

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50384053 (CHEMBL2032453) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at kappa opioid receptor in guinea pig illeum assessed as electric field-stimulated response | Bioorg Med Chem Lett 22: 4173-8 (2012) Article DOI: 10.1016/j.bmcl.2012.04.040 BindingDB Entry DOI: 10.7270/Q2251K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50384059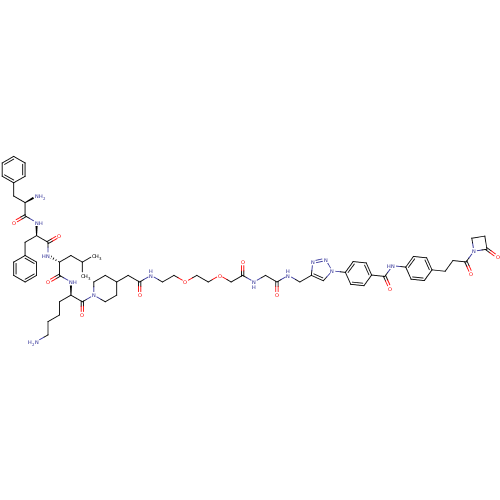 (CHEMBL2032452) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at kappa opioid receptor in guinea pig illeum assessed as electric field-stimulated response | Bioorg Med Chem Lett 22: 4173-8 (2012) Article DOI: 10.1016/j.bmcl.2012.04.040 BindingDB Entry DOI: 10.7270/Q2251K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50384054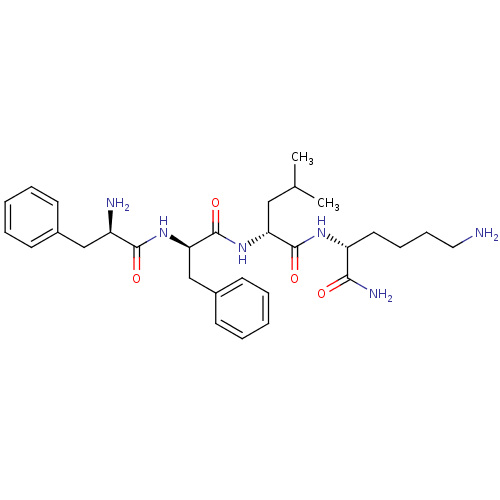 (CHEMBL2032447) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at kappa opioid receptor in guinea pig illeum assessed as electric field-stimulated response | Bioorg Med Chem Lett 22: 4173-8 (2012) Article DOI: 10.1016/j.bmcl.2012.04.040 BindingDB Entry DOI: 10.7270/Q2251K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50059841 ((S)-1-[(S)-2-[2-((S)-2-{(R)-2-[(S)-2-Amino-3-(4-hy...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
University of Naples Curated by ChEMBL | Assay Description Negative logarithm of the molar concentration for agonist activity at mu receptor was determined in guinea pig ileum | Bioorg Med Chem Lett 10: 2745-8 (2000) BindingDB Entry DOI: 10.7270/Q25H7HF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50182939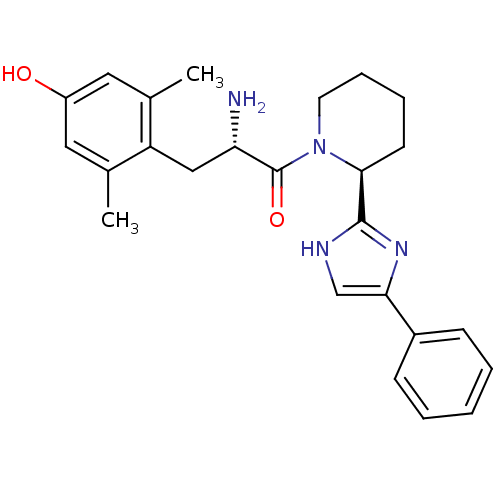 ((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl)-1-((S...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Activity against mu opioid receptor by stimulation of [35S]GTPgammaS binding in CHO-hgamma cells | Bioorg Med Chem Lett 16: 2505-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.082 BindingDB Entry DOI: 10.7270/Q29G5MDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50595849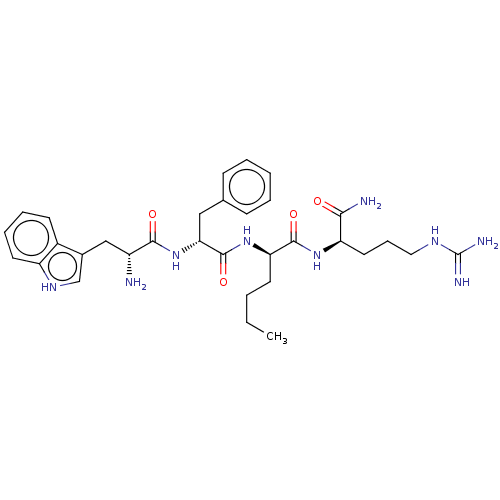 (CHEMBL5169404) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00237 BindingDB Entry DOI: 10.7270/Q21Z48F7 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50595853 (CHEMBL5177451) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00237 BindingDB Entry DOI: 10.7270/Q21Z48F7 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50182946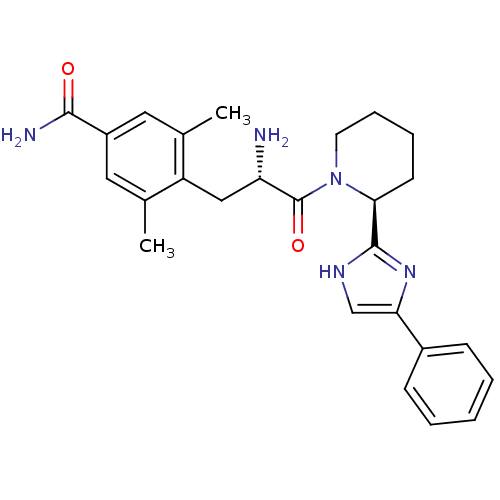 (4-((S)-2-amino-3-oxo-3-((S)-2-(4-phenyl-1H-imidazo...