

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM28802 (2-{[(5-{[2-(4-chlorophenyl)-5-methyl-1,3-oxazol-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 97 | n/a | 4 | n/a | n/a | 8.0 | 22 |
Bristol-Myers Squibb Company | Assay Description For hPPAR alpha, percentage inhibition was calculated relative to unlabeled GW2331, which was used as the active site-specific competitive binder. Fl... | J Pharmacol Exp Ther 327: 716-26 (2008) Article DOI: 10.1124/jpet.108.143271 BindingDB Entry DOI: 10.7270/Q2VD6WT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM28800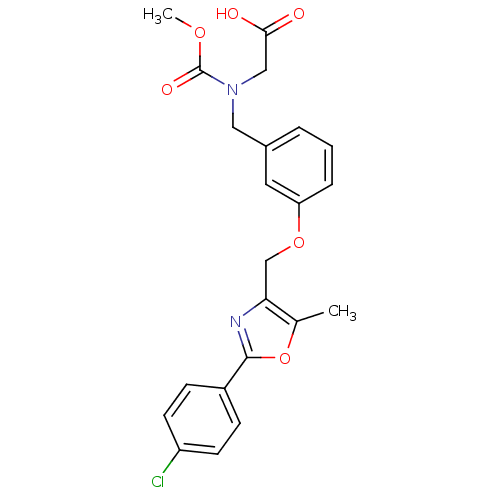 (2-{[(3-{[2-(4-chlorophenyl)-5-methyl-1,3-oxazol-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 260 | n/a | 10 | n/a | n/a | 8.0 | 22 |
Bristol-Myers Squibb Company | Assay Description For hPPAR alpha, percentage inhibition was calculated relative to unlabeled GW2331, which was used as the active site-specific competitive binder. Fl... | J Pharmacol Exp Ther 327: 716-26 (2008) Article DOI: 10.1124/jpet.108.143271 BindingDB Entry DOI: 10.7270/Q2VD6WT9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM28800 (2-{[(3-{[2-(4-chlorophenyl)-5-methyl-1,3-oxazol-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.50E+4 | n/a | 4.10E+3 | n/a | n/a | 8.0 | 22 |
Bristol-Myers Squibb Company | Assay Description For PPARgamma, the percentage inhibition was calculated relative to rosiglitazone, which was used as the active site-specific competitive binder. Flu... | J Pharmacol Exp Ther 327: 716-26 (2008) Article DOI: 10.1124/jpet.108.143271 BindingDB Entry DOI: 10.7270/Q2VD6WT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM28802 (2-{[(5-{[2-(4-chlorophenyl)-5-methyl-1,3-oxazol-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.50E+4 | n/a | 4.51E+3 | n/a | n/a | 8.0 | 22 |
Bristol-Myers Squibb Company | Assay Description For PPARgamma, the percentage inhibition was calculated relative to rosiglitazone, which was used as the active site-specific competitive binder. Flu... | J Pharmacol Exp Ther 327: 716-26 (2008) Article DOI: 10.1124/jpet.108.143271 BindingDB Entry DOI: 10.7270/Q2VD6WT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM28803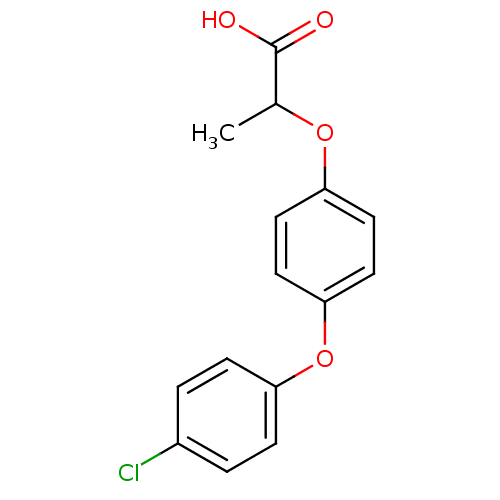 (2-[4-(4-chlorophenoxy)phenoxy]propanoic acid | Clo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >9.84E+4 | n/a | 1.16E+4 | n/a | n/a | 8.0 | 22 |
Bristol-Myers Squibb Company | Assay Description For hPPAR alpha, percentage inhibition was calculated relative to unlabeled GW2331, which was used as the active site-specific competitive binder. Fl... | J Pharmacol Exp Ther 327: 716-26 (2008) Article DOI: 10.1124/jpet.108.143271 BindingDB Entry DOI: 10.7270/Q2VD6WT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM28803 (2-[4-(4-chlorophenoxy)phenoxy]propanoic acid | Clo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >9.84E+4 | n/a | >5.00E+4 | n/a | n/a | 8.0 | 22 |
Bristol-Myers Squibb Company | Assay Description For PPARgamma, the percentage inhibition was calculated relative to rosiglitazone, which was used as the active site-specific competitive binder. Flu... | J Pharmacol Exp Ther 327: 716-26 (2008) Article DOI: 10.1124/jpet.108.143271 BindingDB Entry DOI: 10.7270/Q2VD6WT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Mus musculus) | BDBM28802 (2-{[(5-{[2-(4-chlorophenyl)-5-methyl-1,3-oxazol-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 145 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description EC50 is the concentration of test compounds needed to induce 50% of the maximum luciferase activity in HEK cells transfected with PPAR and Gal4-lucif... | J Pharmacol Exp Ther 327: 716-26 (2008) Article DOI: 10.1124/jpet.108.143271 BindingDB Entry DOI: 10.7270/Q2VD6WT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Mus musculus) | BDBM28803 (2-[4-(4-chlorophenoxy)phenoxy]propanoic acid | Clo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.85E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description EC50 is the concentration of test compounds needed to induce 50% of the maximum luciferase activity in HEK cells transfected with PPAR and Gal4-lucif... | J Pharmacol Exp Ther 327: 716-26 (2008) Article DOI: 10.1124/jpet.108.143271 BindingDB Entry DOI: 10.7270/Q2VD6WT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Rattus norvegicus) | BDBM28800 (2-{[(3-{[2-(4-chlorophenyl)-5-methyl-1,3-oxazol-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | n/a | n/a | 317 