 Found 69 hits Enz. Inhib. hit(s) with all data for entry = 50007641
Found 69 hits Enz. Inhib. hit(s) with all data for entry = 50007641 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
C-C chemokine receptor type 7
(Homo sapiens (Human)) | BDBM50088301

((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...)Show SMILES Cc1ccc(cc1)-c1ccc2CCCC(=Cc2c1)C(=O)Nc1ccc(C[N+](C)(C)C2CCOCC2)cc1 |c:15| Show InChI InChI=1S/C33H38N2O2/c1-24-7-11-27(12-8-24)28-14-13-26-5-4-6-29(22-30(26)21-28)33(36)34-31-15-9-25(10-16-31)23-35(2,3)32-17-19-37-20-18-32/h7-16,21-22,32H,4-6,17-20,23H2,1-3H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CCR7 (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50069756
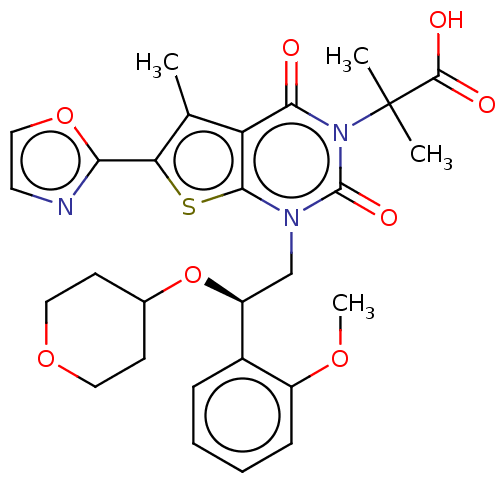
(CHEMBL3407547 | Firsocostat | ND-630 | NDI-010976 ...)Show SMILES COc1ccccc1[C@H](Cn1c2sc(c(C)c2c(=O)n(c1=O)C(C)(C)C(O)=O)-c1ncco1)OC1CCOCC1 |r| Show InChI InChI=1S/C28H31N3O8S/c1-16-21-24(32)31(28(2,3)26(33)34)27(35)30(25(21)40-22(16)23-29-11-14-38-23)15-20(39-17-9-12-37-13-10-17)18-7-5-6-8-19(18)36-4/h5-8,11,14,17,20H,9-10,12-13,15H2,1-4H3,(H,33,34)/t20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of ACC1 (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50069756

(CHEMBL3407547 | Firsocostat | ND-630 | NDI-010976 ...)Show SMILES COc1ccccc1[C@H](Cn1c2sc(c(C)c2c(=O)n(c1=O)C(C)(C)C(O)=O)-c1ncco1)OC1CCOCC1 |r| Show InChI InChI=1S/C28H31N3O8S/c1-16-21-24(32)31(28(2,3)26(33)34)27(35)30(25(21)40-22(16)23-29-11-14-38-23)15-20(39-17-9-12-37-13-10-17)18-7-5-6-8-19(18)36-4/h5-8,11,14,17,20H,9-10,12-13,15H2,1-4H3,(H,33,34)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of ACC2 (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50511117
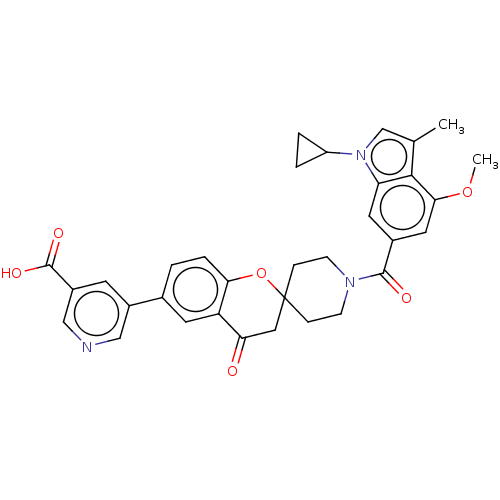
(CHEMBL4557289)Show SMILES COc1cc(cc2n(cc(C)c12)C1CC1)C(=O)N1CCC2(CC1)CC(=O)c1cc(ccc1O2)-c1cncc(c1)C(O)=O Show InChI InChI=1S/C33H31N3O6/c1-19-18-36(24-4-5-24)26-13-21(14-29(41-2)30(19)26)31(38)35-9-7-33(8-10-35)15-27(37)25-12-20(3-6-28(25)42-33)22-11-23(32(39)40)17-34-16-22/h3,6,11-14,16-18,24H,4-5,7-10,15H2,1-2H3,(H,39,40) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of ACC1 (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50511117

(CHEMBL4557289)Show SMILES COc1cc(cc2n(cc(C)c12)C1CC1)C(=O)N1CCC2(CC1)CC(=O)c1cc(ccc1O2)-c1cncc(c1)C(O)=O Show InChI InChI=1S/C33H31N3O6/c1-19-18-36(24-4-5-24)26-13-21(14-29(41-2)30(19)26)31(38)35-9-7-33(8-10-35)15-27(37)25-12-20(3-6-28(25)42-33)22-11-23(32(39)40)17-34-16-22/h3,6,11-14,16-18,24H,4-5,7-10,15H2,1-2H3,(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of ACC2 (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 7
(Homo sapiens (Human)) | BDBM50306033
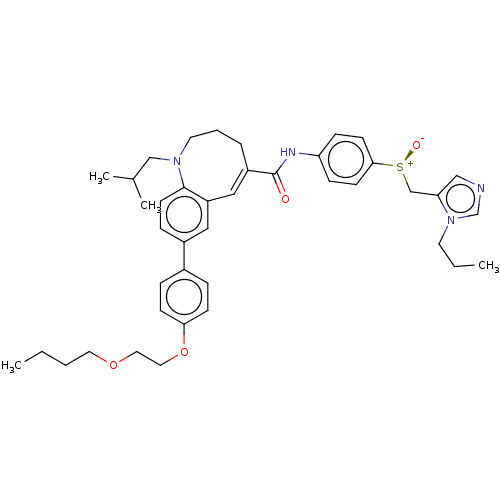
(Cenicriviroc | TAK-652 | TBR-652)Show SMILES CCCCOCCOc1ccc(cc1)-c1ccc2N(CC(C)C)CCC\C(=C/c2c1)C(=O)Nc1ccc(cc1)[S@@+]([O-])Cc1cncn1CCC |r,c:27| Show InChI InChI=1S/C41H52N4O4S/c1-5-7-22-48-23-24-49-38-15-10-32(11-16-38)33-12-19-40-35(25-33)26-34(9-8-21-44(40)28-31(3)4)41(46)43-36-13-17-39(18-14-36)50(47)29-37-27-42-30-45(37)20-6-2/h10-19,25-27,30-31H,5-9,20-24,28-29H2,1-4H3,(H,43,46)/b34-26+/t50-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CCR7 (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50306033

