 Found 55 hits Enz. Inhib. hit(s) with all data for entry = 50010388
Found 55 hits Enz. Inhib. hit(s) with all data for entry = 50010388 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50409039

(CHEMBL2111951)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)C1=CC[C@@H]2[C@H]1CN1 |r,c:19,t:7| Show InChI InChI=1S/C21H31NO/c1-20-9-7-14(23)11-13(20)3-4-15-16-5-6-18(19-12-22-19)21(16,2)10-8-17(15)20/h3,5,14-15,17-19,22-23H,4,6-12H2,1-2H3/t14-,15-,17-,18+,19+,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Binding affinity for Cytochrome P450 17 from human testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50409041

(CHEMBL2111947)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC[C@@H]2[C@H]1CN1 |r,t:7| Show InChI InChI=1S/C21H33NO/c1-20-9-7-14(23)11-13(20)3-4-15-16-5-6-18(19-12-22-19)21(16,2)10-8-17(15)20/h3,14-19,22-23H,4-12H2,1-2H3/t14-,15-,16-,17-,18+,19+,20-,21-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Binding affinity for Cytochrome P450 17 from rat testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50094191
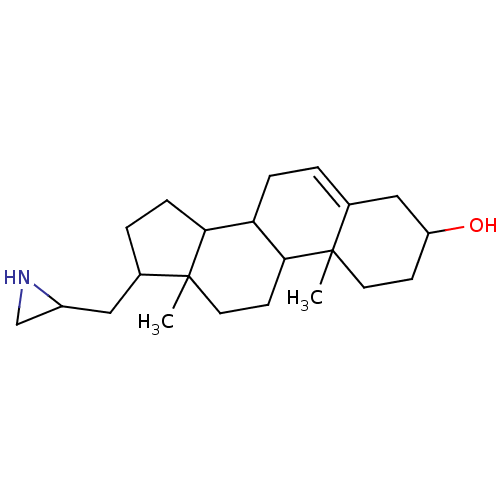
(17-Aziridin-2-ylmethyl-10,13-dimethyl-2,3,4,7,8,9,...)Show SMILES CC12CCC3C(CC=C4CC(O)CCC34C)C1CCC2CC1CN1 |t:7| Show InChI InChI=1S/C22H35NO/c1-21-9-7-17(24)12-15(21)3-5-18-19-6-4-14(11-16-13-23-16)22(19,2)10-8-20(18)21/h3,14,16-20,23-24H,4-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Binding affinity for Cytochrome P450 17 from human testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50409039

(CHEMBL2111951)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)C1=CC[C@@H]2[C@H]1CN1 |r,c:19,t:7| Show InChI InChI=1S/C21H31NO/c1-20-9-7-14(23)11-13(20)3-4-15-16-5-6-18(19-12-22-19)21(16,2)10-8-17(15)20/h3,5,14-15,17-19,22-23H,4,6-12H2,1-2H3/t14-,15-,17-,18+,19+,20-,21-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Binding affinity for Cytochrome P450 17 from rat testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50409041

(CHEMBL2111947)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC[C@@H]2[C@H]1CN1 |r,t:7| Show InChI InChI=1S/C21H33NO/c1-20-9-7-14(23)11-13(20)3-4-15-16-5-6-18(19-12-22-19)21(16,2)10-8-17(15)20/h3,14-19,22-23H,4-12H2,1-2H3/t14-,15-,16-,17-,18+,19+,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Binding affinity for Cytochrome P450 17 from human testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50094191