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Activity against mu opioid receptor by stimulation of [35S]GTPgammaS binding in CHO-hgamma cells | Bioorg Med Chem Lett 16: 2505-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.082 BindingDB Entry DOI: 10.7270/Q29G5MDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM224024 (BDBM50241435 | Dynorphin A (1-13) | YGGFLRRXRPKLK) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity towards k-opioid receptor k-EL-2 mutant (wild-type) expressed in HEK cells | J Med Chem 43: 1251-2 (2001) BindingDB Entry DOI: 10.7270/Q270824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50354578 (BUPRENORPHINE | US10752592, Compound buprenorphine...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Agonist activity at KOR in guinea pig vas deferens | J Med Chem 55: 9868-74 (2012) Article DOI: 10.1021/jm301096s BindingDB Entry DOI: 10.7270/Q2M909TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50354578 (BUPRENORPHINE | US10752592, Compound buprenorphine...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Agonist activity at MOR in guinea pig vas deferens | J Med Chem 55: 9868-74 (2012) Article DOI: 10.1021/jm301096s BindingDB Entry DOI: 10.7270/Q2M909TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50595847 (CHEMBL5199097) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00237 BindingDB Entry DOI: 10.7270/Q21Z48F7 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50595846 (CHEMBL5183917) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00237 BindingDB Entry DOI: 10.7270/Q21Z48F7 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50182942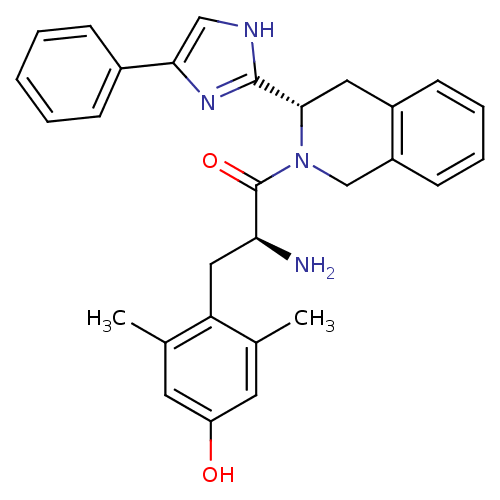 ((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl)-1-((S...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Activity against mu opioid receptor by stimulation of [35S]GTPgammaS binding in CHO-hgamma cells | Bioorg Med Chem Lett 16: 2505-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.082 BindingDB Entry DOI: 10.7270/Q29G5MDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50142621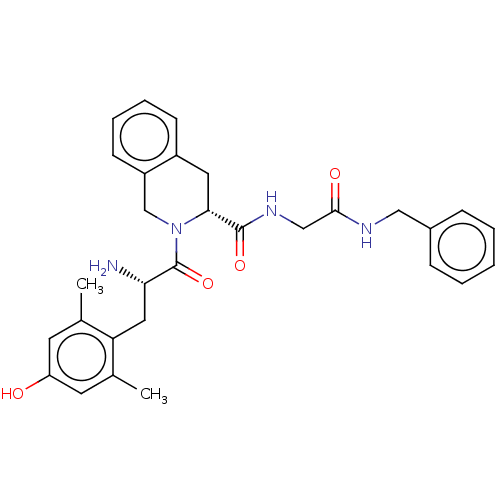 (CHEMBL3759292) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Agonist activity at mu-opioid receptor in guinea pig ileum assessed as inhibition of electrically induced twitches | Eur J Med Chem 108: 211-28 (2016) Article DOI: 10.1016/j.ejmech.2015.11.028 BindingDB Entry DOI: 10.7270/Q2R2137W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50474153 (CHEMBL61630) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 53 | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Agonist activity at mu opioid receptor in guinea pig brain membranes by [35S]-GTPgammaS binding assay | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111701 BindingDB Entry DOI: 10.7270/Q2668HN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50474153 (CHEMBL61630) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 53 | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Agonist activity at kappa opioid receptor in guinea pig brain membranes by [35S]-GTPgammaS binding assay | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111701 BindingDB Entry DOI: 10.7270/Q2668HN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50017698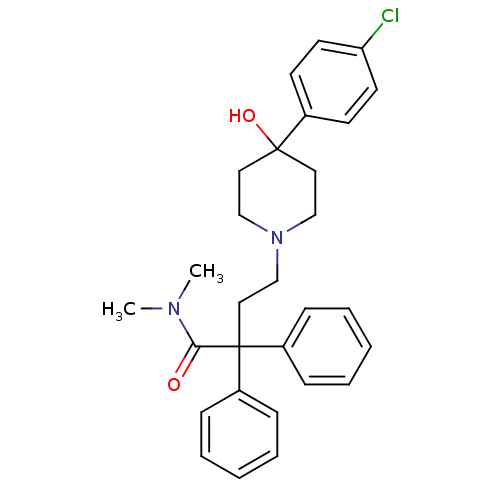 (4-(4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl)-N,N...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 58 | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of mu opioid receptor mediated GTPgammaS binding to CHO cell membranes | J Med Chem 47: 5009-20 (2004) Article DOI: 10.1021/jm030548r BindingDB Entry DOI: 10.7270/Q2H131GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50182944 (4-((S)-2-amino-3-oxo-3-((S)-2-(4-phenyl-1H-imidazo...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Activity against mu opioid receptor by stimulation of [35S]GTPgammaS binding in CHO-hgamma cells | Bioorg Med Chem Lett 16: 2505-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.082 BindingDB Entry DOI: 10.7270/Q29G5MDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50154041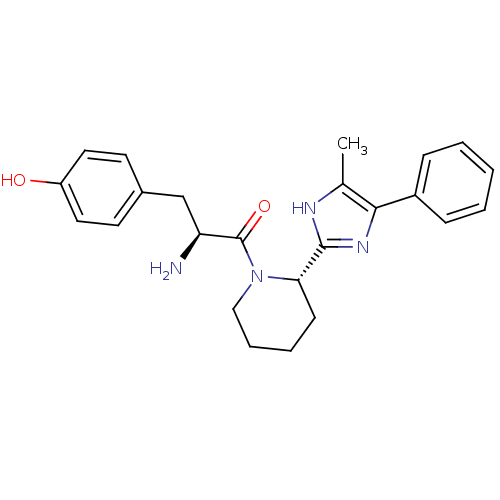 ((S)-2-amino-3-(4-hydroxyphenyl)-1-((S)-2-(5-methyl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 142 | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Activity against mu opioid receptor by stimulation of [35S]GTPgammaS binding in CHO-hgamma cells | Bioorg Med Chem Lett 16: 2505-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.082 BindingDB Entry DOI: 10.7270/Q29G5MDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50154041 ((S)-2-amino-3-(4-hydroxyphenyl)-1-((S)-2-(5-methyl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 153 | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of mu opioid receptor mediated GTPgammaS binding to CHO cell membranes | J Med Chem 47: 5009-20 (2004) Article DOI: 10.1021/jm030548r BindingDB Entry DOI: 10.7270/Q2H131GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50182947 (4-((S)-2-amino-3-oxo-3-((S)-3-(4-phenyl-1H-imidazo...