(Cenicriviroc | TAK-652 | TBR-652)Show SMILES CCCCOCCOc1ccc(cc1)-c1ccc2N(CC(C)C)CCC\C(=C/c2c1)C(=O)Nc1ccc(cc1)[S@@+]([O-])Cc1cncn1CCC |r,c:27| Show InChI InChI=1S/C41H52N4O4S/c1-5-7-22-48-23-24-49-38-15-10-32(11-16-38)33-12-19-40-35(25-33)26-34(9-8-21-44(40)28-31(3)4)41(46)43-36-13-17-39(18-14-36)50(47)29-37-27-42-30-45(37)20-6-2/h10-19,25-27,30-31H,5-9,20-24,28-29H2,1-4H3,(H,43,46)/b34-26+/t50-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CCR2 (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Ketohexokinase
(Homo sapiens (Human)) | BDBM319585

(US10174007, Example 4 | US10787438, Example 4 | US...)Show SMILES C[C@H]1CCN1c1nc(cc(n1)C(F)(F)F)N1C[C@H]2[C@H](CC(O)=O)[C@H]2C1 |r| Show InChI InChI=1S/C16H19F3N4O2/c1-8-2-3-23(8)15-20-12(16(17,18)19)5-13(21-15)22-6-10-9(4-14(24)25)11(10)7-22/h5,8-11H,2-4,6-7H2,1H3,(H,24,25)/t8-,9-,10-,11+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged human KHK expressed in Escherichia coli in presence of NADPH |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50511118
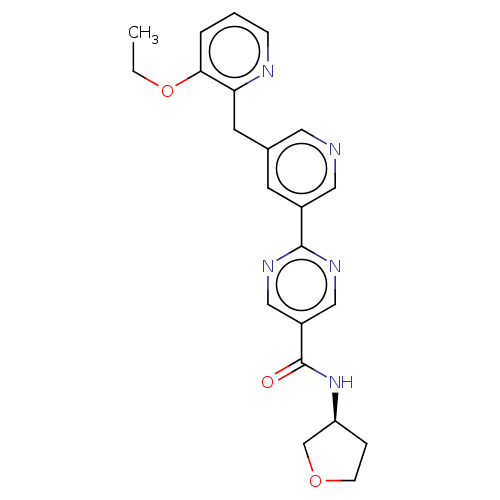
(CHEMBL4456029)Show SMILES CCOc1cccnc1Cc1cncc(c1)-c1ncc(cn1)C(=O)N[C@H]1CCOC1 |r| Show InChI InChI=1S/C22H23N5O3/c1-2-30-20-4-3-6-24-19(20)9-15-8-16(11-23-10-15)21-25-12-17(13-26-21)22(28)27-18-5-7-29-14-18/h3-4,6,8,10-13,18H,2,5,7,9,14H2,1H3,(H,27,28)/t18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FLAG-tagged human DGAT2 expressed in SF9 cells after 1 hr by TopCount assay |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50088301

((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...)Show SMILES Cc1ccc(cc1)-c1ccc2CCCC(=Cc2c1)C(=O)Nc1ccc(C[N+](C)(C)C2CCOCC2)cc1 |c:15| Show InChI InChI=1S/C33H38N2O2/c1-24-7-11-27(12-8-24)28-14-13-26-5-4-6-29(22-30(26)21-28)33(36)34-31-15-9-25(10-16-31)23-35(2,3)32-17-19-37-20-18-32/h7-16,21-22,32H,4-6,17-20,23H2,1-3H3/p+1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CCR2 (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50511112

(CHEMBL4567446)Show SMILES COc1cc(cc(n1)-c1ccc(cc1)C(O)=O)C(=O)N1CCC2(CC1)Cc1cnn(C(C)C)c1C(=O)C2 Show InChI InChI=1S/C28H30N4O5/c1-17(2)32-25-21(16-29-32)14-28(15-23(25)33)8-10-31(11-9-28)26(34)20-12-22(30-24(13-20)37-3)18-4-6-19(7-5-18)27(35)36/h4-7,12-13,16-17H,8-11,14-15H2,1-3H3,(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of ACC2 (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50511112

