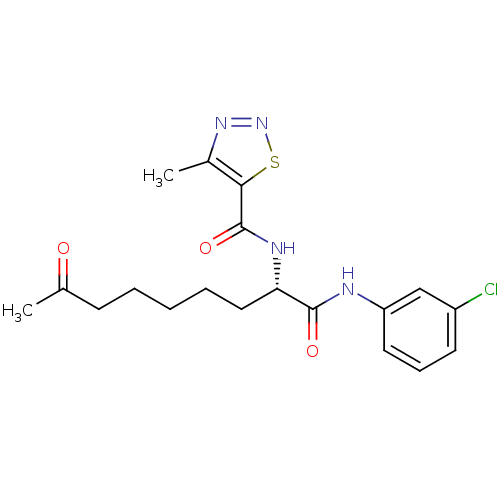
((S)-N-(1-(3-chlorophenylamino)-1,8-dioxononan-2-yl...)Show SMILES CC(=O)CCCCC[C@H](NC(=O)c1snnc1C)C(=O)Nc1cccc(Cl)c1 Show InChI InChI=1S/C19H23ClN4O3S/c1-12(25)7-4-3-5-10-16(22-19(27)17-13(2)23-24-28-17)18(26)21-15-9-6-8-14(20)11-15/h6,8-9,11,16H,3-5,7,10H2,1-2H3,(H,21,26)(H,22,27)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19410

(CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...)Show InChI InChI=1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50195113
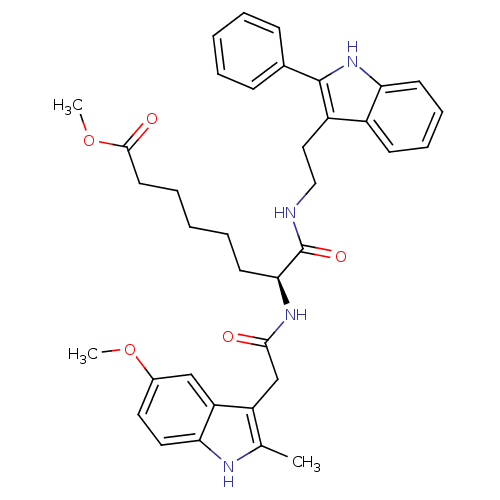
((S)-methyl 7-(2-(5-methoxy-2-methyl-1H-indol-3-yl)...)Show SMILES COC(=O)CCCCC[C@H](NC(=O)Cc1c(C)[nH]c2ccc(OC)cc12)C(=O)NCCc1c([nH]c2ccccc12)-c1ccccc1 Show InChI InChI=1S/C37H42N4O5/c1-24-29(30-22-26(45-2)18-19-32(30)39-24)23-34(42)40-33(16-8-5-9-17-35(43)46-3)37(44)38-21-20-28-27-14-10-11-15-31(27)41-36(28)25-12-6-4-7-13-25/h4,6-7,10-15,18-19,22,33,39,41H,5,8-9,16-17,20-21,23H2,1-3H3,(H,38,44)(H,40,42)/t33-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM25144

((2S)-2-[(1-methylpiperidin-2-yl)formamido]-8-oxo-N...)Show SMILES CN1CCCCC1C(=O)N[C@@H](CCCCCC(C)=O)C(=O)Nc1nc(cs1)-c1ccccc1 |r| Show InChI InChI=1S/C25H34N4O3S/c1-18(30)11-5-3-8-14-20(26-24(32)22-15-9-10-16-29(22)2)23(31)28-25-27-21(17-33-25)19-12-6-4-7-13-19/h4,6-7,12-13,17,20,22H,3,5,8-11,14-16H2,1-2H3,(H,26,32)(H,27,28,31)/t20-,22?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50195128
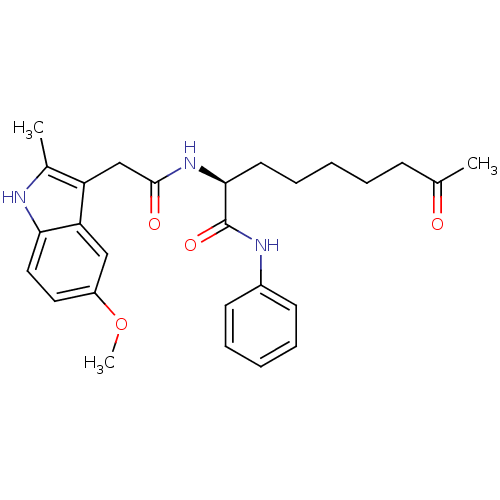
((S)-2-(2-(5-methoxy-2-methyl-1H-indol-3-yl)acetami...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CCCCCC(C)=O)C(=O)Nc3ccccc3)c2c1 Show InChI InChI=1S/C27H33N3O4/c1-18(31)10-6-4-9-13-25(27(33)29-20-11-7-5-8-12-20)30-26(32)17-22-19(2)28-24-15-14-21(34-3)16-23(22)24/h5,7-8,11-12,14-16,25,28H,4,6,9-10,13,17H2,1-3H3,(H,29,33)(H,30,32)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50195107