(17-Aziridin-2-ylmethyl-10,13-dimethyl-2,3,4,7,8,9,...)Show SMILES CC12CCC3C(CC=C4CC(O)CCC34C)C1CCC2CC1CN1 |t:7| Show InChI InChI=1S/C22H35NO/c1-21-9-7-17(24)12-15(21)3-5-18-19-6-4-14(11-16-13-23-16)22(19,2)10-8-20(18)21/h3,14,16-20,23-24H,4-13H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Binding affinity for Cytochrome P450 17 from rat testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50093602
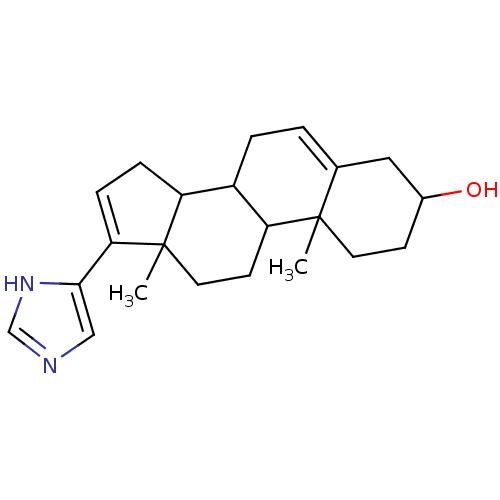
(17-(1H-Imidazol-4-yl)-10,13-dimethyl-2,3,4,7,8,9,1...)Show SMILES CC12CCC3C(CC=C4CC(O)CCC34C)C1CC=C2c1cnc[nH]1 |c:21,t:7| Show InChI InChI=1S/C22H30N2O/c1-21-9-7-15(25)11-14(21)3-4-16-17-5-6-19(20-12-23-13-24-20)22(17,2)10-8-18(16)21/h3,6,12-13,15-18,25H,4-5,7-11H2,1-2H3,(H,23,24) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Inhibition of Cytochrome P450 17 from rat testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(Homo sapiens (Human)) | BDBM50006551
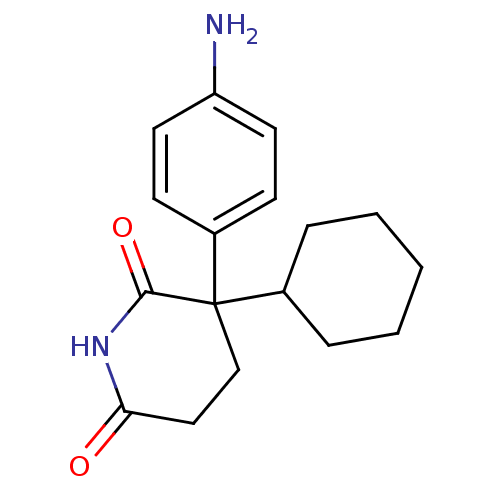
((+)3-(4-Amino-phenyl)-3-cyclohexyl-piperidine-2,6-...)Show InChI InChI=1S/C17H22N2O2/c18-14-8-6-13(7-9-14)17(12-4-2-1-3-5-12)11-10-15(20)19-16(17)21/h6-9,12H,1-5,10-11,18H2,(H,19,20,21) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Inhibition of thromboxane synthase P450 TXA2 |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50094190
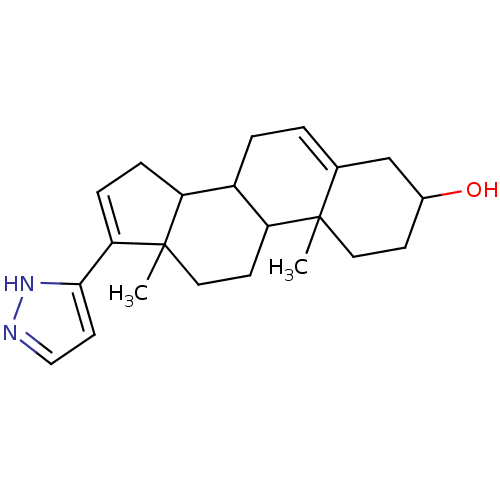
(10,13-Dimethyl-17-(2H-pyrazol-3-yl)-2,3,4,7,8,9,10...)Show SMILES CC12CCC3C(CC=C4CC(O)CCC34C)C1CC=C2c1ccn[nH]1 |c:21,t:7| Show InChI InChI=1S/C22H30N2O/c1-21-10-7-15(25)13-14(21)3-4-16-17-5-6-19(20-9-12-23-24-20)22(17,2)11-8-18(16)21/h3,6,9,12,15-18,25H,4-5,7-8,10-11,13H2,1-2H3,(H,23,24) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Inhibition of Cytochrome P450 17 from rat testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50409041

(CHEMBL2111947)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC[C@@H]2[C@H]1CN1 |r,t:7| Show InChI InChI=1S/C21H33NO/c1-20-9-7-14(23)11-13(20)3-4-15-16-5-6-18(19-12-22-19)21(16,2)10-8-17(15)20/h3,14-19,22-23H,4-12H2,1-2H3/t14-,15-,16-,17-,18+,19+,20-,21-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Inhibition of Cytochrome P450 17 from rat testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50409039

(CHEMBL2111951)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)C1=CC[C@@H]2[C@H]1CN1 |r,c:19,t:7| Show InChI InChI=1S/C21H31NO/c1-20-9-7-14(23)11-13(20)3-4-15-16-5-6-18(19-12-22-19)21(16,2)10-8-17(15)20/h3,5,14-15,17-19,22-23H,4,6-12H2,1-2H3/t14-,15-,17-,18+,19+,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against Cytochrome P450 17 from human testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50094191