(CHEMBL4567446)Show SMILES COc1cc(cc(n1)-c1ccc(cc1)C(O)=O)C(=O)N1CCC2(CC1)Cc1cnn(C(C)C)c1C(=O)C2 Show InChI InChI=1S/C28H30N4O5/c1-17(2)32-25-21(16-29-32)14-28(15-23(25)33)8-10-31(11-9-28)26(34)20-12-22(30-24(13-20)37-3)18-4-6-19(7-5-18)27(35)36/h4-7,12-13,16-17H,8-11,14-15H2,1-3H3,(H,35,36) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of ACC1 (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50399710
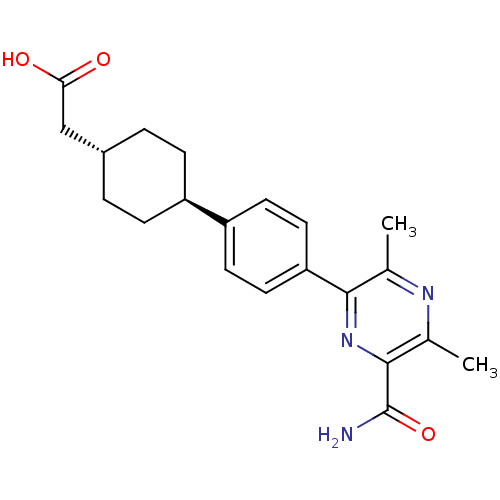
(CHEMBL2178953)Show SMILES Cc1nc(C)c(nc1C(N)=O)-c1ccc(cc1)[C@H]1CC[C@H](CC(O)=O)CC1 |r,wU:17.18,wD:20.22,(61.46,-10.56,;62.79,-9.79,;64.13,-10.56,;65.46,-9.79,;66.8,-10.56,;65.46,-8.26,;64.13,-7.48,;62.79,-8.25,;61.46,-7.48,;60.12,-8.25,;61.46,-5.94,;66.79,-7.49,;68.12,-8.26,;69.46,-7.49,;69.46,-5.94,;68.12,-5.17,;66.79,-5.94,;70.79,-5.16,;70.78,-3.63,;72.12,-2.86,;73.45,-3.62,;74.79,-2.85,;76.12,-3.62,;77.46,-2.85,;76.12,-5.16,;73.45,-5.17,;72.13,-5.93,)| Show InChI InChI=1S/C21H25N3O3/c1-12-19(24-20(21(22)27)13(2)23-12)17-9-7-16(8-10-17)15-5-3-14(4-6-15)11-18(25)26/h7-10,14-15H,3-6,11H2,1-2H3,(H2,22,27)(H,25,26)/t14-,15- | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FLAG-tagged human DGAT2 expressed in SF9 cells after 1 hr by TopCount assay |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Mus musculus) | BDBM50030474
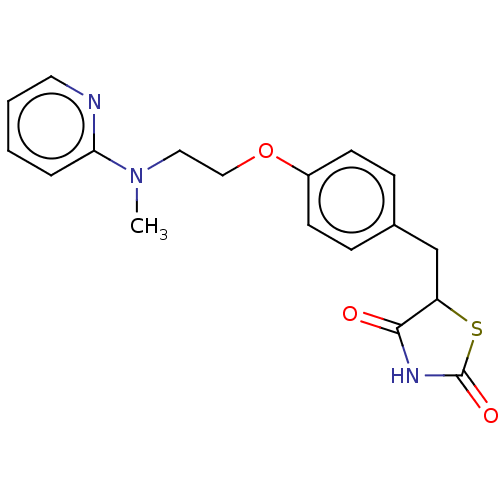
(Avandamet | Avandaryl | Avandia | BRL-49653 | CHEB...)Show InChI InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,15H,10-12H2,1H3,(H,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Activation of mouse liver PPARalpha |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Ketohexokinase
(Rattus norvegicus) | BDBM50241178
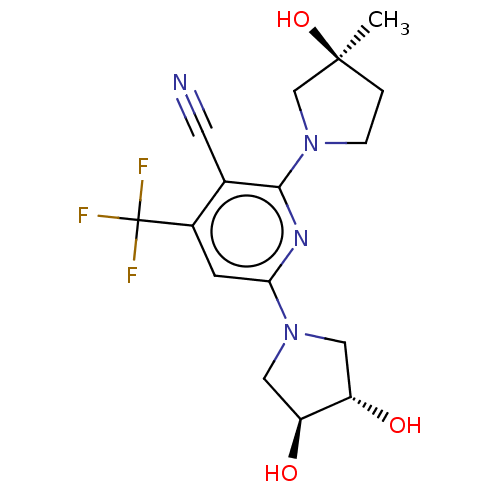
(CHEMBL4070442)Show SMILES C[C@]1(O)CCN(C1)c1nc(cc(c1C#N)C(F)(F)F)N1C[C@H](O)[C@@H](O)C1 |r| Show InChI InChI=1S/C16H19F3N4O3/c1-15(26)2-3-22(8-15)14-9(5-20)10(16(17,18)19)4-13(21-14)23-6-11(24)12(25)7-23/h4,11-12,24-26H,2-3,6-8H2,1H3/t11-,12-,15-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of rat KHK |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ketohexokinase
(Homo sapiens (Human)) | BDBM50241178

(CHEMBL4070442)Show SMILES C[C@]1(O)CCN(C1)c1nc(cc(c1C#N)C(F)(F)F)N1C[C@H](O)[C@@H](O)C1 |r| Show InChI InChI=1S/C16H19F3N4O3/c1-15(26)2-3-22(8-15)14-9(5-20)10(16(17,18)19)4-13(21-14)23-6-11(24)12(25)7-23/h4,11-12,24-26H,2-3,6-8H2,1H3/t11-,12-,15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged human KHK expressed in Escherichia coli in presence of NADPH |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50511110

(CHEMBL4530491)Show SMILES CN1CCN(CC1)c1nc(CNc2ncc(Cl)cn2)cc(Oc2ccc(CS(N)(=O)=O)cc2)n1 Show InChI InChI=1S/C21H25ClN8O3S/c1-29-6-8-30(9-7-29)21-27-17(13-26-20-24-11-16(22)12-25-20)10-19(28-21)33-18-4-2-15(3-5-18)14-34(23,31)32/h2-5,10-12H,6-9,13-14H2,1H3,(H2,23,31,32)(H,24,25,26) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 579 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FLAG-tagged human DGAT2 expressed in SF9 cells after 1 hr by TopCount assay |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Mus musculus) | BDBM50103521
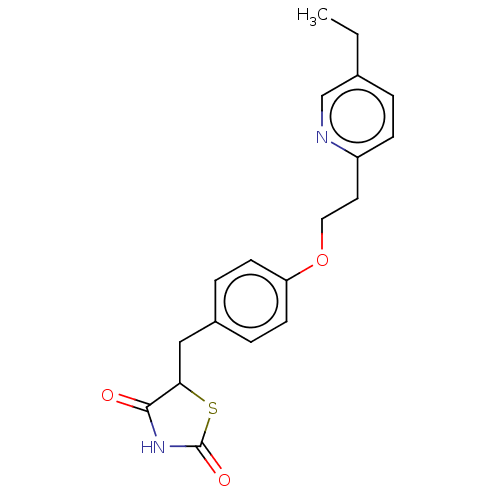
(Actos | CHEBI:8228 | Duetact | Pioglitazone | US10...)Show InChI InChI=1S/C19H20N2O3S/c1-2-13-3-6-15(20-12-13)9-10-24-16-7-4-14(5-8-16)11-17-18(22)21-19(23)25-17/h3-8,12,17H,2,9-11H2,1H3,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Activation of mouse liver PPARalpha |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50511111

(CHEMBL4476176)Show SMILES CCOC(=O)c1sc2n(CCc3ccccc3)c(=O)n(CCO)c(=O)c2c1C Show InChI InChI=1S/C20H22N2O5S/c1-3-27-19(25)16-13(2)15-17(24)21(11-12-23)20(26)22(18(15)28-16)10-9-14-7-5-4-6-8-14/h4-8,23H,3,9-12H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of ACC1 (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50511111