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 155 | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Activity against mu opioid receptor by stimulation of [35S]GTPgammaS binding in CHO-hgamma cells | Bioorg Med Chem Lett 16: 2505-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.082 BindingDB Entry DOI: 10.7270/Q29G5MDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM21015 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 158 | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Agonist activity at MOR in Dunkin-Hartley guinea pig ileum assessed as inhibition of EFS-induced contractions incubated for 60 mins | Bioorg Med Chem 24: 2832-42 (2016) Article DOI: 10.1016/j.bmc.2016.05.005 BindingDB Entry DOI: 10.7270/Q2VH5QR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50182943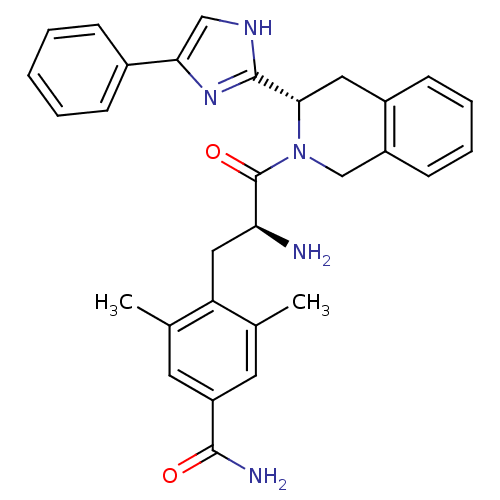 (4-((S)-2-amino-3-oxo-3-((S)-3-(4-phenyl-1H-imidazo...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 161 | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Activity against mu opioid receptor by stimulation of [35S]GTPgammaS binding in CHO-hgamma cells | Bioorg Med Chem Lett 16: 2505-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.082 BindingDB Entry DOI: 10.7270/Q29G5MDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50140900 (6-(2,4-dichlorophenyl)-19-methyl-(2R,10R)-11-oxa-8...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 225 | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonistic activity towards Opioid receptor mu 1 was determined in [35S]GTP-gamma-S, binding assay in guinea pig caudate; g = not active as an agonist | J Med Chem 47: 1400-12 (2004) Article DOI: 10.1021/jm030311v BindingDB Entry DOI: 10.7270/Q2639QGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50474152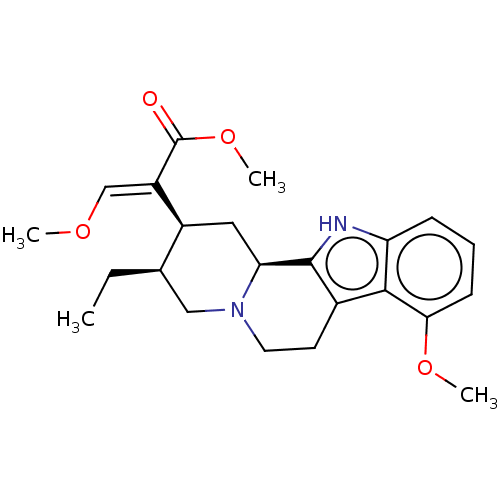 (CHEBI:6956 | CHEMBL299031) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 230 | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Agonist activity at mu opioid receptor in guinea pig brain membranes by [35S]-GTPgammaS binding assay | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111701 BindingDB Entry DOI: 10.7270/Q2668HN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50474152 (CHEBI:6956 | CHEMBL299031) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 230 | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Agonist activity at kappa opioid receptor in guinea pig brain membranes by [35S]-GTPgammaS binding assay | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111701 BindingDB Entry DOI: 10.7270/Q2668HN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50133264 (CHEMBL133719 | [(2S,3S)-TMT1]DPDPE) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 290 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description In vitro bioassay data to determine the effective concentration required for antagonistic activity against Opioid receptor mu 1 in functional assay,G... | J Med Chem 46: 4215-31 (2003) Article DOI: 10.1021/jm0303103 BindingDB Entry DOI: 10.7270/Q2JW8FM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50038422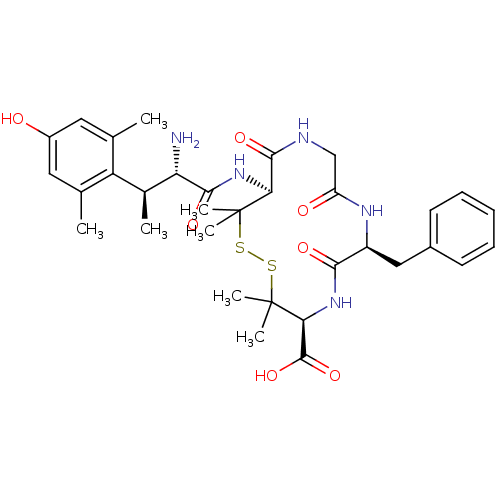 ((4S,7S,13S)-13-[(2S,3S)-2-Amino-3-(4-hydroxy-2,6-d...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 293 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Effective concentration was measured towards mu opioid receptor using guinea pig ileum (GPI,mu) bioassay in vitro | J Med Chem 37: 1746-57 (1994) BindingDB Entry DOI: 10.7270/Q2N58N1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM21015 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 414 | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonistic activity towards Opioid receptor mu 1 was determined in [35S]GTP-gamma-S, binding assay in guinea pig caudate | J Med Chem 47: 1400-12 (2004) Article DOI: 10.1021/jm030311v BindingDB Entry DOI: 10.7270/Q2639QGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50403819 (CHEMBL440881) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 490 | n/a | n/a | n/a | n/a |
University of Naples Curated by ChEMBL | Assay Description Negative logarithm of the molar concentration for agonist activity at mu receptor was determined in guinea pig ileum | Bioorg Med Chem Lett 10: 2745-8 (2000) BindingDB Entry DOI: 10.7270/Q25H7HF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50101612 (2-Amino-1-(3-benzyloxymethyl-3,4-dihydro-1H-isoqui...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 525 | n/a | n/a | n/a | n/a |
