 Found 676 hits of ic50 data for polymerid = 50001875,50004671,9969
Found 676 hits of ic50 data for polymerid = 50001875,50004671,9969 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50024139

(1-Hydroxymethyl-5-phenethylamino-cyclohexane-1,2,3...)Show SMILES OC[C@@]1(O)C[C@H](NCCc2ccccc2)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C15H23NO5/c17-9-15(21)8-11(12(18)13(19)14(15)20)16-7-6-10-4-2-1-3-5-10/h1-5,11-14,16-21H,6-9H2/t11-,12?,13-,14-,15?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50024120

(1-Hydroxymethyl-5-[(thiophen-2-ylmethyl)-amino]-cy...)Show SMILES OC[C@@]1(O)C[C@H](NCc2cccs2)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C12H19NO5S/c14-6-12(18)4-8(9(15)10(16)11(12)17)13-5-7-2-1-3-19-7/h1-3,8-11,13-18H,4-6H2/t8-,9?,10-,11-,12?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50156360
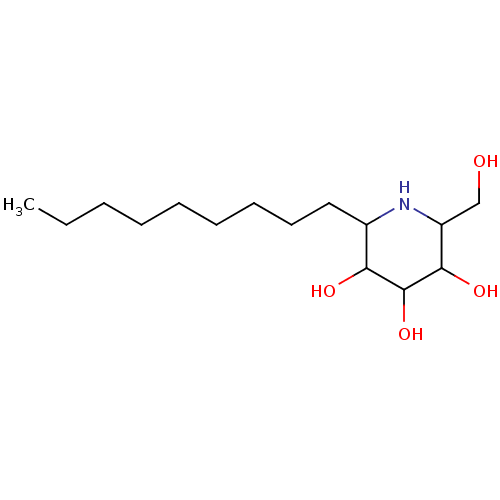
(2-Hydroxymethyl-6-nonyl-piperidine-3,4,5-triol | C...)Show InChI InChI=1S/C15H31NO4/c1-2-3-4-5-6-7-8-9-11-13(18)15(20)14(19)12(10-17)16-11/h11-20H,2-10H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans
Curated by ChEMBL
| Assay Description
Inhibition of rat intestinal isomaltase using disaccharide |
Bioorg Med Chem Lett 14: 5991-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.086
BindingDB Entry DOI: 10.7270/Q27P904W |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50373983
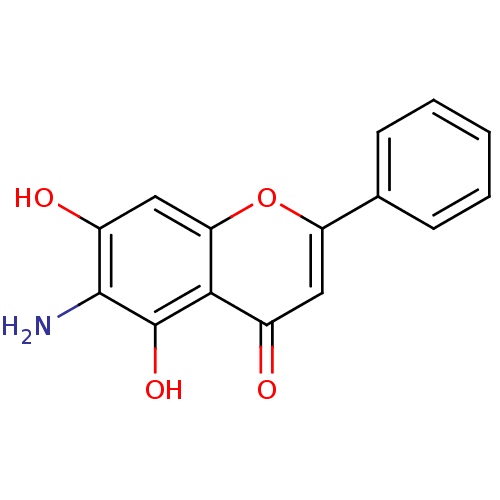
(CHEMBL268675)Show InChI InChI=1S/C15H11NO4/c16-14-10(18)7-12-13(15(14)19)9(17)6-11(20-12)8-4-2-1-3-5-8/h1-7,18-19H,16H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of rat intestinal maltase |
Bioorg Med Chem Lett 18: 812-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.032
BindingDB Entry DOI: 10.7270/Q2MG7QC9 |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50226273
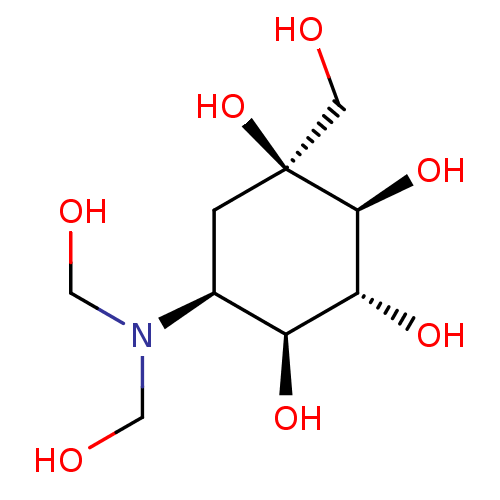
(CHEMBL3349431)Show SMILES OCN(CO)[C@H]1C[C@](O)(CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C9H19NO7/c11-2-9(17)1-5(10(3-12)4-13)6(14)7(15)8(9)16/h5-8,11-17H,1-4H2/t5-,6-,7+,8-,9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50373982
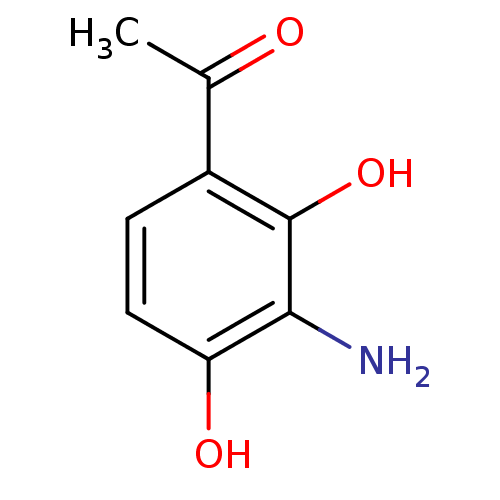