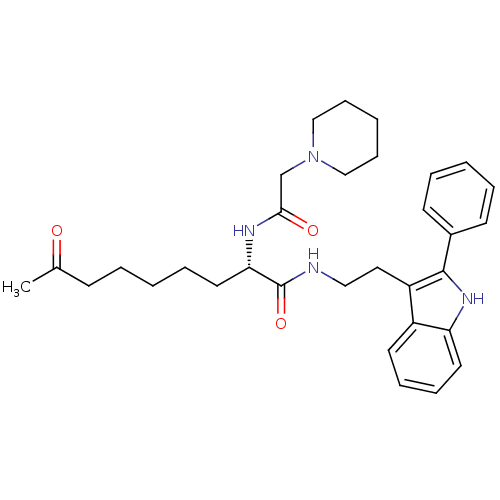
((S)-8-oxo-N-(2-(2-phenyl-1H-indol-3-yl)ethyl)-2-(2...)Show SMILES CC(=O)CCCCC[C@H](NC(=O)CN1CCCCC1)C(=O)NCCc1c([nH]c2ccccc12)-c1ccccc1 Show InChI InChI=1S/C32H42N4O3/c1-24(37)13-5-2-8-18-29(34-30(38)23-36-21-11-4-12-22-36)32(39)33-20-19-27-26-16-9-10-17-28(26)35-31(27)25-14-6-3-7-15-25/h3,6-7,9-10,14-17,29,35H,2,4-5,8,11-13,18-23H2,1H3,(H,33,39)(H,34,38)/t29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50195117
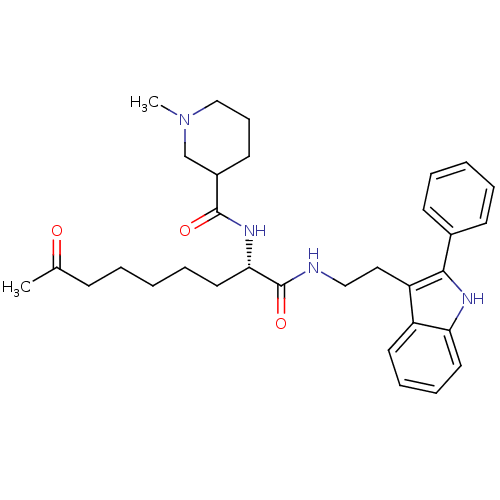
(CHEMBL387224 | N-((S)-1,8-dioxo-1-(2-(2-phenyl-1H-...)Show SMILES CN1CCCC(C1)C(=O)N[C@@H](CCCCCC(C)=O)C(=O)NCCc1c([nH]c2ccccc12)-c1ccccc1 Show InChI InChI=1S/C32H42N4O3/c1-23(37)12-5-3-8-18-29(35-31(38)25-15-11-21-36(2)22-25)32(39)33-20-19-27-26-16-9-10-17-28(26)34-30(27)24-13-6-4-7-14-24/h4,6-7,9-10,13-14,16-17,25,29,34H,3,5,8,11-12,15,18-22H2,1-2H3,(H,33,39)(H,35,38)/t25?,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50195108

((S)-N8-hydroxy-2-(2-(5-methoxy-2-methyl-1H-indol-3...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CCCCCC(=O)NO)C(=O)NCCc3c([nH]c4ccccc34)-c3ccccc3)c2c1 Show InChI InChI=1S/C36H41N5O5/c1-23-28(29-21-25(46-2)17-18-31(29)38-23)22-34(43)39-32(15-7-4-8-16-33(42)41-45)36(44)37-20-19-27-26-13-9-10-14-30(26)40-35(27)24-11-5-3-6-12-24/h3,5-6,9-14,17-18,21,32,38,40,45H,4,7-8,15-16,19-20,22H2,1-2H3,(H,37,44)(H,39,43)(H,41,42)/t32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50195115
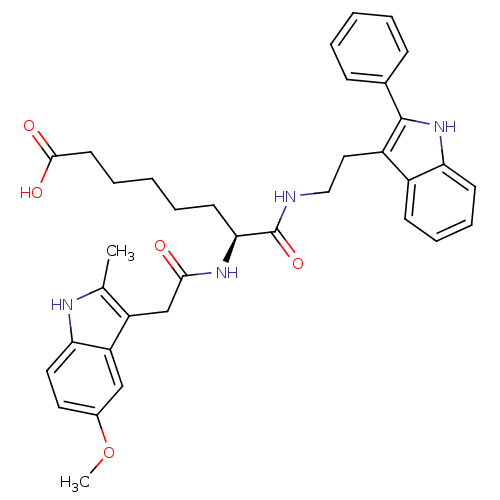
