 Found 138 hits Enz. Inhib. hit(s) with all data for entry = 50009606
Found 138 hits Enz. Inhib. hit(s) with all data for entry = 50009606 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50530438

(Mosapramine | Mosapramine hydrochloride)Show SMILES Clc1ccc2CCc3ccccc3N(CCCN3CCC4(CC3)N3CCCCC3NC4=O)c2c1 Show InChI InChI=1S/C28H35ClN4O/c29-23-12-11-22-10-9-21-6-1-2-7-24(21)32(25(22)20-23)16-5-15-31-18-13-28(14-19-31)27(34)30-26-8-3-4-17-33(26)28/h1-2,6-7,11-12,20,26H,3-5,8-10,13-19H2,(H,30,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Agonist activity at dopamine D4 receptor (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50530438

(Mosapramine | Mosapramine hydrochloride)Show SMILES Clc1ccc2CCc3ccccc3N(CCCN3CCC4(CC3)N3CCCCC3NC4=O)c2c1 Show InChI InChI=1S/C28H35ClN4O/c29-23-12-11-22-10-9-21-6-1-2-7-24(21)32(25(22)20-23)16-5-15-31-18-13-28(14-19-31)27(34)30-26-8-3-4-17-33(26)28/h1-2,6-7,11-12,20,26H,3-5,8-10,13-19H2,(H,30,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Agonist activity at dopamine D4 receptor (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50274351
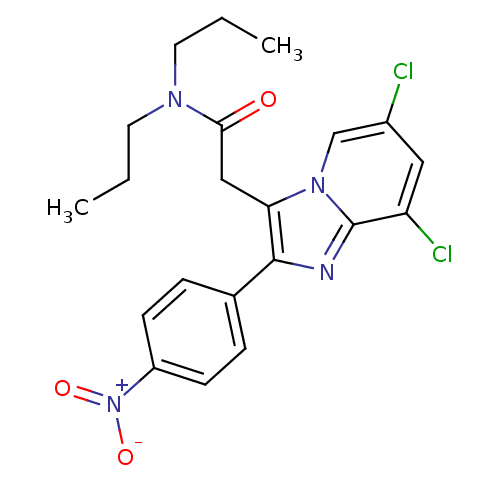
(2-(6,8-Dichloro-2-(4-nitrophenyl)imidazo[1,2-a]pyr...)Show SMILES CCCN(CCC)C(=O)Cc1c(nc2c(Cl)cc(Cl)cn12)-c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C21H22Cl2N4O3/c1-3-9-25(10-4-2)19(28)12-18-20(14-5-7-16(8-6-14)27(29)30)24-21-17(23)11-15(22)13-26(18)21/h5-8,11,13H,3-4,9-10,12H2,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of TSPO (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50274351

(2-(6,8-Dichloro-2-(4-nitrophenyl)imidazo[1,2-a]pyr...)Show SMILES CCCN(CCC)C(=O)Cc1c(nc2c(Cl)cc(Cl)cn12)-c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C21H22Cl2N4O3/c1-3-9-25(10-4-2)19(28)12-18-20(14-5-7-16(8-6-14)27(29)30)24-21-17(23)11-15(22)13-26(18)21/h5-8,11,13H,3-4,9-10,12H2,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of TSPO (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM50247053

(1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...)Show InChI InChI=1S/C17H26ClNO/c18-17-9-7-16(8-10-17)6-4-14-20-15-5-13-19-11-2-1-3-12-19/h7-10H,1-6,11-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of Guinea Pig Ileum H3R |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM50247053

(1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...)Show InChI InChI=1S/C17H26ClNO/c18-17-9-7-16(8-10-17)6-4-14-20-15-5-13-19-11-2-1-3-12-19/h7-10H,1-6,11-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of Guinea Pig Ileum H3R |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50189854

(6-bromo-2-[4-(2-methoxyphenyl)piperazin-1-ylmethyl...)Show InChI InChI=1S/C19H21BrN4O/c1-25-18-5-3-2-4-17(18)23-10-8-22(9-11-23)13-16-14-24-12-15(20)6-7-19(24)21-16/h2-7,12,14H,8-11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Agonist activity at dopamine D4 receptor (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50189854