(CHEMBL402196)Show InChI InChI=1S/C8H9NO3/c1-4(10)5-2-3-6(11)7(9)8(5)12/h2-3,11-12H,9H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of rat intestinal maltase |
Bioorg Med Chem Lett 18: 812-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.032
BindingDB Entry DOI: 10.7270/Q2MG7QC9 |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50024119
(5-(2-Hydroxy-cyclohexylamino)-1-hydroxymethyl-cycl...)Show SMILES OC[C@@]1(O)C[C@H](N[C@@H]2CCCC[C@H]2O)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C13H25NO6/c15-6-13(20)5-8(10(17)11(18)12(13)19)14-7-3-1-2-4-9(7)16/h7-12,14-20H,1-6H2/t7-,8-,9-,10?,11-,12-,13?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50024137
(1-Hydroxymethyl-5-(2-hydroxy-2-phenyl-ethylamino)-...)Show SMILES [H][C@](O)(CN[C@H]1C[C@](O)(CO)[C@H](O)[C@@H](O)[C@H]1O)c1ccccc1 Show InChI InChI=1S/C15H23NO6/c17-8-15(22)6-10(12(19)13(20)14(15)21)16-7-11(18)9-4-2-1-3-5-9/h1-5,10-14,16-22H,6-8H2/t10-,11-,12-,13-,14+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50024136
(5-(3,5-Di-tert-butyl-4-hydroxy-benzylamino)-1-hydr...)Show SMILES CC(C)(C)c1cc(CN[C@H]2C[C@](O)(CO)[C@@H](O)[C@H](O)[C@H]2O)cc(c1O)C(C)(C)C |r| Show InChI InChI=1S/C22H37NO6/c1-20(2,3)13-7-12(8-14(16(13)25)21(4,5)6)10-23-15-9-22(29,11-24)19(28)18(27)17(15)26/h7-8,15,17-19,23-29H,9-11H2,1-6H3/t15-,17?,18-,19-,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50405395
(CHEMBL2051761)Show SMILES C[C@H]1C[C@H](N)[C@H](O)[C@@H](O)[C@@H]1N[C@H]1C[C@](O)(CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C14H28N2O7/c1-5-2-6(15)9(18)11(20)8(5)16-7-3-14(23,4-17)13(22)12(21)10(7)19/h5-13,16-23H,2-4,15H2,1H3/t5-,6-,7-,8+,9-,10-,11-,12+,13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50373981
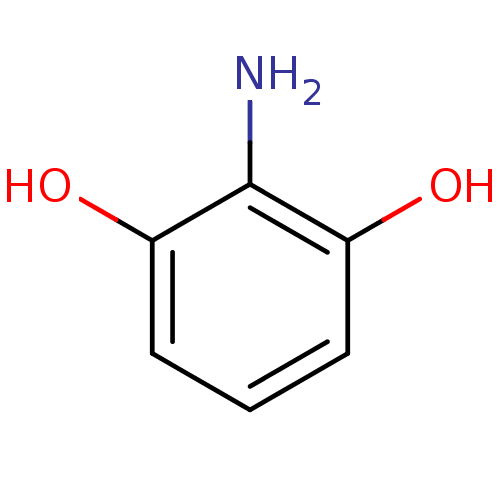
(CHEMBL403239)Show InChI InChI=1S/C6H7NO2/c7-6-4(8)2-1-3-5(6)9/h1-3,8-9H,7H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of rat intestinal maltase |
Bioorg Med Chem Lett 18: 812-5 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.032
BindingDB Entry DOI: 10.7270/Q2MG7QC9 |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50024114