((S)-7-(2-(5-methoxy-2-methyl-1H-indol-3-yl)acetami...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CCCCCC(O)=O)C(=O)NCCc3c([nH]c4ccccc34)-c3ccccc3)c2c1 Show InChI InChI=1S/C36H40N4O5/c1-23-28(29-21-25(45-2)17-18-31(29)38-23)22-33(41)39-32(15-7-4-8-16-34(42)43)36(44)37-20-19-27-26-13-9-10-14-30(26)40-35(27)24-11-5-3-6-12-24/h3,5-6,9-14,17-18,21,32,38,40H,4,7-8,15-16,19-20,22H2,1-2H3,(H,37,44)(H,39,41)(H,42,43)/t32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50195132

(CHEMBL387015 | N-((S)-1,8-dioxo-1-(2-(2-phenyl-1H-...)Show SMILES CN1CCC(C1)C(=O)N[C@@H](CCCCCC(C)=O)C(=O)NCCc1c([nH]c2ccccc12)-c1ccccc1 Show InChI InChI=1S/C31H40N4O3/c1-22(36)11-5-3-8-16-28(34-30(37)24-18-20-35(2)21-24)31(38)32-19-17-26-25-14-9-10-15-27(25)33-29(26)23-12-6-4-7-13-23/h4,6-7,9-10,12-15,24,28,33H,3,5,8,11,16-21H2,1-2H3,(H,32,38)(H,34,37)/t24?,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50195123

((S)-N-(4-chlorophenyl)-2-(2-(5-methoxy-2-methyl-1H...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CCCCCC(C)=O)C(=O)Nc3ccc(Cl)cc3)c2c1 Show InChI InChI=1S/C27H32ClN3O4/c1-17(32)7-5-4-6-8-25(27(34)30-20-11-9-19(28)10-12-20)31-26(33)16-22-18(2)29-24-14-13-21(35-3)15-23(22)24/h9-15,25,29H,4-8,16H2,1-3H3,(H,30,34)(H,31,33)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50195108

((S)-N8-hydroxy-2-(2-(5-methoxy-2-methyl-1H-indol-3...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CCCCCC(=O)NO)C(=O)NCCc3c([nH]c4ccccc34)-c3ccccc3)c2c1 Show InChI InChI=1S/C36H41N5O5/c1-23-28(29-21-25(46-2)17-18-31(29)38-23)22-34(43)39-32(15-7-4-8-16-33(42)41-45)36(44)37-20-19-27-26-13-9-10-14-30(26)40-35(27)24-11-5-3-6-12-24/h3,5-6,9-14,17-18,21,32,38,40,45H,4,7-8,15-16,19-20,22H2,1-2H3,(H,37,44)(H,39,43)(H,41,42)/t32-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC4 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50195127