(CHEMBL4476176)Show SMILES CCOC(=O)c1sc2n(CCc3ccccc3)c(=O)n(CCO)c(=O)c2c1C Show InChI InChI=1S/C20H22N2O5S/c1-3-27-19(25)16-13(2)15-17(24)21(11-12-23)20(26)22(18(15)28-16)10-9-14-7-5-4-6-8-14/h4-8,23H,3,9-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of ACC2 (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Mus musculus) | BDBM50511113
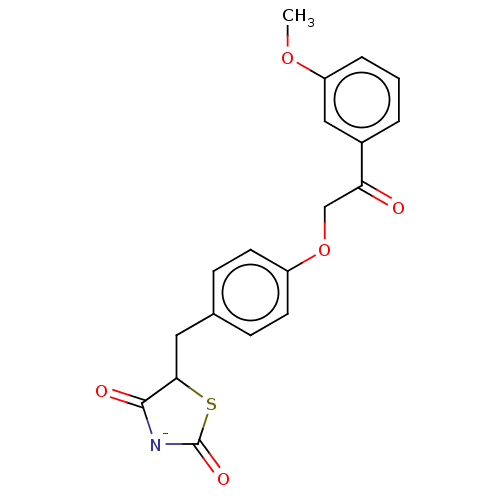
(CHEMBL4593380)Show SMILES [K;v0+].[#6]-[#8]-c1cccc(c1)-[#6](=O)-[#6]-[#8]-c1ccc(-[#6]-[#6]-2-[#16]-[#6](=O)-[#7-]-[#6]-2=O)cc1 Show InChI InChI=1S/C19H17NO5S.K/c1-24-15-4-2-3-13(10-15)16(21)11-25-14-7-5-12(6-8-14)9-17-18(22)20-19(23)26-17;/h2-8,10,17H,9,11H2,1H3,(H,20,22,23);/q;+1/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Activation of mouse liver PPARalpha |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50399710

(CHEMBL2178953)Show SMILES Cc1nc(C)c(nc1C(N)=O)-c1ccc(cc1)[C@H]1CC[C@H](CC(O)=O)CC1 |r,wU:17.18,wD:20.22,(61.46,-10.56,;62.79,-9.79,;64.13,-10.56,;65.46,-9.79,;66.8,-10.56,;65.46,-8.26,;64.13,-7.48,;62.79,-8.25,;61.46,-7.48,;60.12,-8.25,;61.46,-5.94,;66.79,-7.49,;68.12,-8.26,;69.46,-7.49,;69.46,-5.94,;68.12,-5.17,;66.79,-5.94,;70.79,-5.16,;70.78,-3.63,;72.12,-2.86,;73.45,-3.62,;74.79,-2.85,;76.12,-3.62,;77.46,-2.85,;76.12,-5.16,;73.45,-5.17,;72.13,-5.93,)| Show InChI InChI=1S/C21H25N3O3/c1-12-19(24-20(21(22)27)13(2)23-12)17-9-7-16(8-10-17)15-5-3-14(4-6-15)11-18(25)26/h7-10,14-15H,3-6,11H2,1-2H3,(H2,22,27)(H,25,26)/t14-,15- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FLAG-tagged human DGAT1 expressed in SF9 cells after 1 hr by TopCount assay |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Ketohexokinase
(Homo sapiens (Human)) | BDBM50241205

(CHEMBL1463512)Show InChI InChI=1S/C9H5F3N2O/c10-9(11,12)7-8(15)14-6-4-2-1-3-5(6)13-7/h1-4H,(H,14,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.19E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged human KHK expressed in Escherichia coli in presence of NADPH |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Ketohexokinase
(Homo sapiens (Human)) | BDBM50241206
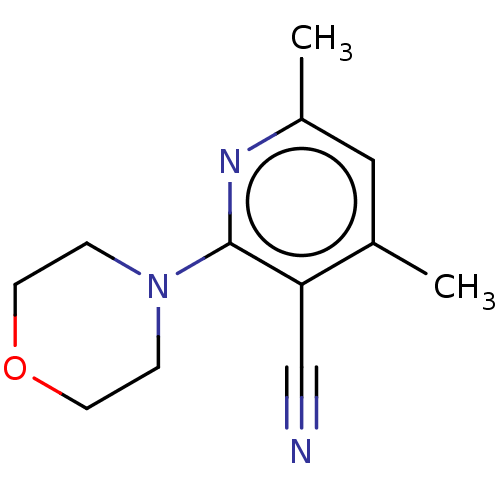
(CHEMBL1500542)Show InChI InChI=1S/C12H15N3O/c1-9-7-10(2)14-12(11(9)8-13)15-3-5-16-6-4-15/h7H,3-6H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 3.19E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His-tagged human KHK expressed in Escherichia coli in presence of NADPH |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bile acid receptor
(Homo sapiens (Human)) | BDBM21674

((4R)-4-[(1S,2S,5R,7S,9R,10R,11S,14R,15R)-5,9-dihyd...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(O)=O Show InChI InChI=1S/C24H40O4/c1-14(4-7-21(27)28)17-5-6-18-22-19(9-11-24(17,18)3)23(2)10-8-16(25)12-15(23)13-20(22)26/h14-20,22,25-26H,4-13H2,1-3H3,(H,27,28)/t14-,15+,16-,17-,18+,19+,20-,22+,23+,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 8.66E+3 | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at FXR (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bile acid receptor
(Homo sapiens (Human)) | BDBM21675
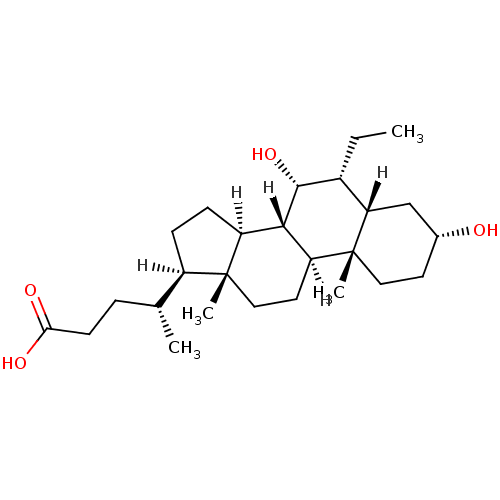
((4R)-4-[(1S,2S,5R,7S,8R,9R,10S,11S,14R,15R)-8-ethy...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)[C@H](CC)[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(O)=O Show InChI InChI=1S/C26H44O4/c1-5-17-21-14-16(27)10-12-26(21,4)20-11-13-25(3)18(15(2)6-9-22(28)29)7-8-19(25)23(20)24(17)30/h15-21,23-24,27,30H,5-14H2,1-4H3,(H,28,29)/t15-,16-,17-,18-,19+,20+,21+,23+,24-,25-,26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 99 | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at FXR (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50090940
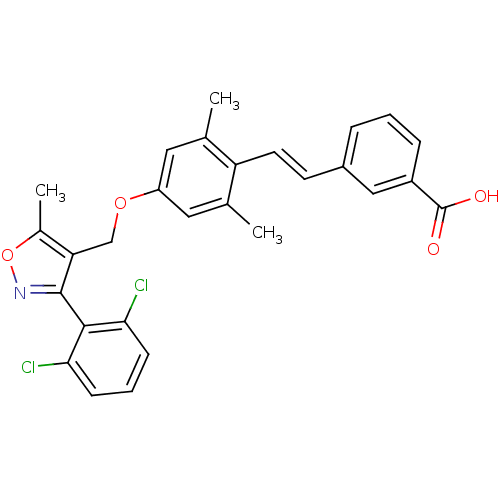
(3-(2-{4-[3-(2,6-Dichloro-phenyl)-5-methyl-isoxazol...)Show SMILES Cc1onc(c1COc1cc(C)c(\C=C\c2cccc(c2)C(O)=O)c(C)c1)-c1c(Cl)cccc1Cl |(23.6,-3.46,;24.75,-4.49,;26.25,-4.16,;27.04,-5.48,;26,-6.65,;24.61,-6.02,;23.28,-6.79,;21.94,-6.02,;20.62,-6.81,;19.25,-6.04,;17.93,-6.82,;16.6,-6.05,;17.94,-8.35,;16.62,-9.12,;15.28,-8.35,;13.95,-9.14,;13.95,-10.69,;12.6,-11.45,;11.26,-10.69,;11.26,-9.15,;12.6,-8.37,;9.94,-8.38,;8.61,-9.15,;9.94,-6.84,;19.28,-9.12,;19.28,-10.65,;20.62,-8.35,;26.34,-8.15,;25.2,-9.17,;23.73,-8.69,;25.52,-10.68,;26.99,-11.15,;28.13,-10.11,;27.81,-8.61,;28.94,-7.57,)| Show InChI InChI=1S/C28H23Cl2NO4/c1-16-12-21(13-17(2)22(16)11-10-19-6-4-7-20(14-19)28(32)33)34-15-23-18(3)35-31-27(23)26-24(29)8-5-9-25(26)30/h4-14H,15H2,1-3H3,(H,32,33)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at FXR (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50185707