(5-(Cyclohexylmethyl-amino)-1-hydroxymethyl-cyclohe...)Show SMILES OC[C@@]1(O)C[C@H](NCC2CCCCC2)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C14H27NO5/c16-8-14(20)6-10(11(17)12(18)13(14)19)15-7-9-4-2-1-3-5-9/h9-13,15-20H,1-8H2/t10-,11?,12-,13-,14?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50405397
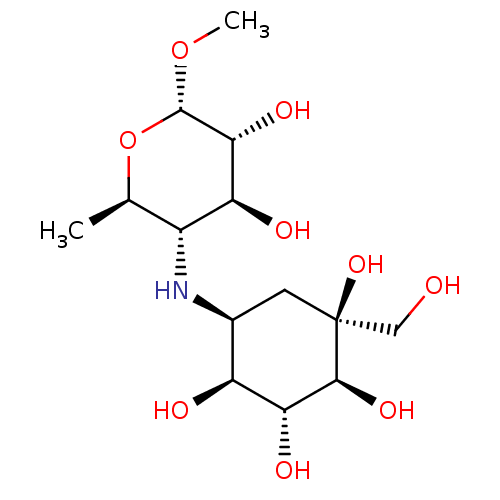
(CHEMBL2051983)Show SMILES CO[C@H]1O[C@H](C)[C@@H](N[C@H]2C[C@](O)(CO)[C@@H](O)[C@H](O)[C@H]2O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C14H27NO9/c1-5-7(9(18)11(20)13(23-2)24-5)15-6-3-14(22,4-16)12(21)10(19)8(6)17/h5-13,15-22H,3-4H2,1-2H3/t5-,6+,7-,8+,9+,10-,11-,12+,13+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50024118
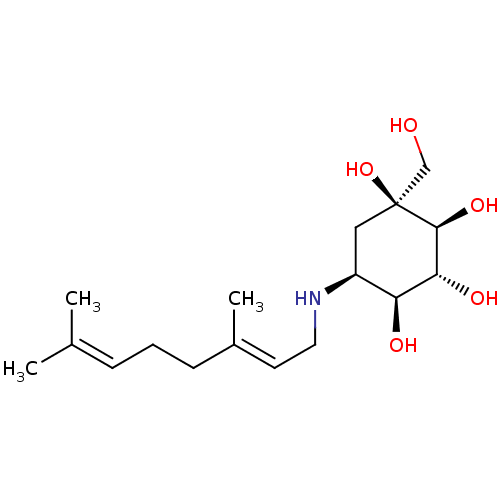
(5-(3,7-Dimethyl-octa-2,6-dienylamino)-1-hydroxymet...)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#7]-[#6@H]-1-[#6][C@]([#8])([#6]-[#8])[#6@@H](-[#8])-[#6@H](-[#8])-[#6@H]-1-[#8] |r| Show InChI InChI=1S/C17H31NO5/c1-11(2)5-4-6-12(3)7-8-18-13-9-17(23,10-19)16(22)15(21)14(13)20/h5,7,13-16,18-23H,4,6,8-10H2,1-3H3/b12-7+/t13-,14?,15-,16-,17?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50024124
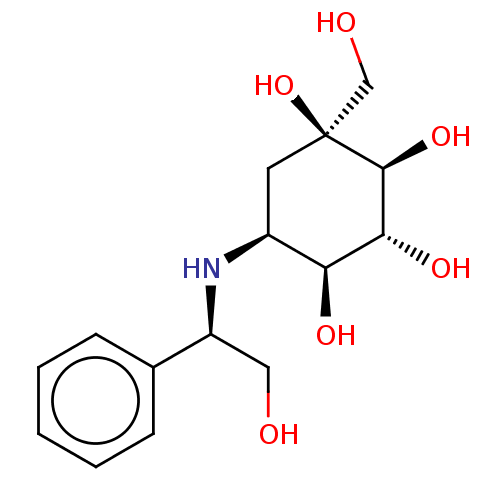
(1-Hydroxymethyl-5-(2-hydroxy-1-phenyl-ethylamino)-...)Show SMILES [H][C@@](CO)(N[C@H]1C[C@](O)(CO)[C@@H](O)[C@H](O)[C@H]1O)c1ccccc1 Show InChI InChI=1S/C15H23NO6/c17-7-11(9-4-2-1-3-5-9)16-10-6-15(22,8-18)14(21)13(20)12(10)19/h1-5,10-14,16-22H,6-8H2/t10-,11-,12-,13+,14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50024133