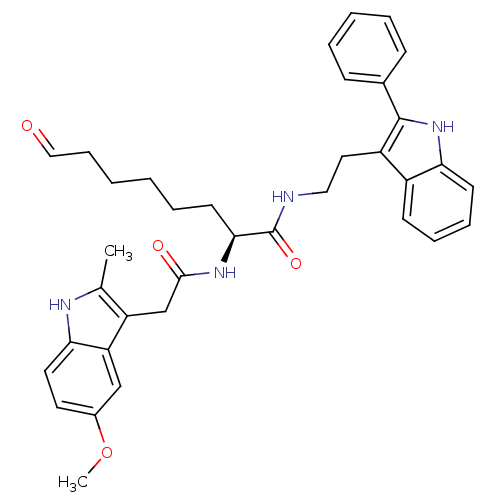
((S)-2-(2-(5-methoxy-2-methyl-1H-indol-3-yl)acetami...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CCCCCC=O)C(=O)NCCc3c([nH]c4ccccc34)-c3ccccc3)c2c1 Show InChI InChI=1S/C36H40N4O4/c1-24-29(30-22-26(44-2)17-18-32(30)38-24)23-34(42)39-33(16-8-3-4-11-21-41)36(43)37-20-19-28-27-14-9-10-15-31(27)40-35(28)25-12-6-5-7-13-25/h5-7,9-10,12-15,17-18,21-22,33,38,40H,3-4,8,11,16,19-20,23H2,1-2H3,(H,37,43)(H,39,42)/t33-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
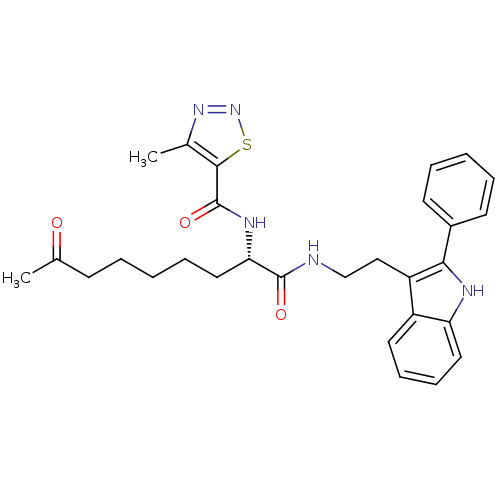
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50195131
((S)-N-(1,8-dioxo-1-(2-(2-phenyl-1H-indol-3-yl)ethy...)Show SMILES CC(=O)CCCCC[C@H](NC(=O)c1snnc1C)C(=O)NCCc1c([nH]c2ccccc12)-c1ccccc1 Show InChI InChI=1S/C29H33N5O3S/c1-19(35)11-5-3-8-16-25(32-29(37)27-20(2)33-34-38-27)28(36)30-18-17-23-22-14-9-10-15-24(22)31-26(23)21-12-6-4-7-13-21/h4,6-7,9-10,12-15,25,31H,3,5,8,11,16-18H2,1-2H3,(H,30,36)(H,32,37)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50195125
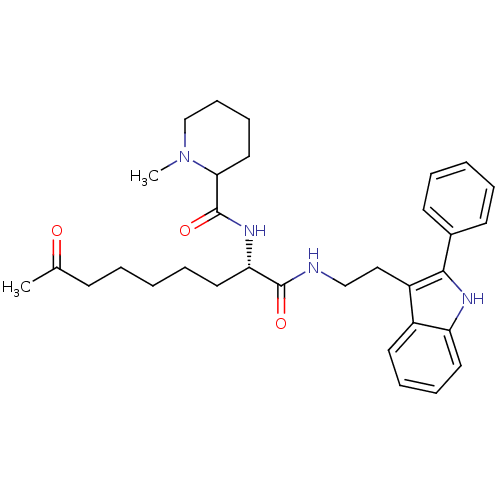
(CHEMBL218129 | N-((S)-1,8-dioxo-1-(2-(2-phenyl-1H-...)Show SMILES CN1CCCCC1C(=O)N[C@@H](CCCCCC(C)=O)C(=O)NCCc1c([nH]c2ccccc12)-c1ccccc1 Show InChI InChI=1S/C32H42N4O3/c1-23(37)13-5-3-8-18-28(35-32(39)29-19-11-12-22-36(29)2)31(38)33-21-20-26-25-16-9-10-17-27(25)34-30(26)24-14-6-4-7-15-24/h4,6-7,9-10,14-17,28-29,34H,3,5,8,11-13,18-22H2,1-2H3,(H,33,38)(H,35,39)/t28-,29?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50195122
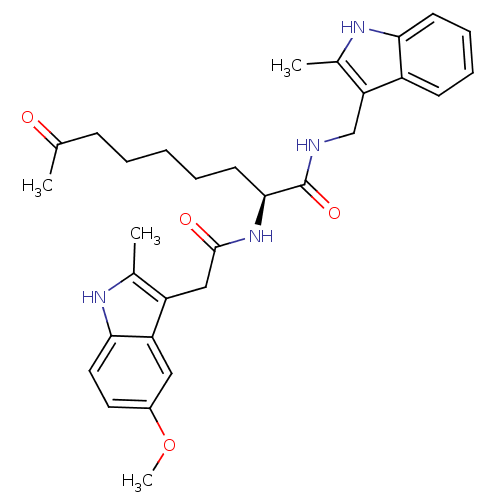