(17-Aziridin-2-ylmethyl-10,13-dimethyl-2,3,4,7,8,9,...)Show SMILES CC12CCC3C(CC=C4CC(O)CCC34C)C1CCC2CC1CN1 |t:7| Show InChI InChI=1S/C22H35NO/c1-21-9-7-17(24)12-15(21)3-5-18-19-6-4-14(11-16-13-23-16)22(19,2)10-8-20(18)21/h3,14,16-20,23-24H,4-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against Cytochrome P450 17 from human testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50409040
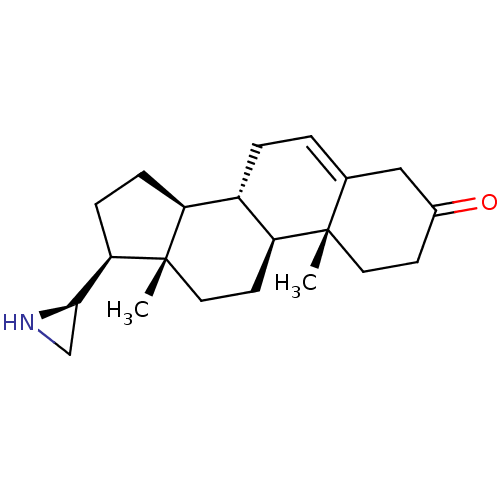
(CHEMBL2111948)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4CC(=O)CC[C@]34C)[C@@H]1CC[C@@H]2[C@H]1CN1 |r,t:7| Show InChI InChI=1S/C21H31NO/c1-20-9-7-14(23)11-13(20)3-4-15-16-5-6-18(19-12-22-19)21(16,2)10-8-17(15)20/h3,15-19,22H,4-12H2,1-2H3/t15-,16-,17-,18+,19+,20-,21-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against Cytochrome P450 17 from rat testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50409039

(CHEMBL2111951)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)C1=CC[C@@H]2[C@H]1CN1 |r,c:19,t:7| Show InChI InChI=1S/C21H31NO/c1-20-9-7-14(23)11-13(20)3-4-15-16-5-6-18(19-12-22-19)21(16,2)10-8-17(15)20/h3,5,14-15,17-19,22-23H,4,6-12H2,1-2H3/t14-,15-,17-,18+,19+,20-,21-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against Cytochrome P450 17 from rat testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50094191

(17-Aziridin-2-ylmethyl-10,13-dimethyl-2,3,4,7,8,9,...)Show SMILES CC12CCC3C(CC=C4CC(O)CCC34C)C1CCC2CC1CN1 |t:7| Show InChI InChI=1S/C22H35NO/c1-21-9-7-17(24)12-15(21)3-5-18-19-6-4-14(11-16-13-23-16)22(19,2)10-8-20(18)21/h3,14,16-20,23-24H,4-13H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against Cytochrome P450 17 from rat testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50409041

(CHEMBL2111947)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC[C@@H]2[C@H]1CN1 |r,t:7| Show InChI InChI=1S/C21H33NO/c1-20-9-7-14(23)11-13(20)3-4-15-16-5-6-18(19-12-22-19)21(16,2)10-8-17(15)20/h3,14-19,22-23H,4-12H2,1-2H3/t14-,15-,16-,17-,18+,19+,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against Cytochrome P450 17 from human testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against Cytochrome P450 17 from human testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50409046
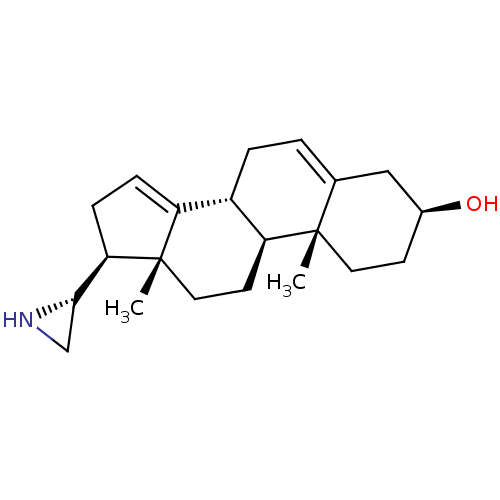
(CHEMBL2112782)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)C1=CC[C@@H]2[C@@H]1CN1 |r,c:19,t:7| Show InChI InChI=1S/C21H31NO/c1-20-9-7-14(23)11-13(20)3-4-15-16-5-6-18(19-12-22-19)21(16,2)10-8-17(15)20/h3,5,14-15,17-19,22-23H,4,6-12H2,1-2H3/t14-,15-,17-,18+,19-,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against Cytochrome P450 17 from human testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Bos taurus (Bovine)) | BDBM50094191