(1-Hydroxymethyl-5-(3-phenyl-allylamino)-cyclohexan...)Show SMILES OC[C@@]1(O)C[C@H](NC\C=C\c2ccccc2)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C16H23NO5/c18-10-16(22)9-12(13(19)14(20)15(16)21)17-8-4-7-11-5-2-1-3-6-11/h1-7,12-15,17-22H,8-10H2/b7-4+/t12-,13?,14-,15-,16?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50024128
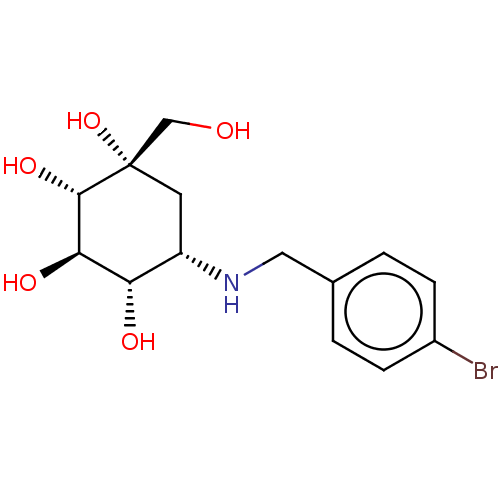
(5-(4-Bromo-benzylamino)-1-hydroxymethyl-cyclohexan...)Show SMILES OC[C@@]1(O)C[C@H](NCc2ccc(Br)cc2)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C14H20BrNO5/c15-9-3-1-8(2-4-9)6-16-10-5-14(21,7-17)13(20)12(19)11(10)18/h1-4,10-13,16-21H,5-7H2/t10-,11?,12-,13-,14?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50024126
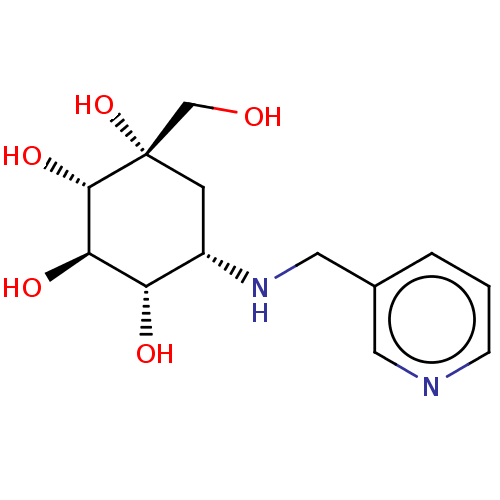
(1-Hydroxymethyl-5-[(pyridin-3-ylmethyl)-amino]-cyc...)Show SMILES OC[C@@]1(O)C[C@H](NCc2cccnc2)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C13H20N2O5/c16-7-13(20)4-9(10(17)11(18)12(13)19)15-6-8-2-1-3-14-5-8/h1-3,5,9-12,15-20H,4,6-7H2/t9-,10?,11-,12-,13?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50024117

(5-Cyclohexylamino-1-hydroxymethyl-cyclohexane-1,2,...)Show SMILES OC[C@@]1(O)C[C@H](NC2CCCCC2)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C13H25NO5/c15-7-13(19)6-9(10(16)11(17)12(13)18)14-8-4-2-1-3-5-8/h8-12,14-19H,1-7H2/t9-,10?,11-,12-,13?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50048090
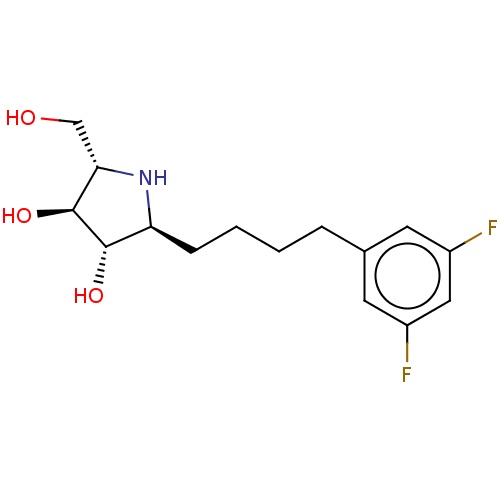
(CHEMBL3311519)Show SMILES OC[C@@H]1N[C@@H](CCCCc2cc(F)cc(F)c2)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H21F2NO3/c16-10-5-9(6-11(17)7-10)3-1-2-4-12-14(20)15(21)13(8-19)18-12/h5-7,12-15,18-21H,1-4,8H2/t12-,13-,14-,15-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Wistar rat intestinal sucrase assessed as inhibition of D-glucose release after 30 mins by spectrophotometry |
Bioorg Med Chem Lett 24: 3298-301 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.001
BindingDB Entry DOI: 10.7270/Q2T43VRS |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50333455
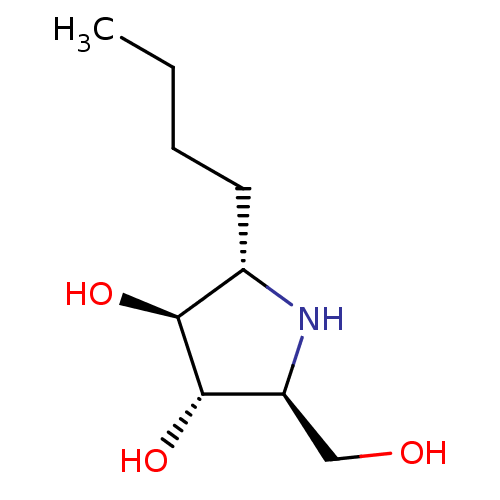
((2S,3S,4S,5S)-2-butyl-5-(hydroxymethyl)pyrrolidine...)Show InChI InChI=1S/C9H19NO3/c1-2-3-4-6-8(12)9(13)7(5-11)10-6/h6-13H,2-5H2,1H3/t6-,7-,8-,9-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Wistar rat small intestine sucrase after 30 mins |
Bioorg Med Chem Lett 21: 738-41 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.112
BindingDB Entry DOI: 10.7270/Q2D21XVS |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50333455