((S)-2-(2-(5-methoxy-2-methyl-1H-indol-3-yl)acetami...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CCCCCC(C)=O)C(=O)NCc3c(C)[nH]c4ccccc34)c2c1 Show InChI InChI=1S/C31H38N4O4/c1-19(36)10-6-5-7-13-29(31(38)32-18-26-21(3)34-27-12-9-8-11-23(26)27)35-30(37)17-24-20(2)33-28-15-14-22(39-4)16-25(24)28/h8-9,11-12,14-16,29,33-34H,5-7,10,13,17-18H2,1-4H3,(H,32,38)(H,35,37)/t29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50195121
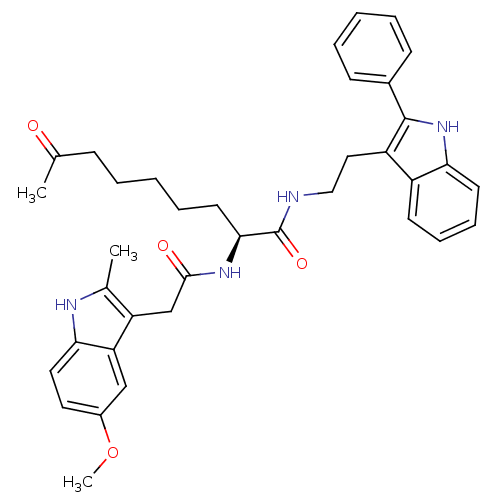
((S)-2-(2-(5-methoxy-2-methyl-1H-indol-3-yl)acetami...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CCCCCC(C)=O)C(=O)NCCc3c([nH]c4ccccc34)-c3ccccc3)c2c1 Show InChI InChI=1S/C37H42N4O4/c1-24(42)12-6-4-9-17-34(40-35(43)23-30-25(2)39-33-19-18-27(45-3)22-31(30)33)37(44)38-21-20-29-28-15-10-11-16-32(28)41-36(29)26-13-7-5-8-14-26/h5,7-8,10-11,13-16,18-19,22,34,39,41H,4,6,9,12,17,20-21,23H2,1-3H3,(H,38,44)(H,40,43)/t34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50195134
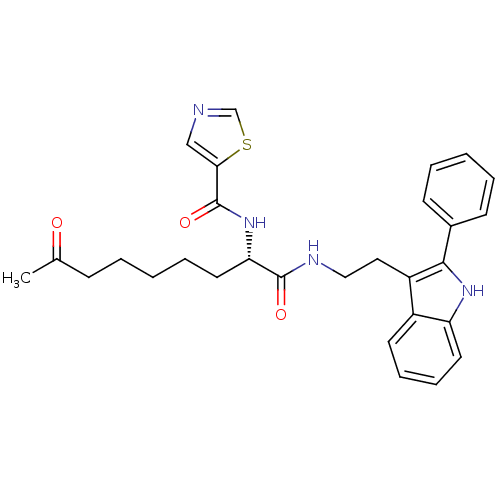
((S)-N-(1,8-dioxo-1-(2-(2-phenyl-1H-indol-3-yl)ethy...)Show SMILES CC(=O)CCCCC[C@H](NC(=O)c1cncs1)C(=O)NCCc1c([nH]c2ccccc12)-c1ccccc1 Show InChI InChI=1S/C29H32N4O3S/c1-20(34)10-4-2-7-15-25(33-29(36)26-18-30-19-37-26)28(35)31-17-16-23-22-13-8-9-14-24(22)32-27(23)21-11-5-3-6-12-21/h3,5-6,8-9,11-14,18-19,25,32H,2,4,7,10,15-17H2,1H3,(H,31,35)(H,33,36)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50195110
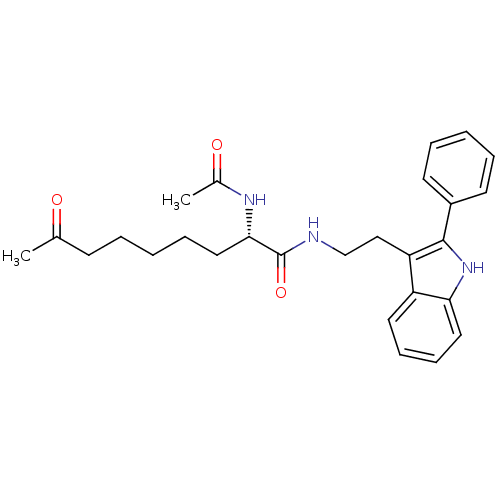
((S)-2-acetamido-8-oxo-N-(2-(2-phenyl-1H-indol-3-yl...)Show SMILES CC(=O)CCCCC[C@H](NC(C)=O)C(=O)NCCc1c([nH]c2ccccc12)-c1ccccc1 Show InChI InChI=1S/C27H33N3O3/c1-19(31)11-5-3-8-16-25(29-20(2)32)27(33)28-18-17-23-22-14-9-10-15-24(22)30-26(23)21-12-6-4-7-13-21/h4,6-7,9-10,12-15,25,30H,3,5,8,11,16-18H2,1-2H3,(H,28,33)(H,29,32)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50195112