(17-Aziridin-2-ylmethyl-10,13-dimethyl-2,3,4,7,8,9,...)Show SMILES CC12CCC3C(CC=C4CC(O)CCC34C)C1CCC2CC1CN1 |t:7| Show InChI InChI=1S/C22H35NO/c1-21-9-7-17(24)12-15(21)3-5-18-19-6-4-14(11-16-13-23-16)22(19,2)10-8-20(18)21/h3,14,16-20,23-24H,4-13H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Inhibition of P450 scc |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(Homo sapiens (Human)) | BDBM7962
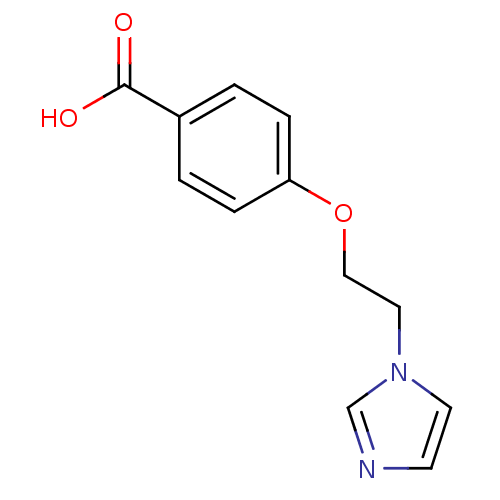
(4-(2-Imidazol-1-yl-ethoxy)-benzoic acid; hydrochlo...)Show InChI InChI=1S/C12H12N2O3/c15-12(16)10-1-3-11(4-2-10)17-8-7-14-6-5-13-9-14/h1-6,9H,7-8H2,(H,15,16) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Inhibition of thromboxane synthase P450 TXA2 |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50409042
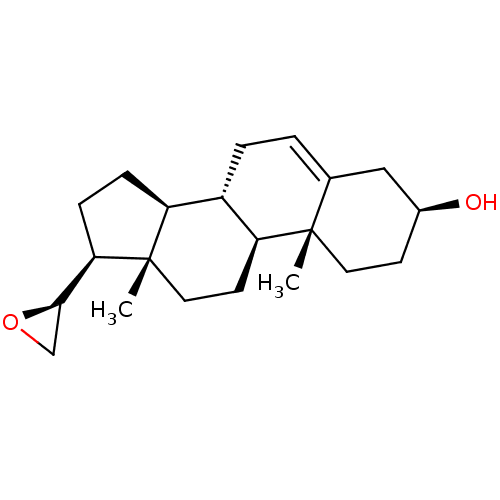
(CHEMBL2112781)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC[C@@H]2[C@H]1CO1 |r,t:7| Show InChI InChI=1S/C21H32O2/c1-20-9-7-14(22)11-13(20)3-4-15-16-5-6-18(19-12-23-19)21(16,2)10-8-17(15)20/h3,14-19,22H,4-12H2,1-2H3/t14-,15-,16-,17-,18+,19+,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against Cytochrome P450 17 from human testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50094189
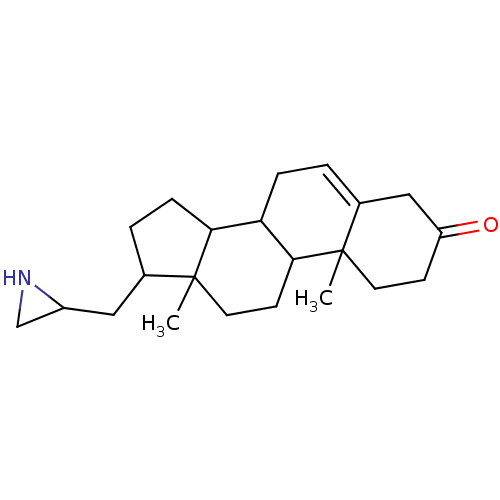
(17-Aziridin-2-ylmethyl-10,13-dimethyl-1,2,4,7,8,9,...)Show SMILES CC12CCC3C(CC=C4CC(=O)CCC34C)C1CCC2CC1CN1 |t:7| Show InChI InChI=1S/C22H33NO/c1-21-9-7-17(24)12-15(21)3-5-18-19-6-4-14(11-16-13-23-16)22(19,2)10-8-20(18)21/h3,14,16,18-20,23H,4-13H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against Cytochrome P450 17 from rat testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50409048
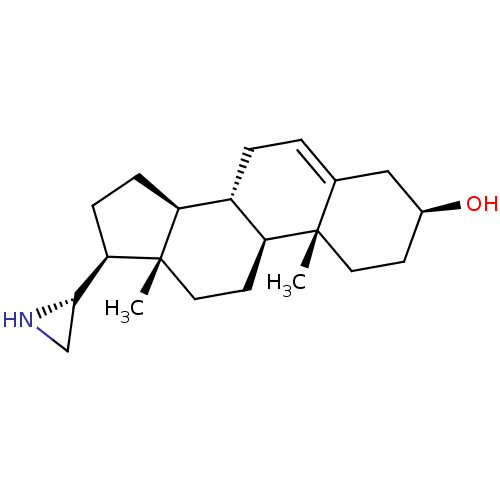
(CHEMBL2112772)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC[C@@H]2[C@@H]1CN1 |r,t:7| Show InChI InChI=1S/C21H33NO/c1-20-9-7-14(23)11-13(20)3-4-15-16-5-6-18(19-12-22-19)21(16,2)10-8-17(15)20/h3,14-19,22-23H,4-12H2,1-2H3/t14-,15-,16-,17-,18+,19-,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against Cytochrome P450 17 from human testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50409040

(CHEMBL2111948)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4CC(=O)CC[C@]34C)[C@@H]1CC[C@@H]2[C@H]1CN1 |r,t:7| Show InChI InChI=1S/C21H31NO/c1-20-9-7-14(23)11-13(20)3-4-15-16-5-6-18(19-12-22-19)21(16,2)10-8-17(15)20/h3,15-19,22H,4-12H2,1-2H3/t15-,16-,17-,18+,19+,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against Cytochrome P450 17 from human testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 1
(Homo sapiens (Human)) | BDBM50094189