University of Naples Curated by ChEMBL | Assay Description Negative logarithm of the molar concentration for agonist activity at mu receptor was determined in guinea pig ileum | Bioorg Med Chem Lett 10: 2745-8 (2000) BindingDB Entry DOI: 10.7270/Q25H7HF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50403820 (CHEMBL92707) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 631 | n/a | n/a | n/a | n/a |
University of Naples Curated by ChEMBL | Assay Description Negative logarithm of the molar concentration for agonist activity at mu receptor was determined in guinea pig ileum | Bioorg Med Chem Lett 10: 2745-8 (2000) BindingDB Entry DOI: 10.7270/Q25H7HF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50140903 (19-methyl-6-phenyl-(2S,10R)-11-oxa-8,19-diazahexac...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 660 | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonistic activity towards Opioid receptor mu 1 was determined in [35S]GTP-gamma-S, binding assay in guinea pig caudate | J Med Chem 47: 1400-12 (2004) Article DOI: 10.1021/jm030311v BindingDB Entry DOI: 10.7270/Q2639QGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50142692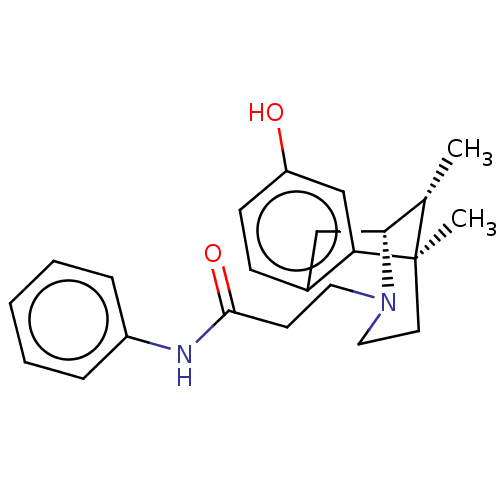 (CHEMBL3759092) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 794 | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Agonist activity at MOR in Dunkin-Hartley guinea pig ileum assessed as inhibition of EFS-induced contractions incubated for 60 mins | Bioorg Med Chem 24: 2832-42 (2016) Article DOI: 10.1016/j.bmc.2016.05.005 BindingDB Entry DOI: 10.7270/Q2VH5QR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50403821 (CHEMBL93891) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 813 | n/a | n/a | n/a | n/a |
University of Naples Curated by ChEMBL | Assay Description Negative logarithm of the molar concentration for agonist activity at mu receptor was determined in guinea pig ileum | Bioorg Med Chem Lett 10: 2745-8 (2000) BindingDB Entry DOI: 10.7270/Q25H7HF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50140901 (6-(4-chlorophenyl)-19-methyl-(2R,10R)-11-oxa-8,19-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 900 | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonistic activity towards Opioid receptor mu 1 was determined in [35S]GTP-gamma-S, binding assay in guinea pig caudate; g = not active as an agonist | J Med Chem 47: 1400-12 (2004) Article DOI: 