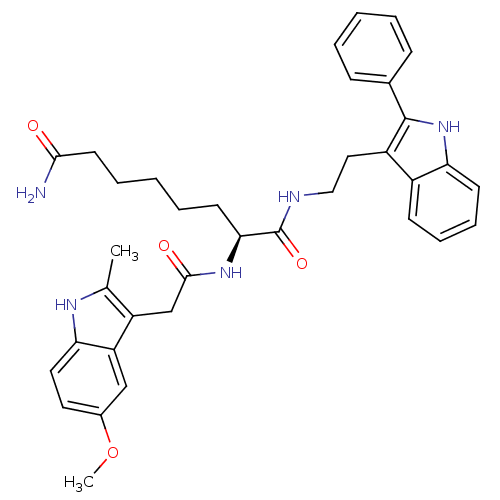
((S)-2-(2-(5-methoxy-2-methyl-1H-indol-3-yl)acetami...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CCCCCC(N)=O)C(=O)NCCc3c([nH]c4ccccc34)-c3ccccc3)c2c1 Show InChI InChI=1S/C36H41N5O4/c1-23-28(29-21-25(45-2)17-18-31(29)39-23)22-34(43)40-32(15-7-4-8-16-33(37)42)36(44)38-20-19-27-26-13-9-10-14-30(26)41-35(27)24-11-5-3-6-12-24/h3,5-6,9-14,17-18,21,32,39,41H,4,7-8,15-16,19-20,22H2,1-2H3,(H2,37,42)(H,38,44)(H,40,43)/t32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM25144

((2S)-2-[(1-methylpiperidin-2-yl)formamido]-8-oxo-N...)Show SMILES CN1CCCCC1C(=O)N[C@@H](CCCCCC(C)=O)C(=O)Nc1nc(cs1)-c1ccccc1 |r| Show InChI InChI=1S/C25H34N4O3S/c1-18(30)11-5-3-8-14-20(26-24(32)22-15-9-10-16-29(22)2)23(31)28-25-27-21(17-33-25)19-12-6-4-7-13-19/h4,6-7,12-13,17,20,22H,3,5,8-11,14-16H2,1-2H3,(H,26,32)(H,27,28,31)/t20-,22?/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC5 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM50195108

((S)-N8-hydroxy-2-(2-(5-methoxy-2-methyl-1H-indol-3...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CCCCCC(=O)NO)C(=O)NCCc3c([nH]c4ccccc34)-c3ccccc3)c2c1 Show InChI InChI=1S/C36H41N5O5/c1-23-28(29-21-25(46-2)17-18-31(29)38-23)22-34(43)39-32(15-7-4-8-16-33(42)41-45)36(44)37-20-19-27-26-13-9-10-14-30(26)40-35(27)24-11-5-3-6-12-24/h3,5-6,9-14,17-18,21,32,38,40,45H,4,7-8,15-16,19-20,22H2,1-2H3,(H,37,44)(H,39,43)(H,41,42)/t32-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC5 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM25144