(17-Aziridin-2-ylmethyl-10,13-dimethyl-1,2,4,7,8,9,...)Show SMILES CC12CCC3C(CC=C4CC(=O)CCC34C)C1CCC2CC1CN1 |t:7| Show InChI InChI=1S/C22H33NO/c1-21-9-7-17(24)12-15(21)3-5-18-19-6-4-14(11-16-13-23-16)22(19,2)10-8-20(18)21/h3,14,16,18-20,23H,4-13H2,1-2H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Inhibition of steroid 5-alpha-reductase type I isozyme |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50094189

(17-Aziridin-2-ylmethyl-10,13-dimethyl-1,2,4,7,8,9,...)Show SMILES CC12CCC3C(CC=C4CC(=O)CCC34C)C1CCC2CC1CN1 |t:7| Show InChI InChI=1S/C22H33NO/c1-21-9-7-17(24)12-15(21)3-5-18-19-6-4-14(11-16-13-23-16)22(19,2)10-8-20(18)21/h3,14,16,18-20,23H,4-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against Cytochrome P450 17 from human testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50094193
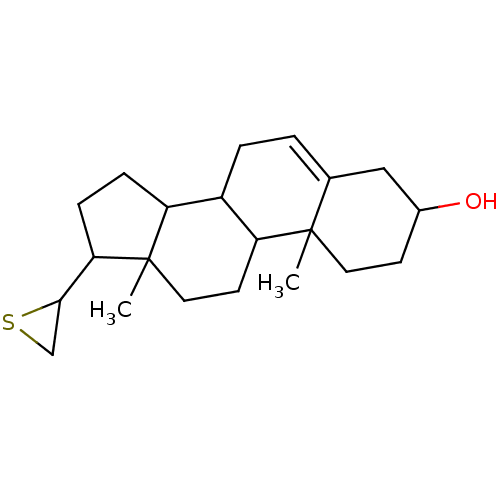
((R)-10,13-Dimethyl-17-thiiranyl-2,3,4,7,8,9,10,11,...)Show SMILES CC12CCC3C(CC=C4CC(O)CCC34C)C1CCC2C1CS1 |t:7| Show InChI InChI=1S/C21H32OS/c1-20-9-7-14(22)11-13(20)3-4-15-16-5-6-18(19-12-23-19)21(16,2)10-8-17(15)20/h3,14-19,22H,4-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against Cytochrome P450 17 from human testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50409045
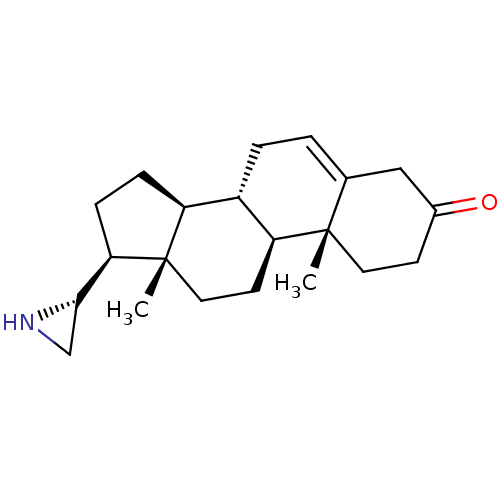
(CHEMBL2112777)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4CC(=O)CC[C@]34C)[C@@H]1CC[C@@H]2[C@@H]1CN1 |r,t:7| Show InChI InChI=1S/C21H31NO/c1-20-9-7-14(23)11-13(20)3-4-15-16-5-6-18(19-12-22-19)21(16,2)10-8-17(15)20/h3,15-19,22H,4-12H2,1-2H3/t15-,16-,17-,18+,19-,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against Cytochrome P450 17 from human testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50409044
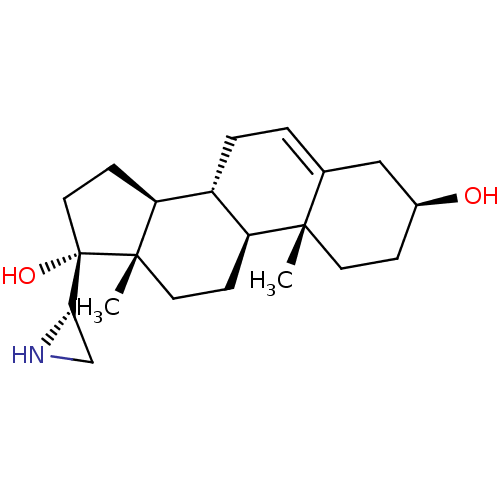
(CHEMBL2111950)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC[C@]2(O)[C@@H]1CN1 |r,t:7| Show InChI InChI=1S/C21H33NO2/c1-19-8-5-14(23)11-13(19)3-4-15-16(19)6-9-20(2)17(15)7-10-21(20,24)18-12-22-18/h3,14-18,22-24H,4-12H2,1-2H3/t14-,15+,16-,17-,18-,19-,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against Cytochrome P450 17 from human testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50409043
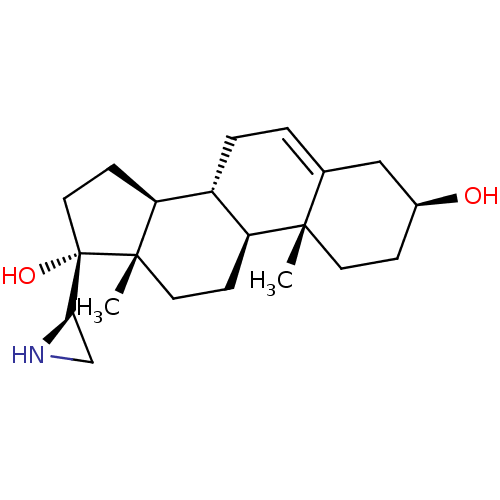
(CHEMBL2112775)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC[C@]2(O)[C@H]1CN1 |r,t:7| Show InChI InChI=1S/C21H33NO2/c1-19-8-5-14(23)11-13(19)3-4-15-16(19)6-9-20(2)17(15)7-10-21(20,24)18-12-22-18/h3,14-18,22-24H,4-12H2,1-2H3/t14-,15+,16-,17-,18+,19-,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against Cytochrome P450 17 from human testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50094193