10.1021/jm030311v BindingDB Entry DOI: 10.7270/Q2639QGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50595851 (CHEMBL5192735) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 955 | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00237 BindingDB Entry DOI: 10.7270/Q21Z48F7 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50140905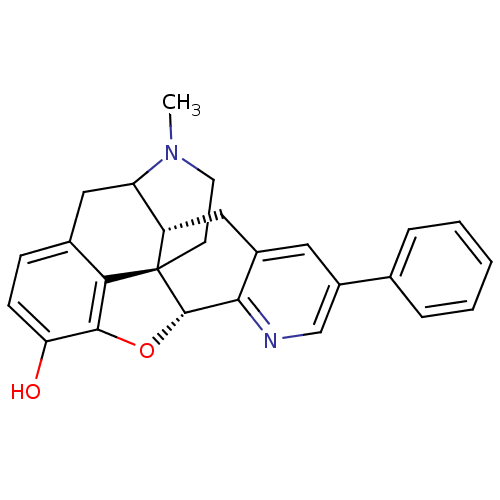 (19-methyl-6-phenyl-(2R,10R)-11-oxa-8,19-diazahexac...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Antagonism of compound towards Opioid receptor mu 1 was determined using PL-017 in guinea pig ileum (GPI) smooth muscle contraction assays; d = agoni... | J Med Chem 47: 1400-12 (2004) Article DOI: 10.1021/jm030311v BindingDB Entry DOI: 10.7270/Q2639QGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50068136 (3-[(4-Benzyl-piperazin-1-yl)-phenyl-methyl]-phenol...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a |
The University of Arizona Curated by ChEMBL | Assay Description Tested for electrically induced smooth muscle contractions from guinea pig ileum expressed in mu opioid receptors | J Med Chem 41: 4767-76 (1998) Article DOI: 10.1021/jm980374r BindingDB Entry DOI: 10.7270/Q2DZ07FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50154037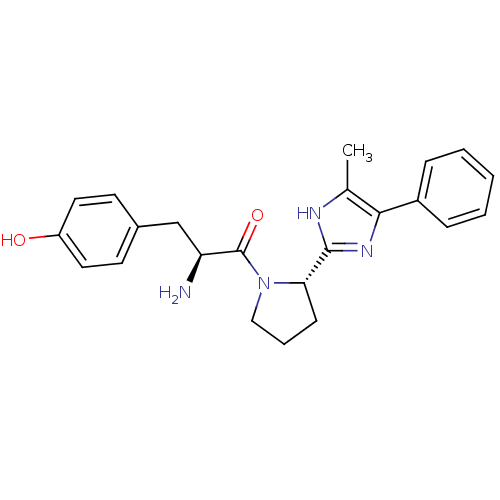 (2-Amino-3-(4-hydroxy-phenyl)-1-[2-(5-methyl-4-phen...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of mu opioid receptor mediated GTPgammaS binding to CHO cell membranes | J Med Chem 47: 5009-20 (2004) Article DOI: 10.1021/jm030548r BindingDB Entry DOI: 10.7270/Q2H131GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50140909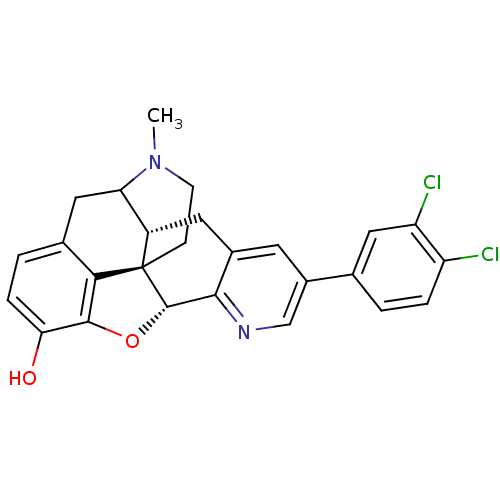 (6-(3,4-dichlorophenyl)-19-methyl-(2R,10R)-11-oxa-8...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.31E+3 | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonistic activity towards Opioid receptor mu 1 was determined in [35S]GTP-gamma-S, binding assay in guinea pig caudate; g = not active as an agonist | J Med Chem 47: 1400-12 (2004) Article DOI: 10.1021/jm030311v BindingDB Entry DOI: 10.7270/Q2639QGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50595848 (CHEMBL5179970) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00237 BindingDB Entry DOI: 10.7270/Q21Z48F7 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM21130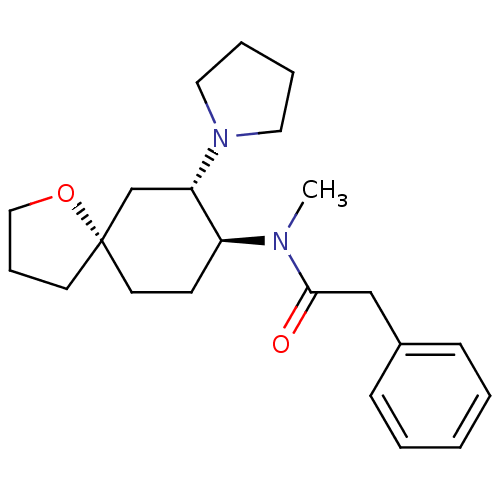 (N-methyl-2-phenyl-N-[(5R,7S,8S)-7-(pyrrolidin-1-yl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00237 BindingDB Entry DOI: 10.7270/Q21Z48F7 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50393720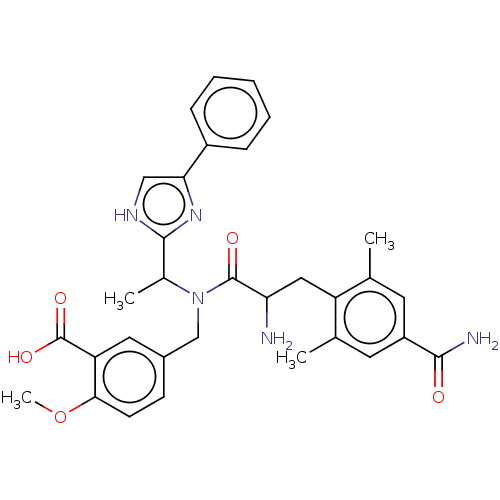 (CHEMBL2159122 | CHEMBL3216329) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Agonist activity at kappa opioid receptor guinea pig colon tissue | Bioorg Med Chem Lett 22: 4869-72 (2012) Article DOI: 10.1016/j.bmcl.2012.05.042 BindingDB Entry DOI: 10.7270/Q2F76DP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50595852 (CHEMBL5194818) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.86E+3 | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00237 BindingDB Entry DOI: 10.7270/Q21Z48F7 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50154042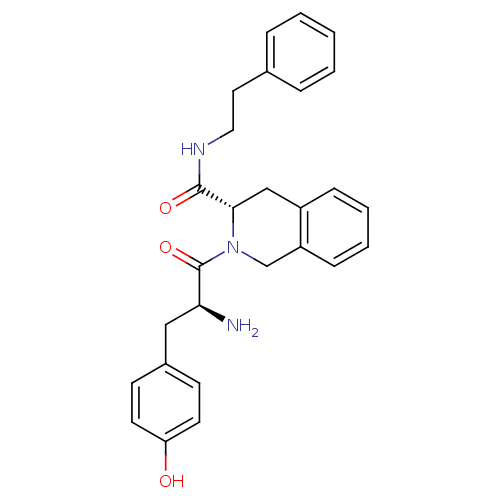 (2-[2-Amino-3-(4-hydroxy-phenyl)-propionyl]-1,2,3,4...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.12E+3 | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of mu opioid receptor mediated GTPgammaS binding to CHO cell membranes | J Med Chem 47: 5009-20 (2004) Article DOI: 10.1021/jm030548r BindingDB Entry DOI: 10.7270/Q2H131GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50154036 ((S)-2-amino-3-(4-hydroxyphenyl)-1-((S)-3-(4-phenyl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of mu opioid receptor mediated GTPgammaS binding to CHO cell membranes | J Med Chem 47: 5009-20 (2004) Article DOI: 10.1021/jm030548r BindingDB Entry DOI: 10.7270/Q2H131GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50154036 ((S)-2-amino-3-(4-hydroxyphenyl)-1-((S)-3-(4-phenyl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.45E+3 | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Activity against mu opioid receptor by stimulation of [35S]GTPgammaS binding in CHO-hgamma cells | Bioorg Med Chem Lett 16: 2505-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.082 BindingDB Entry DOI: 10.7270/Q29G5MDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50038424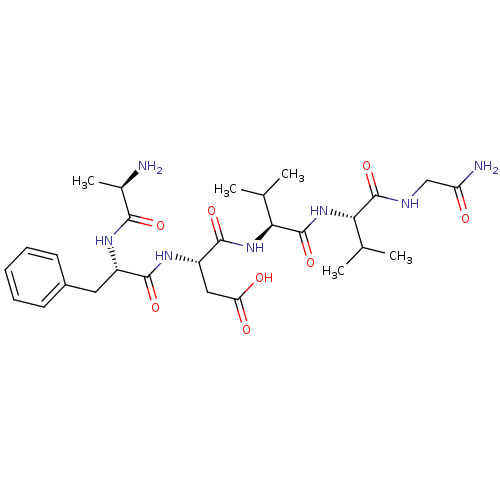 ((S)-3-[(S)-2-((R)-2-Amino-propionylamino)-3-phenyl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Effective concentration was measured towards mu opioid receptor using guinea pig ileum (GPI,mu) bioassay in vitro | J Med Chem 37: 1746-57 (1994) BindingDB Entry DOI: 10.7270/Q2N58N1K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 67 total ) | Next | Last >> |