(6-bromo-2-[4-(2-methoxyphenyl)piperazin-1-ylmethyl...)Show InChI InChI=1S/C19H21BrN4O/c1-25-18-5-3-2-4-17(18)23-10-8-22(9-11-23)13-16-14-24-12-15(20)6-7-19(24)21-16/h2-7,12,14H,8-11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Agonist activity at dopamine D4 receptor (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50189841
(6-iodo-2-[4-(2-methoxyphenyl)piperazin-1-ylmethyl]...)Show InChI InChI=1S/C19H21IN4O/c1-25-18-5-3-2-4-17(18)23-10-8-22(9-11-23)13-16-14-24-12-15(20)6-7-19(24)21-16/h2-7,12,14H,8-11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Agonist activity at dopamine D4 receptor (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50189841

(6-iodo-2-[4-(2-methoxyphenyl)piperazin-1-ylmethyl]...)Show InChI InChI=1S/C19H21IN4O/c1-25-18-5-3-2-4-17(18)23-10-8-22(9-11-23)13-16-14-24-12-15(20)6-7-19(24)21-16/h2-7,12,14H,8-11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Agonist activity at dopamine D4 receptor (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50060095

(2-[6,8-Dichloro-2-(4-chloro-phenyl)-imidazo[1,2-a]...)Show SMILES CCCN(CCC)C(=O)Cc1c(nc2c(Cl)cc(Cl)cn12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C21H22Cl3N3O/c1-3-9-26(10-4-2)19(28)12-18-20(14-5-7-15(22)8-6-14)25-21-17(24)11-16(23)13-27(18)21/h5-8,11,13H,3-4,9-10,12H2,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of TSPO (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50060095

(2-[6,8-Dichloro-2-(4-chloro-phenyl)-imidazo[1,2-a]...)Show SMILES CCCN(CCC)C(=O)Cc1c(nc2c(Cl)cc(Cl)cn12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C21H22Cl3N3O/c1-3-9-26(10-4-2)19(28)12-18-20(14-5-7-15(22)8-6-14)25-21-17(24)11-16(23)13-27(18)21/h5-8,11,13H,3-4,9-10,12H2,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of TSPO (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM22041
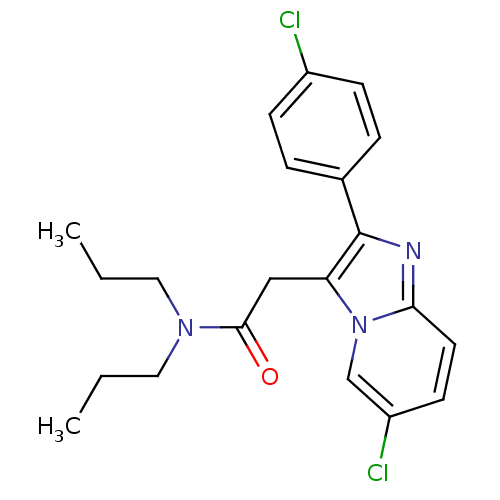
(2-[6-chloro-2-(4-chlorophenyl)imidazo[1,2-a]pyridi...)Show SMILES CCCN(CCC)C(=O)Cc1c(nc2ccc(Cl)cn12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23Cl2N3O/c1-3-11-25(12-4-2)20(27)13-18-21(15-5-7-16(22)8-6-15)24-19-10-9-17(23)14-26(18)19/h5-10,14H,3-4,11-13H2,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of TSPO (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50530421
(CHEMBL4585201)Show InChI InChI=1S/C24H31N3O/c1-19-10-14-27-18-23(25-24(27)16-19)22-9-8-21(17-20(22)2)28-15-7-13-26-11-5-3-4-6-12-26/h8-10,14,16-18H,3-7,11-13,15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of H3R (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM22041

(2-[6-chloro-2-(4-chlorophenyl)imidazo[1,2-a]pyridi...)Show SMILES CCCN(CCC)C(=O)Cc1c(nc2ccc(Cl)cn12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23Cl2N3O/c1-3-11-25(12-4-2)20(27)13-18-21(15-5-7-16(22)8-6-15)24-19-10-9-17(23)14-26(18)19/h5-10,14H,3-4,11-13H2,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of TSPO (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50530421