((2S)-2-[(1-methylpiperidin-2-yl)formamido]-8-oxo-N...)Show SMILES CN1CCCCC1C(=O)N[C@@H](CCCCCC(C)=O)C(=O)Nc1nc(cs1)-c1ccccc1 |r| Show InChI InChI=1S/C25H34N4O3S/c1-18(30)11-5-3-8-14-20(26-24(32)22-15-9-10-16-29(22)2)23(31)28-25-27-21(17-33-25)19-12-6-4-7-13-19/h4,6-7,12-13,17,20,22H,3,5,8-11,14-16H2,1-2H3,(H,26,32)(H,27,28,31)/t20-,22?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50195124
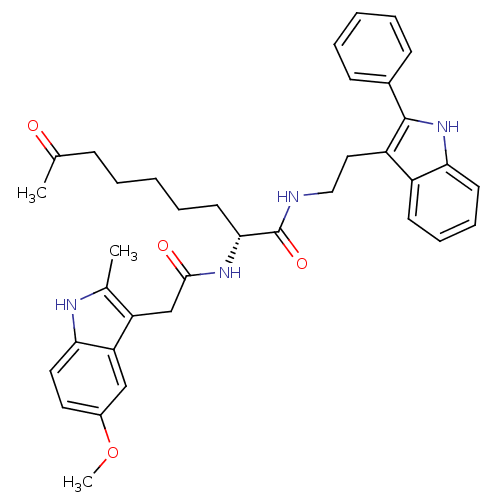
((R)-2-(2-(5-methoxy-2-methyl-1H-indol-3-yl)acetami...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@H](CCCCCC(C)=O)C(=O)NCCc3c([nH]c4ccccc34)-c3ccccc3)c2c1 Show InChI InChI=1S/C37H42N4O4/c1-24(42)12-6-4-9-17-34(40-35(43)23-30-25(2)39-33-19-18-27(45-3)22-31(30)33)37(44)38-21-20-29-28-15-10-11-16-32(28)41-36(29)26-13-7-5-8-14-26/h5,7-8,10-11,13-16,18-19,22,34,39,41H,4,6,9,12,17,20-21,23H2,1-3H3,(H,38,44)(H,40,43)/t34-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50195133
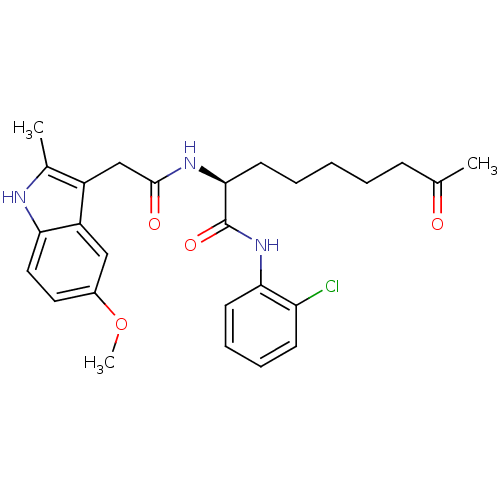
((S)-N-(2-chlorophenyl)-2-(2-(5-methoxy-2-methyl-1H...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CCCCCC(C)=O)C(=O)Nc3ccccc3Cl)c2c1 Show InChI InChI=1S/C27H32ClN3O4/c1-17(32)9-5-4-6-12-25(27(34)31-24-11-8-7-10-22(24)28)30-26(33)16-20-18(2)29-23-14-13-19(35-3)15-21(20)23/h7-8,10-11,13-15,25,29H,4-6,9,12,16H2,1-3H3,(H,30,33)(H,31,34)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 7
(Homo sapiens (Human)) | BDBM50195108

((S)-N8-hydroxy-2-(2-(5-methoxy-2-methyl-1H-indol-3...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CCCCCC(=O)NO)C(=O)NCCc3c([nH]c4ccccc34)-c3ccccc3)c2c1 Show InChI InChI=1S/C36H41N5O5/c1-23-28(29-21-25(46-2)17-18-31(29)38-23)22-34(43)39-32(15-7-4-8-16-33(42)41-45)36(44)37-20-19-27-26-13-9-10-14-30(26)40-35(27)24-11-5-3-6-12-24/h3,5-6,9-14,17-18,21,32,38,40,45H,4,7-8,15-16,19-20,22H2,1-2H3,(H,37,44)(H,39,43)(H,41,42)/t32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC7 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50195114