(CHEMBL3822773)Show SMILES OC(=O)c1ccc(cc1)[C@H]1C[C@@H]1c1ccc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)cc1Cl |r,wU:9.9,wD:11.13,(10.44,1.17,;10,.02,;10.78,-.94,;8.48,-.23,;7.51,.96,;5.99,.71,;5.45,-.71,;6.42,-1.92,;7.94,-1.67,;4.13,-1.5,;3.35,-2.83,;2.67,-1.54,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;-2.67,1.54,;-4,.77,;-5.34,1.53,;-5.48,3.05,;-6.99,3.37,;-7.76,2.04,;-6.73,.9,;-7.04,-.61,;-8.54,-.98,;-9.39,-.09,;-8.97,-2.45,;-7.9,-3.57,;-6.41,-3.2,;-5.98,-1.72,;-4.78,-1.43,;-4.33,4.08,;-2.93,4.46,;-4.05,5.51,;,1.54,;1.33,.77,;2.4,1.39,)| Show InChI InChI=1S/C29H22Cl3NO4/c30-23-2-1-3-24(31)26(23)27-22(28(37-33-27)16-6-7-16)14-36-18-10-11-19(25(32)12-18)21-13-20(21)15-4-8-17(9-5-15)29(34)35/h1-5,8-12,16,20-21H,6-7,13-14H2,(H,34,35)/t20-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at FXR (unknown origin) by cell based assay |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50511109

(Cilofexor | GS 9674 | Gs-9674 | PX-104 | PX104)Show SMILES OC(=O)c1ccnc(c1)N1CC(O)(C1)c1ccc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)cc1Cl |(3.31,-11.16,;4.8,-10.76,;5.2,-9.28,;5.89,-11.85,;5.49,-13.35,;6.58,-14.43,;8.06,-14.03,;8.46,-12.54,;7.38,-11.45,;9.95,-12.14,;11.28,-12.91,;12.06,-11.55,;12.83,-10.22,;10.73,-10.78,;13.55,-11.16,;13.95,-9.67,;15.43,-9.27,;16.52,-10.36,;17.86,-9.59,;19.19,-10.36,;20.53,-9.59,;20.69,-8.06,;22.19,-7.75,;22.95,-9.08,;21.93,-10.21,;22.33,-11.7,;21.24,-12.79,;19.75,-12.39,;21.65,-14.28,;23.13,-14.67,;24.22,-13.58,;23.82,-12.1,;24.91,-11.01,;19.6,-6.97,;19.2,-5.48,;18.12,-6.57,;16.13,-11.84,;14.65,-12.25,;14.25,-13.74,)| Show InChI InChI=1S/C28H22Cl3N3O5/c29-20-2-1-3-21(30)24(20)25-18(26(39-33-25)15-4-5-15)12-38-17-6-7-19(22(31)11-17)28(37)13-34(14-28)23-10-16(27(35)36)8-9-32-23/h1-3,6-11,15,37H,4-5,12-14H2,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at FXR (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ketohexokinase
(Homo sapiens (Human)) | BDBM50241206

(CHEMBL1500542)Show InChI InChI=1S/C12H15N3O/c1-9-7-10(2)14-12(11(9)8-13)15-3-5-16-6-4-15/h7H,3-6H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 6.20E+4 | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant His-tagged human KHK expressed in Escherichia coli in presence of NADPH |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ketohexokinase
(Homo sapiens (Human)) | BDBM50241205

(CHEMBL1463512)Show InChI InChI=1S/C9H5F3N2O/c10-9(11,12)7-8(15)14-6-4-2-1-3-5(6)13-7/h1-4H,(H,14,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant His-tagged human KHK expressed in Escherichia coli in presence of NADPH |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM50511114
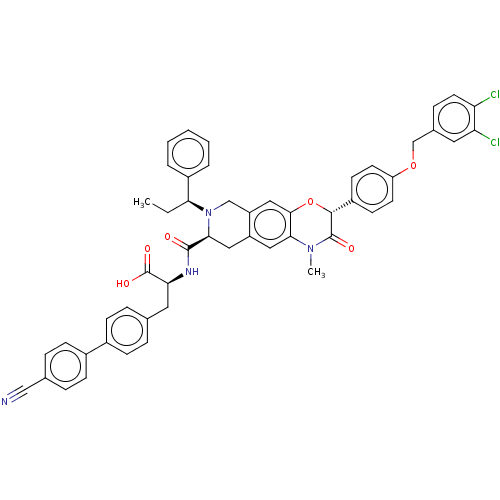
(CHEMBL4438585)Show SMILES CC[C@H](N1Cc2cc3O[C@@H](C(=O)N(C)c3cc2C[C@H]1C(=O)N[C@@H](Cc1ccc(cc1)-c1ccc(cc1)C#N)C(O)=O)c1ccc(OCc2ccc(Cl)c(Cl)c2)cc1)c1ccccc1 |r| Show InChI InChI=1S/C51H44Cl2N4O6/c1-3-44(36-7-5-4-6-8-36)57-29-39-27-47-45(56(2)50(59)48(63-47)37-18-20-40(21-19-37)62-30-33-13-22-41(52)42(53)23-33)25-38(39)26-46(57)49(58)55-43(51(60)61)24-31-9-14-34(15-10-31)35-16-11-32(28-54)12-17-35/h4-23,25,27,43-44,46,48H,3,24,26,29-30H2,1-2H3,(H,55,58)(H,60,61)/t43-,44-,46-,48+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 59 | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]GLP-1 from human GLP-1R (7 to 36 residues) expressed in HEK293 or CHO cell membranes after 120 mins by radioligand binding assa... |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 1
(Homo sapiens (Human)) | BDBM50511115