(CHEMBL4585201)Show InChI InChI=1S/C24H31N3O/c1-19-10-14-27-18-23(25-24(27)16-19)22-9-8-21(17-20(22)2)28-15-7-13-26-11-5-3-4-6-12-26/h8-10,14,16-18H,3-7,11-13,15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of H3R (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50274258
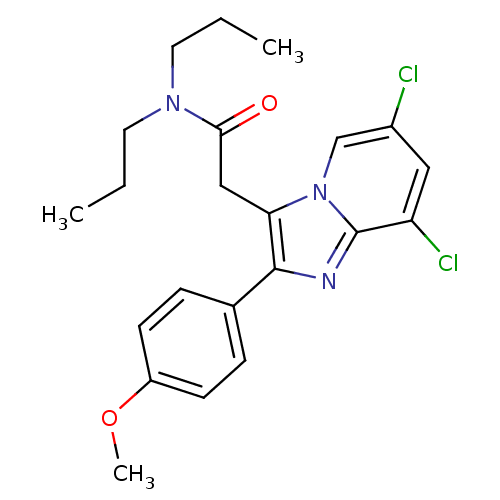
(2-(6,8-Dichloro-2-(4-methoxyphenyl)imidazo[1,2-a]p...)Show SMILES CCCN(CCC)C(=O)Cc1c(nc2c(Cl)cc(Cl)cn12)-c1ccc(OC)cc1 Show InChI InChI=1S/C22H25Cl2N3O2/c1-4-10-26(11-5-2)20(28)13-19-21(15-6-8-17(29-3)9-7-15)25-22-18(24)12-16(23)14-27(19)22/h6-9,12,14H,4-5,10-11,13H2,1-3H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.537 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of TSPO (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50274258

(2-(6,8-Dichloro-2-(4-methoxyphenyl)imidazo[1,2-a]p...)Show SMILES CCCN(CCC)C(=O)Cc1c(nc2c(Cl)cc(Cl)cn12)-c1ccc(OC)cc1 Show InChI InChI=1S/C22H25Cl2N3O2/c1-4-10-26(11-5-2)20(28)13-19-21(15-6-8-17(29-3)9-7-15)25-22-18(24)12-16(23)14-27(19)22/h6-9,12,14H,4-5,10-11,13H2,1-3H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.537 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of TSPO (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50530440
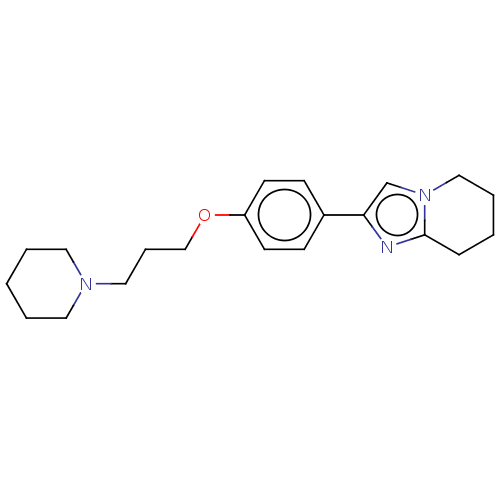
(CHEMBL4548796)Show InChI InChI=1S/C21H29N3O/c1-3-12-23(13-4-1)14-6-16-25-19-10-8-18(9-11-19)20-17-24-15-5-2-7-21(24)22-20/h8-11,17H,1-7,12-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of H3R (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50530440

(CHEMBL4548796)Show InChI InChI=1S/C21H29N3O/c1-3-12-23(13-4-1)14-6-16-25-19-10-8-18(9-11-19)20-17-24-15-5-2-7-21(24)22-20/h8-11,17H,1-7,12-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of H3R (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50274561

(4-(2-(4-Chlorophenyl)-3-(2-(dipropylamino)-2-oxoet...)Show SMILES CCCN(CCC)C(=O)Cc1c(nc2c(NC(=O)CCC(=O)OCC)cccn12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C27H33ClN4O4/c1-4-15-31(16-5-2)24(34)18-22-26(19-9-11-20(28)12-10-19)30-27-21(8-7-17-32(22)27)29-23(33)13-14-25(35)36-6-3/h7-12,17H,4-6,13-16,18H2,1-3H3,(H,29,33) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of TSPO (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50274561