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description EC50 is the concentration of test compounds needed to induce 50% of the maximum luciferase activity in HEK cells transfected with PPAR and Gal4-lucif... | J Pharmacol Exp Ther 327: 716-26 (2008) Article DOI: 10.1124/jpet.108.143271 BindingDB Entry DOI: 10.7270/Q2VD6WT9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor alpha (Rattus norvegicus) | BDBM28802 (2-{[(5-{[2-(4-chlorophenyl)-5-methyl-1,3-oxazol-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 123 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description EC50 is the concentration of test compounds needed to induce 50% of the maximum luciferase activity in HEK cells transfected with PPAR and Gal4-lucif... | J Pharmacol Exp Ther 327: 716-26 (2008) Article DOI: 10.1124/jpet.108.143271 BindingDB Entry DOI: 10.7270/Q2VD6WT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Mus musculus) | BDBM28800 (2-{[(3-{[2-(4-chlorophenyl)-5-methyl-1,3-oxazol-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | n/a | n/a | 426 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description EC50 is the concentration of test compounds needed to induce 50% of the maximum luciferase activity in HEK cells transfected with PPAR and Gal4-lucif... | J Pharmacol Exp Ther 327: 716-26 (2008) Article DOI: 10.1124/jpet.108.143271 BindingDB Entry DOI: 10.7270/Q2VD6WT9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor alpha (Mesocricetus auratus (golden hamster)) | BDBM28803 (2-[4-(4-chlorophenoxy)phenoxy]propanoic acid | Clo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.87E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description EC50 is the concentration of test compounds needed to induce 50% of the maximum luciferase activity in HEK cells transfected with PPAR and Gal4-lucif... | J Pharmacol Exp Ther 327: 716-26 (2008) Article DOI: 10.1124/jpet.108.143271 BindingDB Entry DOI: 10.7270/Q2VD6WT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Mesocricetus auratus (golden hamster)) | BDBM28802 (2-{[(5-{[2-(4-chlorophenyl)-5-methyl-1,3-oxazol-4-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 178 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description EC50 is the concentration of test compounds needed to induce 50% of the maximum luciferase activity in HEK cells transfected with PPAR and Gal4-lucif... | J Pharmacol Exp Ther 327: 716-26 (2008) Article DOI: 10.1124/jpet.108.143271 BindingDB Entry DOI: 10.7270/Q2VD6WT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Rattus norvegicus) | BDBM28803 (2-[4-(4-chlorophenoxy)phenoxy]propanoic acid | Clo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.77E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description EC50 is the concentration of test compounds needed to induce 50% of the maximum luciferase activity in HEK cells transfected with PPAR and Gal4-lucif... | J Pharmacol Exp Ther 327: 716-26 (2008) Article DOI: 10.1124/jpet.108.143271 BindingDB Entry DOI: 10.7270/Q2VD6WT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28802 (2-{[(5-{[2-(4-chlorophenyl)-5-methyl-1,3-oxazol-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description EC50 is the concentration of test compounds needed to induce 50% of the maximum luciferase activity in HEK cells transfected with PPAR and Gal4-lucif... | J Pharmacol Exp Ther 327: 716-26 (2008) Article DOI: 10.1124/jpet.108.143271 BindingDB Entry DOI: 10.7270/Q2VD6WT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM28800 (2-{[(3-{[2-(4-chlorophenyl)-5-methyl-1,3-oxazol-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description EC50 is the concentration of test compounds needed to induce 50% of the maximum luciferase activity in HEK cells transfected with PPAR and Gal4-lucif... | J Pharmacol Exp Ther 327: 716-26 (2008) Article DOI: 10.1124/jpet.108.143271 BindingDB Entry DOI: 10.7270/Q2VD6WT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Mesocricetus auratus (golden hamster)) | BDBM28800 (2-{[(3-{[2-(4-chlorophenyl)-5-methyl-1,3-oxazol-4-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | n/a | n/a | 488 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description EC50 is the concentration of test compounds needed to induce 50% of the maximum luciferase activity in HEK cells transfected with PPAR and Gal4-lucif... | J Pharmacol Exp Ther 327: 716-26 (2008) Article DOI: 10.1124/jpet.108.143271 BindingDB Entry DOI: 10.7270/Q2VD6WT9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||