(CHEMBL4552590)Show SMILES OC[C@H]1O[C@@H](Oc2ccccc2C(=O)CCc2ccc(O)cc2)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C21H24O8/c22-11-17-18(25)19(26)20(27)21(29-17)28-16-4-2-1-3-14(16)15(24)10-7-12-5-8-13(23)9-6-12/h1-6,8-9,17-23,25-27H,7,10-11H2/t17-,18-,19+,20-,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 330 | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of SGLT1 (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 1
(Homo sapiens (Human)) | BDBM20880
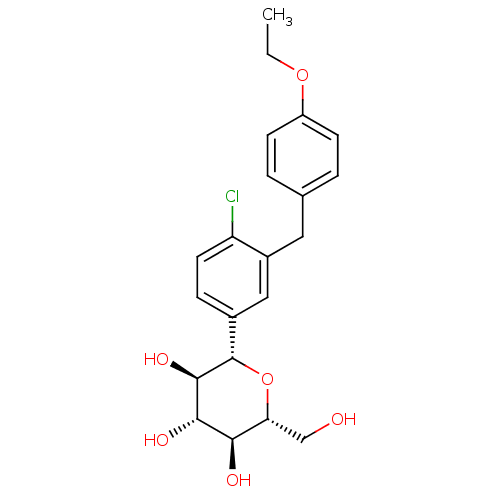
((2S,3R,4R,5S,6R)-2-{4-chloro-3-[(4-ethoxyphenyl)me...)Show SMILES CCOc1ccc(Cc2cc(ccc2Cl)[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)cc1 Show InChI InChI=1S/C21H25ClO6/c1-2-27-15-6-3-12(4-7-15)9-14-10-13(5-8-16(14)22)21-20(26)19(25)18(24)17(11-23)28-21/h3-8,10,17-21,23-26H,2,9,11H2,1H3/t17-,18-,19+,20-,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of SGLT1 (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM20880

((2S,3R,4R,5S,6R)-2-{4-chloro-3-[(4-ethoxyphenyl)me...)Show SMILES CCOc1ccc(Cc2cc(ccc2Cl)[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)cc1 Show InChI InChI=1S/C21H25ClO6/c1-2-27-15-6-3-12(4-7-15)9-14-10-13(5-8-16(14)22)21-20(26)19(25)18(24)17(11-23)28-21/h3-8,10,17-21,23-26H,2,9,11H2,1H3/t17-,18-,19+,20-,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of SGLT2 (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium/glucose cotransporter 1
(Homo sapiens (Human)) | BDBM50386885
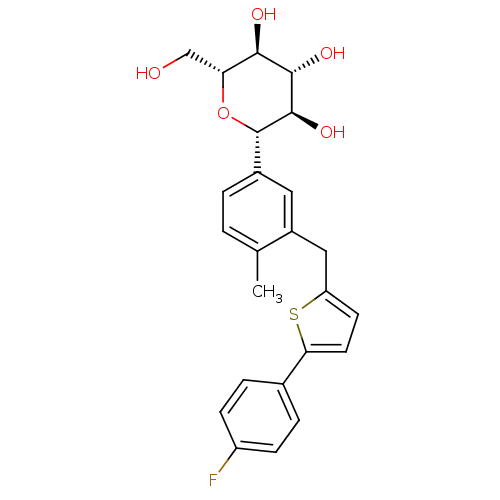
(CANAGLIFLOZIN | CANAGLIFLOZIN HYDRATE | US10752604...)Show SMILES Cc1ccc(cc1Cc1ccc(s1)-c1ccc(F)cc1)[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C24H25FO5S/c1-13-2-3-15(24-23(29)22(28)21(27)19(12-26)30-24)10-16(13)11-18-8-9-20(31-18)14-4-6-17(25)7-5-14/h2-10,19,21-24,26-29H,11-12H2,1H3/t19-,21-,22+,23-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 910 | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of SGLT1 (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50386885

(CANAGLIFLOZIN | CANAGLIFLOZIN HYDRATE | US10752604...)Show SMILES Cc1ccc(cc1Cc1ccc(s1)-c1ccc(F)cc1)[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C24H25FO5S/c1-13-2-3-15(24-23(29)22(28)21(27)19(12-26)30-24)10-16(13)11-18-8-9-20(31-18)14-4-6-17(25)7-5-14/h2-10,19,21-24,26-29H,11-12H2,1H3/t19-,21-,22+,23-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of SGLT2 (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM150162