(4-(2-(4-Chlorophenyl)-3-(2-(dipropylamino)-2-oxoet...)Show SMILES CCCN(CCC)C(=O)Cc1c(nc2c(NC(=O)CCC(=O)OCC)cccn12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C27H33ClN4O4/c1-4-15-31(16-5-2)24(34)18-22-26(19-9-11-20(28)12-10-19)30-27-21(8-7-17-32(22)27)29-23(33)13-14-25(35)36-6-3/h7-12,17H,4-6,13-16,18H2,1-3H3,(H,29,33) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of TSPO (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50274259
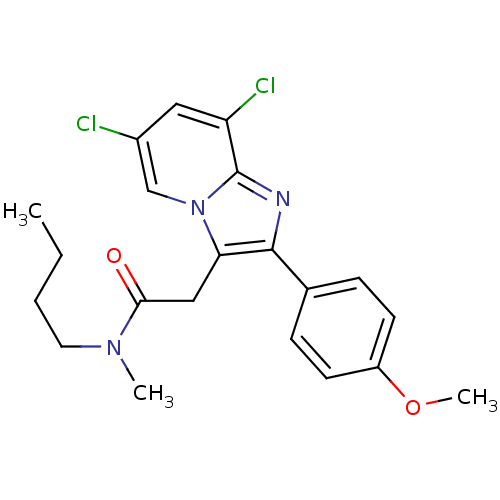
(CHEMBL521186 | N-Butyl-2-(6,8-dichloro-2-(4-methox...)Show SMILES CCCCN(C)C(=O)Cc1c(nc2c(Cl)cc(Cl)cn12)-c1ccc(OC)cc1 Show InChI InChI=1S/C21H23Cl2N3O2/c1-4-5-10-25(2)19(27)12-18-20(14-6-8-16(28-3)9-7-14)24-21-17(23)11-15(22)13-26(18)21/h6-9,11,13H,4-5,10,12H2,1-3H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.882 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of TSPO (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50274259

(CHEMBL521186 | N-Butyl-2-(6,8-dichloro-2-(4-methox...)Show SMILES CCCCN(C)C(=O)Cc1c(nc2c(Cl)cc(Cl)cn12)-c1ccc(OC)cc1 Show InChI InChI=1S/C21H23Cl2N3O2/c1-4-5-10-25(2)19(27)12-18-20(14-6-8-16(28-3)9-7-14)24-21-17(23)11-15(22)13-26(18)21/h6-9,11,13H,4-5,10,12H2,1-3H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.882 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of TSPO (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50189848

(2-[4-(3,4-dichlorophenyl)piperazin-1-ylmethyl]imid...)Show InChI InChI=1S/C18H18Cl2N4/c19-16-5-4-15(11-17(16)20)23-9-7-22(8-10-23)12-14-13-24-6-2-1-3-18(24)21-14/h1-6,11,13H,7-10,12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Agonist activity at dopamine D4 receptor (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50189848

(2-[4-(3,4-dichlorophenyl)piperazin-1-ylmethyl]imid...)Show InChI InChI=1S/C18H18Cl2N4/c19-16-5-4-15(11-17(16)20)23-9-7-22(8-10-23)12-14-13-24-6-2-1-3-18(24)21-14/h1-6,11,13H,7-10,12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Agonist activity at dopamine D4 receptor (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50408728

(CHEMBL339816)Show SMILES CCCN(CCC)C(=O)Cc1c(nc2c(N)cccn12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C21H25ClN4O/c1-3-11-25(12-4-2)19(27)14-18-20(15-7-9-16(22)10-8-15)24-21-17(23)6-5-13-26(18)21/h5-10,13H,3-4,11-12,14,23H2,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of TSPO (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50408728

(CHEMBL339816)Show SMILES CCCN(CCC)C(=O)Cc1c(nc2c(N)cccn12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C21H25ClN4O/c1-3-11-25(12-4-2)19(27)14-18-20(15-7-9-16(22)10-8-15)24-21-17(23)6-5-13-26(18)21/h5-10,13H,3-4,11-12,14,23H2,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of TSPO (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50120543
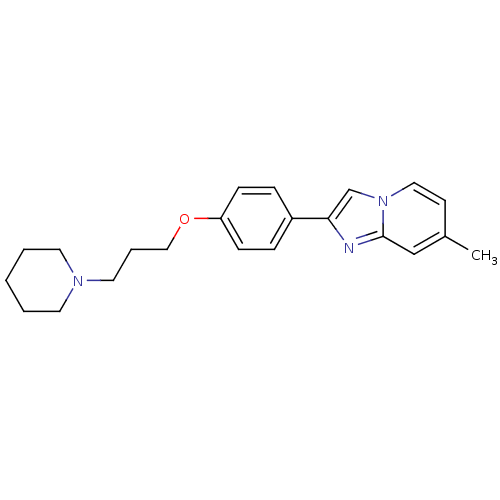
(7-Methyl-2-[4-(3-piperidin-1-yl-propoxy)-phenyl]-i...)Show InChI InChI=1S/C22H27N3O/c1-18-10-14-25-17-21(23-22(25)16-18)19-6-8-20(9-7-19)26-15-5-13-24-11-3-2-4-12-24/h6-10,14,16-17H,2-5,11-13,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of H3R (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50120543