((R)-10,13-Dimethyl-17-thiiranyl-2,3,4,7,8,9,10,11,...)Show SMILES CC12CCC3C(CC=C4CC(O)CCC34C)C1CCC2C1CS1 |t:7| Show InChI InChI=1S/C21H32OS/c1-20-9-7-14(22)11-13(20)3-4-15-16-5-6-18(19-12-23-19)21(16,2)10-8-17(15)20/h3,14-19,22H,4-12H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against Cytochrome P450 17 from rat testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50094201
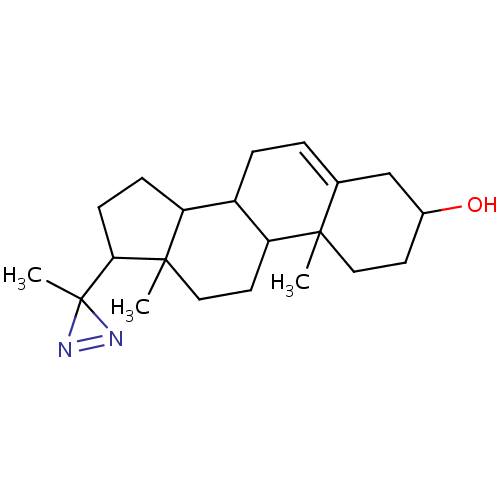
(10,13-Dimethyl-17-(3-methyl-3H-diazirin-3-yl)-2,3,...)Show SMILES CC1(N=N1)C1CCC2C3CC=C4CC(O)CCC4(C)C3CCC12C |c:2,t:11| Show InChI InChI=1S/C21H32N2O/c1-19-10-8-14(24)12-13(19)4-5-15-16-6-7-18(21(3)22-23-21)20(16,2)11-9-17(15)19/h4,14-18,24H,5-12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against Cytochrome P450 17 from human testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(Homo sapiens (Human)) | BDBM50409041

(CHEMBL2111947)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC[C@@H]2[C@H]1CN1 |r,t:7| Show InChI InChI=1S/C21H33NO/c1-20-9-7-14(23)11-13(20)3-4-15-16-5-6-18(19-12-22-19)21(16,2)10-8-17(15)20/h3,14-19,22-23H,4-12H2,1-2H3/t14-,15-,16-,17-,18+,19+,20-,21-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Inhibition of thromboxane synthase P450 TXA2 |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50094191

(17-Aziridin-2-ylmethyl-10,13-dimethyl-2,3,4,7,8,9,...)Show SMILES CC12CCC3C(CC=C4CC(O)CCC34C)C1CCC2CC1CN1 |t:7| Show InChI InChI=1S/C22H35NO/c1-21-9-7-17(24)12-15(21)3-5-18-19-6-4-14(11-16-13-23-16)22(19,2)10-8-20(18)21/h3,14,16-20,23-24H,4-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Inhibition of Cytochrome P450 19A1 |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50094189

(17-Aziridin-2-ylmethyl-10,13-dimethyl-1,2,4,7,8,9,...)Show SMILES CC12CCC3C(CC=C4CC(=O)CCC34C)C1CCC2CC1CN1 |t:7| Show InChI InChI=1S/C22H33NO/c1-21-9-7-17(24)12-15(21)3-5-18-19-6-4-14(11-16-13-23-16)22(19,2)10-8-20(18)21/h3,14,16,18-20,23H,4-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Inhibition of steroid 5-alpha-reductase type II isozyme |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50409046