(BI-10773 | Jardiance | US8980829, EMPAGLIFLOZIN | ...)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1ccc(Cl)c(Cc2ccc(O[C@H]3CCOC3)cc2)c1 Show InChI InChI=1S/C23H27ClO7/c24-18-6-3-14(23-22(28)21(27)20(26)19(11-25)31-23)10-15(18)9-13-1-4-16(5-2-13)30-17-7-8-29-12-17/h1-6,10,17,19-23,25-28H,7-9,11-12H2/t17-,19+,20+,21-,22+,23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of SGLT2 (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50381554
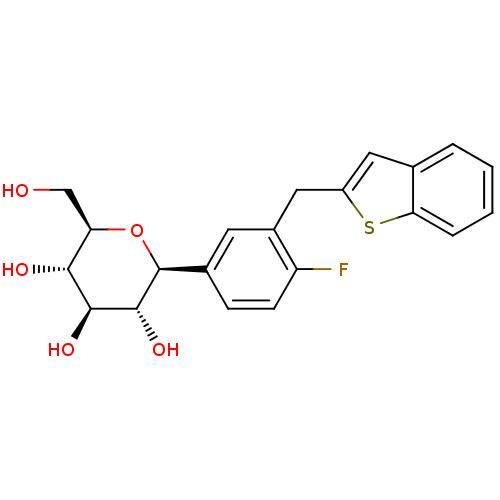
(CHEMBL2018096)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)c1ccc(F)c(Cc2cc3ccccc3s2)c1 |r| Show InChI InChI=1S/C21H21FO5S/c22-15-6-5-12(21-20(26)19(25)18(24)16(10-23)27-21)7-13(15)9-14-8-11-3-1-2-4-17(11)28-14/h1-8,16,18-21,23-26H,9-10H2/t16-,18-,19+,20-,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of SGLT2 (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 1
(Homo sapiens (Human)) | BDBM50315426
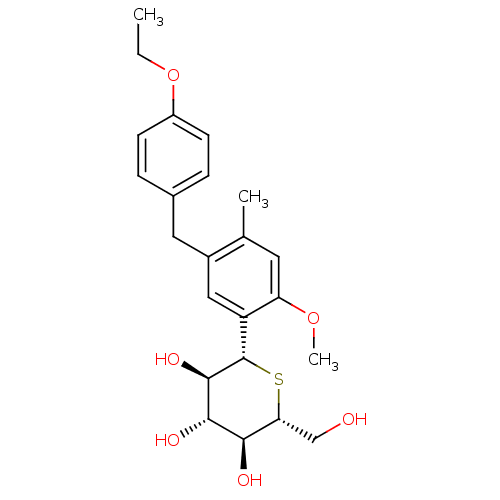
((1S)-1,5-Anhydro-1-[5-(4-ethoxybenzyl)-2-methoxy-4...)Show SMILES CCOc1ccc(Cc2cc([C@@H]3S[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)c(OC)cc2C)cc1 |r| Show InChI InChI=1S/C23H30O6S/c1-4-29-16-7-5-14(6-8-16)10-15-11-17(18(28-3)9-13(15)2)23-22(27)21(26)20(25)19(12-24)30-23/h5-9,11,19-27H,4,10,12H2,1-3H3/t19-,20-,21+,22-,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of SGLT1 (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
5'-AMP-activated protein kinase catalytic subunit alpha-1/subunit beta-2/subunit gamma-1
(Rattus norvegicus) | BDBM237920
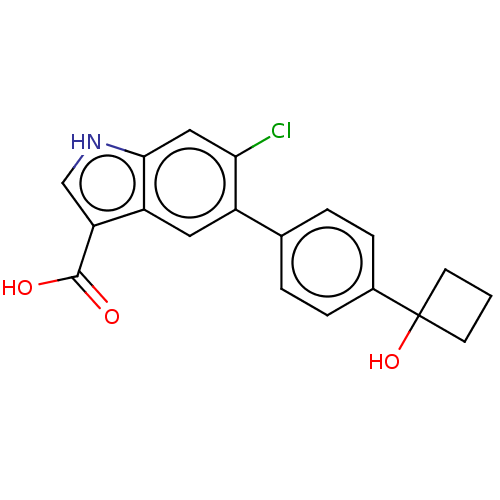
(US9394285, 1)Show SMILES OC(=O)c1c[nH]c2cc(Cl)c(cc12)-c1ccc(cc1)C1(O)CCC1 Show InChI InChI=1S/C19H16ClNO3/c20-16-9-17-14(15(10-21-17)18(22)23)8-13(16)11-2-4-12(5-3-11)19(24)6-1-7-19/h2-5,8-10,21,24H,1,6-7H2,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Activation of rat hepatocytes AMPK alpha1/beta2/gamma1 expressed in baculovirus infected Sf9 cells |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5'-AMP-activated protein kinase catalytic subunit alpha-1/subunit beta-2/subunit gamma-1
(Rattus norvegicus) | BDBM50511116

(CHEMBL4167177)Show SMILES [H][C@]12OC[C@@H](Oc3nc4nc(c(Cl)cc4[nH]3)-c3ccc(cc3)-c3ccccc3)[C@@]1([H])OC[C@H]2O |r| Show InChI InChI=1S/C24H20ClN3O4/c25-16-10-17-23(28-24(26-17)32-19-12-31-21-18(29)11-30-22(19)21)27-20(16)15-8-6-14(7-9-15)13-4-2-1-3-5-13/h1-10,18-19,21-22,29H,11-12H2,(H,26,27,28)/t18-,19-,21-,22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Activation of rat hepatocytes AMPK alpha1/beta2/gamma1 expressed in baculovirus infected Sf9 cells |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM21724

(3-[(E)-2-(2-chloro-4-{[3-(2,6-dichlorophenyl)-5-(1...)Show SMILES CC(C)c1onc(c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1)-c1c(Cl)cccc1Cl |(-5,9.13,;-4.28,7.76,;-2.74,7.7,;-5.11,6.46,;-6.65,6.36,;-7.03,4.87,;-5.73,4.05,;-4.54,5.03,;-3.21,4.26,;-1.87,5.03,;-.54,4.26,;.79,5.03,;2.13,4.26,;2.13,2.72,;3.46,1.95,;4.79,2.72,;6.13,1.95,;6.13,.41,;7.46,-.36,;8.79,.41,;8.79,1.95,;7.46,2.72,;10.13,2.72,;11.46,1.95,;10.13,4.26,;.79,1.95,;.79,.41,;-.54,2.72,;-5.63,2.51,;-4.28,1.78,;-2.97,2.59,;-4.23,.24,;-5.54,-.57,;-6.9,.16,;-6.94,1.7,;-8.3,2.43,)| Show InChI InChI=1S/C28H22Cl3NO4/c1-16(2)27-21(26(32-36-27)25-22(29)7-4-8-23(25)30)15-35-20-12-11-18(24(31)14-20)10-9-17-5-3-6-19(13-17)28(33)34/h3-14,16H,15H2,1-2H3,(H,33,34)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at FXR (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bile acid receptor
(Homo sapiens (Human)) | BDBM21724

(3-[(E)-2-(2-chloro-4-{[3-(2,6-dichlorophenyl)-5-(1...)Show SMILES CC(C)c1onc(c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1)-c1c(Cl)cccc1Cl |(-5,9.13,;-4.28,7.76,;-2.74,7.7,;-5.11,6.46,;-6.65,6.36,;-7.03,4.87,;-5.73,4.05,;-4.54,5.03,;-3.21,4.26,;-1.87,5.03,;-.54,4.26,;.79,5.03,;2.13,4.26,;2.13,2.72,;3.46,1.95,;4.79,2.72,;6.13,1.95,;6.13,.41,;7.46,-.36,;8.79,.41,;8.79,1.95,;7.46,2.72,;10.13,2.72,;11.46,1.95,;10.13,4.26,;.79,1.95,;.79,.41,;-.54,2.72,;-5.63,2.51,;-4.28,1.78,;-2.97,2.59,;-4.23,.24,;-5.54,-.57,;-6.9,.16,;-6.94,1.7,;-8.3,2.43,)| Show InChI InChI=1S/C28H22Cl3NO4/c1-16(2)27-21(26(32-36-27)25-22(29)7-4-8-23(25)30)15-35-20-12-11-18(24(31)14-20)10-9-17-5-3-6-19(13-17)28(33)34/h3-14,16H,15H2,1-2H3,(H,33,34)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at FXR (unknown origin) by cell based assay |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50103521