(7-Methyl-2-[4-(3-piperidin-1-yl-propoxy)-phenyl]-i...)Show InChI InChI=1S/C22H27N3O/c1-18-10-14-25-17-21(23-22(25)16-18)19-6-8-20(9-7-19)26-15-5-13-24-11-3-2-4-12-24/h6-10,14,16-17H,2-5,11-13,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of H3R (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50530442
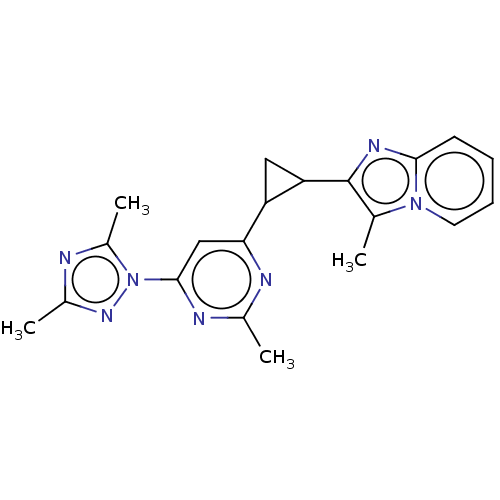
(CHEMBL4461114)Show SMILES Cc1nc(C)n(n1)-c1cc(nc(C)n1)C1CC1c1nc2ccccn2c1C Show InChI InChI=1S/C20H21N7/c1-11-20(24-18-7-5-6-8-26(11)18)16-9-15(16)17-10-19(23-12(2)22-17)27-14(4)21-13(3)25-27/h5-8,10,15-16H,9H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10 |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50530442

(CHEMBL4461114)Show SMILES Cc1nc(C)n(n1)-c1cc(nc(C)n1)C1CC1c1nc2ccccn2c1C Show InChI InChI=1S/C20H21N7/c1-11-20(24-18-7-5-6-8-26(11)18)16-9-15(16)17-10-19(23-12(2)22-17)27-14(4)21-13(3)25-27/h5-8,10,15-16H,9H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10 |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50189845
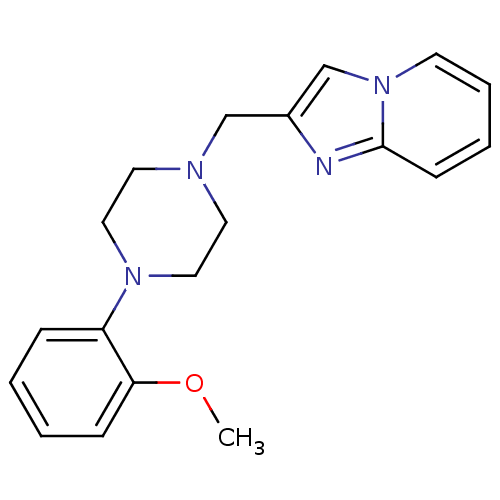
(2-[4-(2-methoxyphenyl)piperazin-1-ylmethyl]imidazo...)Show InChI InChI=1S/C19H22N4O/c1-24-18-7-3-2-6-17(18)22-12-10-21(11-13-22)14-16-15-23-9-5-4-8-19(23)20-16/h2-9,15H,10-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Agonist activity at dopamine D4 receptor (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50189845

(2-[4-(2-methoxyphenyl)piperazin-1-ylmethyl]imidazo...)Show InChI InChI=1S/C19H22N4O/c1-24-18-7-3-2-6-17(18)22-12-10-21(11-13-22)14-16-15-23-9-5-4-8-19(23)20-16/h2-9,15H,10-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Agonist activity at dopamine D4 receptor (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50045880

(2-[5-Chloro-2-(4-chloro-phenyl)-1H-indol-3-yl]-N,N...)Show SMILES CCCN(CCC)C(=O)Cc1c([nH]c2ccc(Cl)cc12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H24Cl2N2O/c1-3-11-26(12-4-2)21(27)14-19-18-13-17(24)9-10-20(18)25-22(19)15-5-7-16(23)8-6-15/h5-10,13,25H,3-4,11-12,14H2,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of TSPO (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50045880