((S)-N-ethyl-2-(2-(5-methoxy-2-methyl-1H-indol-3-yl...)Show SMILES CCNC(=O)[C@H](CCCCCC(C)=O)NC(=O)Cc1c(C)[nH]c2ccc(OC)cc12 Show InChI InChI=1S/C23H33N3O4/c1-5-24-23(29)21(10-8-6-7-9-15(2)27)26-22(28)14-18-16(3)25-20-12-11-17(30-4)13-19(18)20/h11-13,21,25H,5-10,14H2,1-4H3,(H,24,29)(H,26,28)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50195109
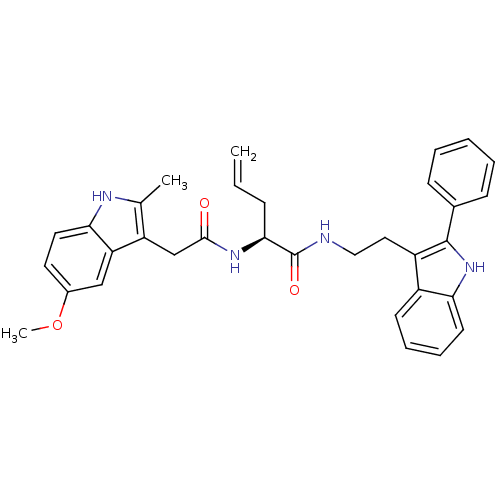
((S)-2-(2-(5-methoxy-2-methyl-1H-indol-3-yl)acetami...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CC=C)C(=O)NCCc3c([nH]c4ccccc34)-c3ccccc3)c2c1 Show InChI InChI=1S/C33H34N4O3/c1-4-10-30(36-31(38)20-26-21(2)35-29-16-15-23(40-3)19-27(26)29)33(39)34-18-17-25-24-13-8-9-14-28(24)37-32(25)22-11-6-5-7-12-22/h4-9,11-16,19,30,35,37H,1,10,17-18,20H2,2-3H3,(H,34,39)(H,36,38)/t30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50195126
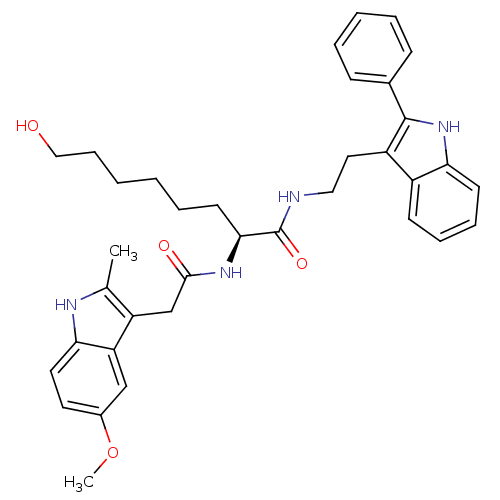
((S)-8-hydroxy-2-(2-(5-methoxy-2-methyl-1H-indol-3-...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CCCCCCO)C(=O)NCCc3c([nH]c4ccccc34)-c3ccccc3)c2c1 Show InChI InChI=1S/C36H42N4O4/c1-24-29(30-22-26(44-2)17-18-32(30)38-24)23-34(42)39-33(16-8-3-4-11-21-41)36(43)37-20-19-28-27-14-9-10-15-31(27)40-35(28)25-12-6-5-7-13-25/h5-7,9-10,12-15,17-18,22,33,38,40-41H,3-4,8,11,16,19-21,23H2,1-2H3,(H,37,43)(H,39,42)/t33-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (mean IC50) |
Bioorg Med Chem Lett 16: 5948-52 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.002
BindingDB Entry DOI: 10.7270/Q2RN38PD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data