(CHEMBL2112782)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)C1=CC[C@@H]2[C@@H]1CN1 |r,c:19,t:7| Show InChI InChI=1S/C21H31NO/c1-20-9-7-14(23)11-13(20)3-4-15-16-5-6-18(19-12-22-19)21(16,2)10-8-17(15)20/h3,5,14-15,17-19,22-23H,4,6-12H2,1-2H3/t14-,15-,17-,18+,19-,20-,21-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against Cytochrome P450 17 from rat testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50409041

(CHEMBL2111947)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC[C@@H]2[C@H]1CN1 |r,t:7| Show InChI InChI=1S/C21H33NO/c1-20-9-7-14(23)11-13(20)3-4-15-16-5-6-18(19-12-22-19)21(16,2)10-8-17(15)20/h3,14-19,22-23H,4-12H2,1-2H3/t14-,15-,16-,17-,18+,19+,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Inhibition of Steroid 5-alpha-reductase type 2 isozyme |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50409040

(CHEMBL2111948)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4CC(=O)CC[C@]34C)[C@@H]1CC[C@@H]2[C@H]1CN1 |r,t:7| Show InChI InChI=1S/C21H31NO/c1-20-9-7-14(23)11-13(20)3-4-15-16-5-6-18(19-12-22-19)21(16,2)10-8-17(15)20/h3,15-19,22H,4-12H2,1-2H3/t15-,16-,17-,18+,19+,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Inhibition of steroid 5-alpha-reductase type II isozyme |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50094192
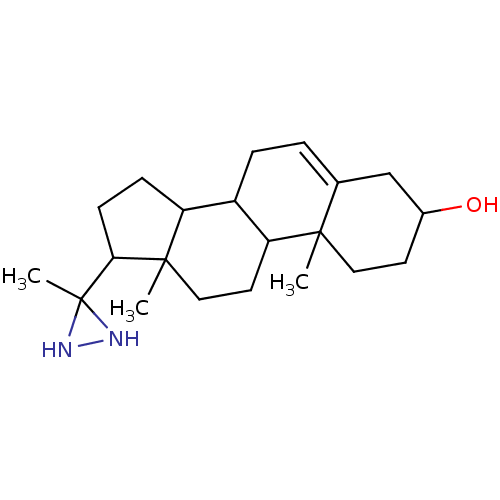
(10,13-Dimethyl-17-(3-methyl-diaziridin-3-yl)-2,3,4...)Show SMILES CC1(NN1)C1CCC2C3CC=C4CC(O)CCC4(C)C3CCC12C |t:11| Show InChI InChI=1S/C21H34N2O/c1-19-10-8-14(24)12-13(19)4-5-15-16-6-7-18(21(3)22-23-21)20(16,2)11-9-17(15)19/h4,14-18,22-24H,5-12H2,1-3H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against Cytochrome P450 17 from rat testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50409047
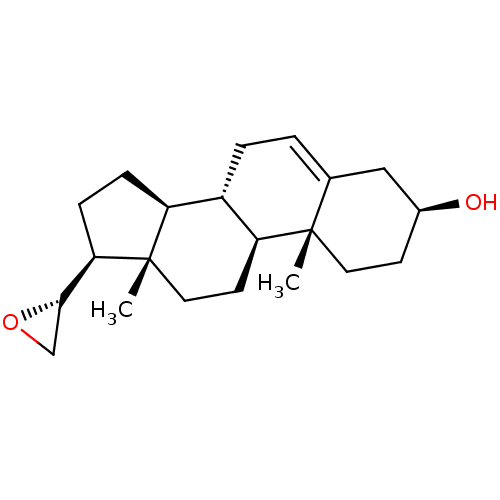
(CHEMBL2111949)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC[C@@H]2[C@@H]1CO1 |r,t:7| Show InChI InChI=1S/C21H32O2/c1-20-9-7-14(22)11-13(20)3-4-15-16-5-6-18(19-12-23-19)21(16,2)10-8-17(15)20/h3,14-19,22H,4-12H2,1-2H3/t14-,15-,16-,17-,18+,19-,20-,21-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against Cytochrome P450 17 from rat testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50094192