(2-[5-Chloro-2-(4-chloro-phenyl)-1H-indol-3-yl]-N,N...)Show SMILES CCCN(CCC)C(=O)Cc1c([nH]c2ccc(Cl)cc12)-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H24Cl2N2O/c1-3-11-26(12-4-2)21(27)14-19-18-13-17(24)9-10-20(18)25-22(19)15-5-7-16(23)8-6-15/h5-10,13,25H,3-4,11-12,14H2,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of TSPO (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50189842
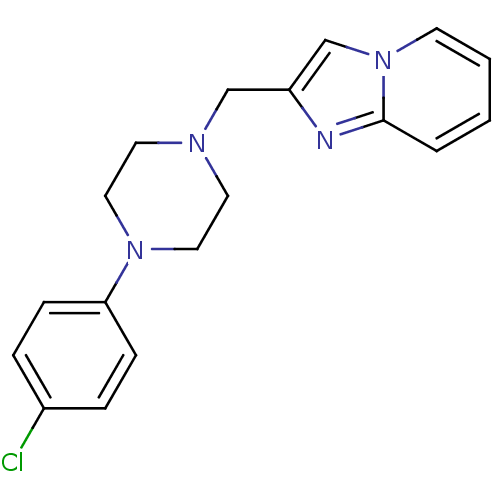
(2-[4-(4-chlorophenyl)piperazin-1-yl)methyl]imidazo...)Show InChI InChI=1S/C18H19ClN4/c19-15-4-6-17(7-5-15)22-11-9-21(10-12-22)13-16-14-23-8-2-1-3-18(23)20-16/h1-8,14H,9-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Agonist activity at dopamine D4 receptor (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50189842

(2-[4-(4-chlorophenyl)piperazin-1-yl)methyl]imidazo...)Show InChI InChI=1S/C18H19ClN4/c19-15-4-6-17(7-5-15)22-11-9-21(10-12-22)13-16-14-23-8-2-1-3-18(23)20-16/h1-8,14H,9-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Agonist activity at dopamine D4 receptor (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50530422
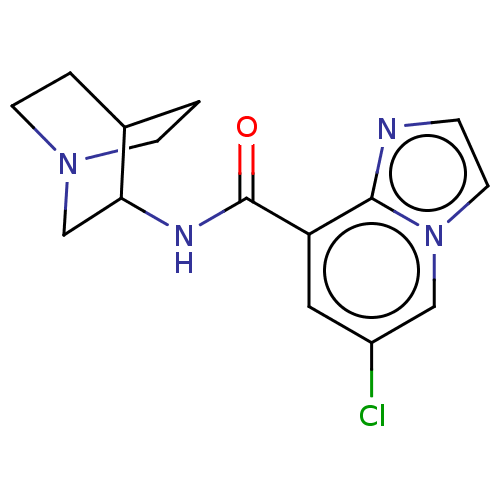
(CHEMBL4468363)Show SMILES Clc1cc(C(=O)NC2CN3CCC2CC3)c2nccn2c1 |(8.45,-32.6,;9.78,-31.83,;9.78,-30.3,;11.11,-29.53,;11.11,-27.99,;9.78,-27.22,;12.45,-27.22,;12.45,-25.68,;13.78,-24.92,;13.78,-23.37,;12.45,-22.6,;11.11,-23.37,;11.11,-24.92,;12.85,-24.59,;12.17,-23.45,;12.45,-30.3,;13.91,-29.82,;14.82,-31.06,;13.91,-32.31,;12.45,-31.83,;11.11,-32.6,)| Show InChI InChI=1S/C15H17ClN4O/c16-11-7-12(14-17-3-6-20(14)8-11)15(21)18-13-9-19-4-1-10(13)2-5-19/h3,6-8,10,13H,1-2,4-5,9H2,(H,18,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Antagonist activity at 5-HT3 receptor (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50011297
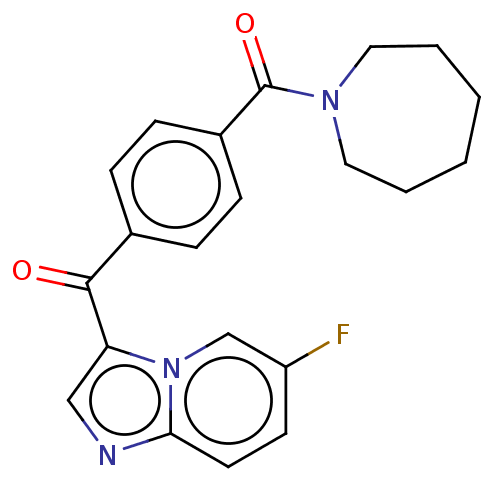
(CHEMBL3260729 | US9029393, 14)Show SMILES Fc1ccc2ncc(C(=O)c3ccc(cc3)C(=O)N3CCCCCC3)n2c1 Show InChI InChI=1S/C21H20FN3O2/c22-17-9-10-19-23-13-18(25(19)14-17)20(26)15-5-7-16(8-6-15)21(27)24-11-3-1-2-4-12-24/h5-10,13-14H,1-4,11-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of GFP-tagged human A2A adenosine receptor expressed in HEK cells by FRET-binding assay |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50530422