(Actos | CHEBI:8228 | Duetact | Pioglitazone | US10...)Show InChI InChI=1S/C19H20N2O3S/c1-2-13-3-6-15(20-12-13)9-10-24-16-7-4-14(5-8-16)11-17-18(22)21-19(23)25-17/h3-8,12,17H,2,9-11H2,1H3,(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 970 | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50185707

(CHEMBL3822773)Show SMILES OC(=O)c1ccc(cc1)[C@H]1C[C@@H]1c1ccc(OCc2c(onc2-c2c(Cl)cccc2Cl)C2CC2)cc1Cl |r,wU:9.9,wD:11.13,(10.44,1.17,;10,.02,;10.78,-.94,;8.48,-.23,;7.51,.96,;5.99,.71,;5.45,-.71,;6.42,-1.92,;7.94,-1.67,;4.13,-1.5,;3.35,-2.83,;2.67,-1.54,;1.33,-.77,;,-1.54,;-1.33,-.77,;-1.33,.77,;-2.67,1.54,;-4,.77,;-5.34,1.53,;-5.48,3.05,;-6.99,3.37,;-7.76,2.04,;-6.73,.9,;-7.04,-.61,;-8.54,-.98,;-9.39,-.09,;-8.97,-2.45,;-7.9,-3.57,;-6.41,-3.2,;-5.98,-1.72,;-4.78,-1.43,;-4.33,4.08,;-2.93,4.46,;-4.05,5.51,;,1.54,;1.33,.77,;2.4,1.39,)| Show InChI InChI=1S/C29H22Cl3NO4/c30-23-2-1-3-24(31)26(23)27-22(28(37-33-27)16-6-7-16)14-36-18-10-11-19(25(32)12-18)21-13-20(21)15-4-8-17(9-5-15)29(34)35/h1-5,8-12,16,20-21H,6-7,13-14H2,(H,34,35)/t20-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 209 | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at FXR (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 1
(Homo sapiens (Human)) | BDBM20878
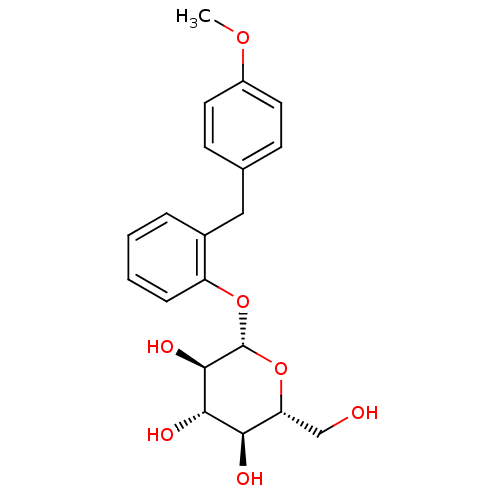
((2R,3S,4S,5R,6S)-2-(hydroxymethyl)-6-{2-[(4-methox...)Show SMILES COc1ccc(Cc2ccccc2O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)cc1 Show InChI InChI=1S/C20H24O7/c1-25-14-8-6-12(7-9-14)10-13-4-2-3-5-15(13)26-20-19(24)18(23)17(22)16(11-21)27-20/h2-9,16-24H,10-11H2,1H3/t16-,17-,18+,19-,20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of SGLT1 (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50213714
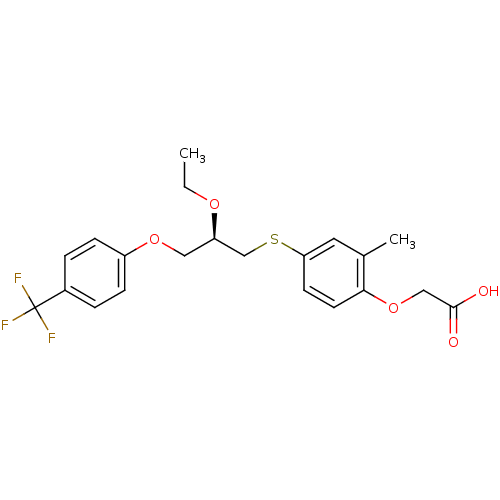
((R)-2-(4-(2-ethoxy-3-(4-(trifluoromethyl)phenoxy)p...)Show SMILES CCO[C@H](COc1ccc(cc1)C(F)(F)F)CSc1ccc(OCC(O)=O)c(C)c1 Show InChI InChI=1S/C21H23F3O5S/c1-3-27-17(11-28-16-6-4-15(5-7-16)21(22,23)24)13-30-18-8-9-19(14(2)10-18)29-12-20(25)26/h4-10,17H,3,11-13H2,1-2H3,(H,25,26)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bile acid receptor
(Homo sapiens (Human)) | BDBM21675

((4R)-4-[(1S,2S,5R,7S,8R,9R,10S,11S,14R,15R)-8-ethy...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)[C@H](CC)[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(O)=O Show InChI InChI=1S/C26H44O4/c1-5-17-21-14-16(27)10-12-26(21,4)20-11-13-25(3)18(15(2)6-9-22(28)29)7-8-19(25)23(20)24(17)30/h15-21,23-24,27,30H,5-14H2,1-4H3,(H,28,29)/t15-,16-,17-,18-,19+,20+,21+,23+,24-,25-,26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 85 | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at FXR (unknown origin) by cell based assay |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium/glucose cotransporter 2
(Homo sapiens (Human)) | BDBM50511115

(CHEMBL4552590)Show SMILES OC[C@H]1O[C@@H](Oc2ccccc2C(=O)CCc2ccc(O)cc2)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C21H24O8/c22-11-17-18(25)19(26)20(27)21(29-17)28-16-4-2-1-3-14(16)15(24)10-7-12-5-8-13(23)9-6-12/h1-6,8-9,17-23,25-27H,7,10-11H2/t17-,18-,19+,20-,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 36 | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of SGLT2 (unknown origin) |
J Med Chem 63: 5031-5073 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01701
BindingDB Entry DOI: 10.7270/Q2DJ5JXG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data