(10,13-Dimethyl-17-(3-methyl-diaziridin-3-yl)-2,3,4...)Show SMILES CC1(NN1)C1CCC2C3CC=C4CC(O)CCC4(C)C3CCC12C |t:11| Show InChI InChI=1S/C21H34N2O/c1-19-10-8-14(24)12-13(19)4-5-15-16-6-7-18(21(3)22-23-21)20(16,2)11-9-17(15)19/h4,14-18,22-24H,5-12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against Cytochrome P450 17 from human testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50094202
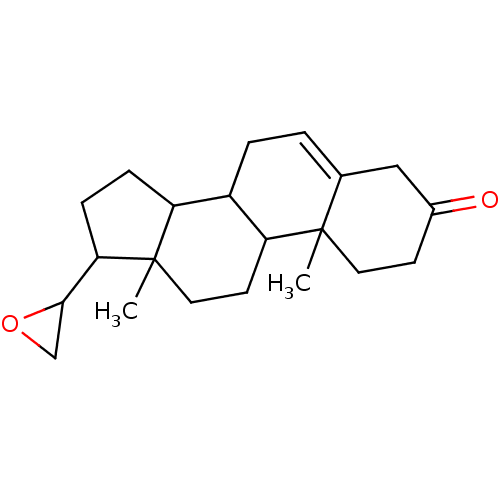
((R)-10,13-Dimethyl-17-oxiranyl-1,2,4,7,8,9,10,11,1...)Show SMILES CC12CCC3C(CC=C4CC(=O)CCC34C)C1CCC2C1CO1 |t:7| Show InChI InChI=1S/C21H30O2/c1-20-9-7-14(22)11-13(20)3-4-15-16-5-6-18(19-12-23-19)21(16,2)10-8-17(15)20/h3,15-19H,4-12H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against Cytochrome P450 17 from rat testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Cholesterol side-chain cleavage enzyme, mitochondrial
(Bos taurus (Bovine)) | BDBM50409041

(CHEMBL2111947)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC[C@@H]2[C@H]1CN1 |r,t:7| Show InChI InChI=1S/C21H33NO/c1-20-9-7-14(23)11-13(20)3-4-15-16-5-6-18(19-12-22-19)21(16,2)10-8-17(15)20/h3,14-19,22-23H,4-12H2,1-2H3/t14-,15-,16-,17-,18+,19+,20-,21-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Inhibition of P450 scc |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50409044

(CHEMBL2111950)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC[C@]2(O)[C@@H]1CN1 |r,t:7| Show InChI InChI=1S/C21H33NO2/c1-19-8-5-14(23)11-13(19)3-4-15-16(19)6-9-20(2)17(15)7-10-21(20,24)18-12-22-18/h3,14-18,22-24H,4-12H2,1-2H3/t14-,15+,16-,17-,18-,19-,20-,21-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against Cytochrome P450 17 from rat testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50409048

(CHEMBL2112772)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC[C@@H]2[C@@H]1CN1 |r,t:7| Show InChI InChI=1S/C21H33NO/c1-20-9-7-14(23)11-13(20)3-4-15-16-5-6-18(19-12-22-19)21(16,2)10-8-17(15)20/h3,14-19,22-23H,4-12H2,1-2H3/t14-,15-,16-,17-,18+,19-,20-,21-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against Cytochrome P450 17 from rat testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(Homo sapiens (Human)) | BDBM50094191

(17-Aziridin-2-ylmethyl-10,13-dimethyl-2,3,4,7,8,9,...)Show SMILES CC12CCC3C(CC=C4CC(O)CCC34C)C1CCC2CC1CN1 |t:7| Show InChI InChI=1S/C22H35NO/c1-21-9-7-17(24)12-15(21)3-5-18-19-6-4-14(11-16-13-23-16)22(19,2)10-8-20(18)21/h3,14,16-20,23-24H,4-13H2,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Inhibition of thromboxane synthase P450 TXA2 |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50409041

(CHEMBL2111947)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC[C@@H]2[C@H]1CN1 |r,t:7| Show InChI InChI=1S/C21H33NO/c1-20-9-7-14(23)11-13(20)3-4-15-16-5-6-18(19-12-22-19)21(16,2)10-8-17(15)20/h3,14-19,22-23H,4-12H2,1-2H3/t14-,15-,16-,17-,18+,19+,20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Inhibition of Cytochrome P450 19A1 |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against Cytochrome P450 17 from rat testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50409042

(CHEMBL2112781)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC[C@@H]2[C@H]1CO1 |r,t:7| Show InChI InChI=1S/C21H32O2/c1-20-9-7-14(22)11-13(20)3-4-15-16-5-6-18(19-12-23-19)21(16,2)10-8-17(15)20/h3,14-19,22H,4-12H2,1-2H3/t14-,15-,16-,17-,18+,19+,20-,21-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against Cytochrome P450 17 from rat testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50409043

(CHEMBL2112775)Show SMILES C[C@]12CC[C@H]3[C@@H](CC=C4C[C@@H](O)CC[C@]34C)[C@@H]1CC[C@]2(O)[C@H]1CN1 |r,t:7| Show InChI InChI=1S/C21H33NO2/c1-19-8-5-14(23)11-13(19)3-4-15-16(19)6-9-20(2)17(15)7-10-21(20,24)18-12-22-18/h3,14-18,22-24H,4-12H2,1-2H3/t14-,15+,16-,17-,18+,19-,20-,21-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Saarland
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against Cytochrome P450 17 from rat testicular microsomes |
J Med Chem 43: 4437-45 (2000)
BindingDB Entry DOI: 10.7270/Q25H7FH3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data