(CHEMBL4468363)Show SMILES Clc1cc(C(=O)NC2CN3CCC2CC3)c2nccn2c1 |(8.45,-32.6,;9.78,-31.83,;9.78,-30.3,;11.11,-29.53,;11.11,-27.99,;9.78,-27.22,;12.45,-27.22,;12.45,-25.68,;13.78,-24.92,;13.78,-23.37,;12.45,-22.6,;11.11,-23.37,;11.11,-24.92,;12.85,-24.59,;12.17,-23.45,;12.45,-30.3,;13.91,-29.82,;14.82,-31.06,;13.91,-32.31,;12.45,-31.83,;11.11,-32.6,)| Show InChI InChI=1S/C15H17ClN4O/c16-11-7-12(14-17-3-6-20(14)8-11)15(21)18-13-9-19-4-1-10(13)2-5-19/h3,6-8,10,13H,1-2,4-5,9H2,(H,18,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Antagonist activity at 5-HT3 receptor (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM159248
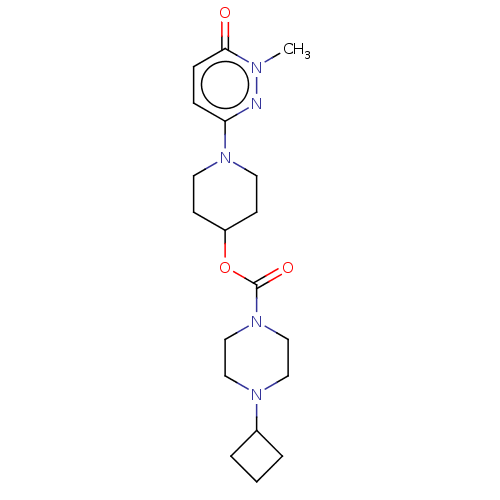
(US9034874, 1.5)Show SMILES Cn1nc(ccc1=O)N1CCC(CC1)OC(=O)N1CCN(CC1)C1CCC1 Show InChI InChI=1S/C19H29N5O3/c1-21-18(25)6-5-17(20-21)23-9-7-16(8-10-23)27-19(26)24-13-11-22(12-14-24)15-3-2-4-15/h5-6,15-16H,2-4,7-14H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of Guinea Pig Ileum H3R |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM159248

(US9034874, 1.5)Show SMILES Cn1nc(ccc1=O)N1CCC(CC1)OC(=O)N1CCN(CC1)C1CCC1 Show InChI InChI=1S/C19H29N5O3/c1-21-18(25)6-5-17(20-21)23-9-7-16(8-10-23)27-19(26)24-13-11-22(12-14-24)15-3-2-4-15/h5-6,15-16H,2-4,7-14H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of Guinea Pig Ileum H3R |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50011297

(CHEMBL3260729 | US9029393, 14)Show SMILES Fc1ccc2ncc(C(=O)c3ccc(cc3)C(=O)N3CCCCCC3)n2c1 Show InChI InChI=1S/C21H20FN3O2/c22-17-9-10-19-23-13-18(25(19)14-17)20(26)15-5-7-16(8-6-15)21(27)24-11-3-1-2-4-12-24/h5-10,13-14H,1-4,11-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of GFP-tagged human A2A adenosine receptor expressed in HEK cells by FRET-binding assay |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50530420
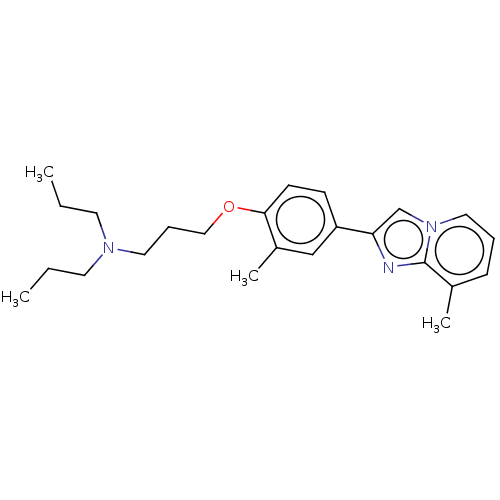
(CHEMBL4450928)Show InChI InChI=1S/C24H33N3O/c1-5-12-26(13-6-2)14-8-16-28-23-11-10-21(17-20(23)4)22-18-27-15-7-9-19(3)24(27)25-22/h7,9-11,15,17-18H,5-6,8,12-14,16H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of H3R (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50530420

(CHEMBL4450928)Show InChI InChI=1S/C24H33N3O/c1-5-12-26(13-6-2)14-8-16-28-23-11-10-21(17-20(23)4)22-18-27-15-7-9-19(3)24(27)25-22/h7,9-11,15,17-18H,5-6,8,12-14,16H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of H3R (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Homo sapiens (Human)) | BDBM26266
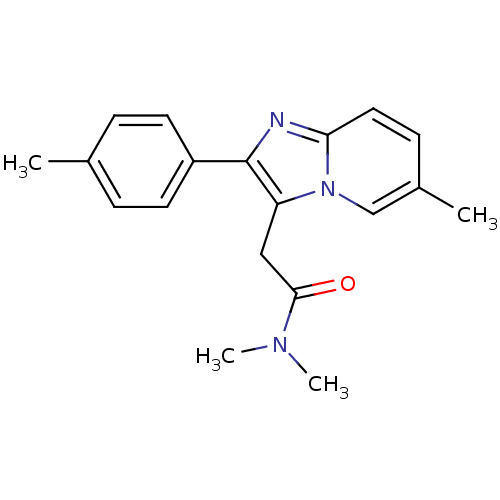
(Ambien | CHEMBL911 | Dalparan | N,N-dimethyl-2-[6-...)Show InChI InChI=1S/C19H21N3O/c1-13-5-8-15(9-6-13)19-16(11-18(23)21(3)4)22-12-14(2)7-10-17(22)20-19/h5-10,12H,11H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of GABAA receptor alpha1 (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gamma-aminobutyric acid receptor subunit alpha-1
(Homo sapiens (Human)) | BDBM26266

(Ambien | CHEMBL911 | Dalparan | N,N-dimethyl-2-[6-...)Show InChI InChI=1S/C19H21N3O/c1-13-5-8-15(9-6-13)19-16(11-18(23)21(3)4)22-12-14(2)7-10-17(22)20-19/h5-10,12H,11H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Inhibition of GABAA receptor alpha1 (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50530424
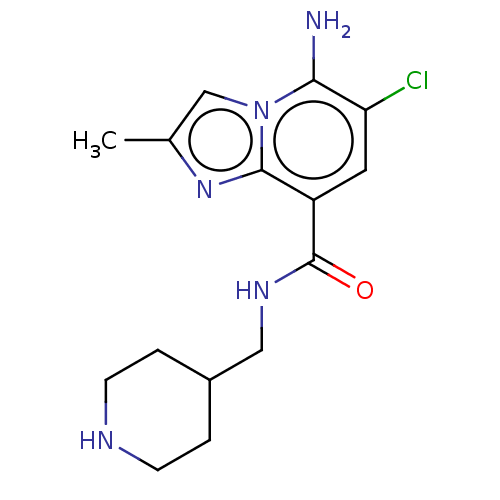
(CHEMBL4533922)Show InChI InChI=1S/C15H20ClN5O/c1-9-8-21-13(17)12(16)6-11(14(21)20-9)15(22)19-7-10-2-4-18-5-3-10/h6,8,10,18H,2-5,7,17H2,1H3,(H,19,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Antagonist activity at 5-HT3 receptor (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50530424

(CHEMBL4533922)Show InChI InChI=1S/C15H20ClN5O/c1-9-8-21-13(17)12(16)6-11(14(21)20-9)15(22)19-7-10-2-4-18-5-3-10/h6,8,10,18H,2-5,7,17H2,1H3,(H,19,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Palack£ University
Curated by ChEMBL
| Assay Description
Antagonist activity at 5-HT3 receptor (unknown origin) |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.111569
BindingDB Entry DOI: 10.7270/Q2H41VXG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data