((2S,3S,4S,5S)-2-butyl-5-(hydroxymethyl)pyrrolidine...)Show InChI InChI=1S/C9H19NO3/c1-2-3-4-6-8(12)9(13)7(5-11)10-6/h6-13H,2-5H2,1H3/t6-,7-,8-,9-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama
Curated by ChEMBL
| Assay Description
Inhibition of rat intestinal sucrase using sucrose as substrate |
J Med Chem 55: 10347-62 (2012)
Article DOI: 10.1021/jm301304e
BindingDB Entry DOI: 10.7270/Q2K35VTX |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50333455

((2S,3S,4S,5S)-2-butyl-5-(hydroxymethyl)pyrrolidine...)Show InChI InChI=1S/C9H19NO3/c1-2-3-4-6-8(12)9(13)7(5-11)10-6/h6-13H,2-5H2,1H3/t6-,7-,8-,9-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Wistar rat intestinal sucrase assessed as inhibition of D-glucose release after 30 mins by spectrophotometry |
Bioorg Med Chem Lett 24: 3298-301 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.001
BindingDB Entry DOI: 10.7270/Q2T43VRS |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50405389
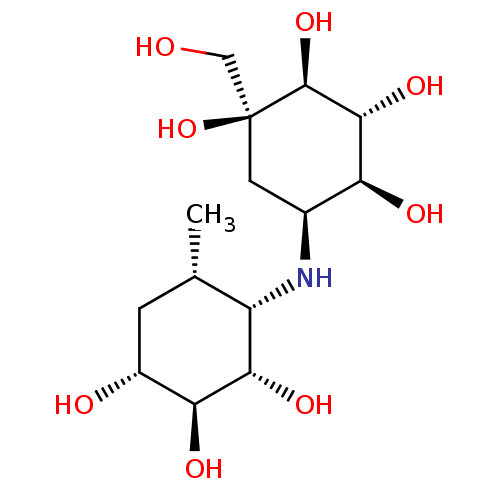
(CHEMBL2051982)Show SMILES C[C@H]1C[C@@H](O)[C@H](O)[C@@H](O)[C@H]1N[C@H]1C[C@](O)(CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C14H27NO8/c1-5-2-7(17)10(19)11(20)8(5)15-6-3-14(23,4-16)13(22)12(21)9(6)18/h5-13,15-23H,2-4H2,1H3/t5-,6-,7+,8-,9-,10-,11-,12+,13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50405396

(CHEMBL2051762)Show SMILES C[C@H]1C[C@H](O)[C@H](O)[C@@H](O)[C@H]1N[C@H]1C[C@](O)(CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C14H27NO8/c1-5-2-7(17)10(19)11(20)8(5)15-6-3-14(23,4-16)13(22)12(21)9(6)18/h5-13,15-23H,2-4H2,1H3/t5-,6-,7-,8-,9-,10-,11-,12+,13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM18353

((2R,3R,4R,5S)-2-(hydroxymethyl)-1-methylpiperidine...)Show InChI InChI=1S/C7H15NO4/c1-8-2-5(10)7(12)6(11)4(8)3-9/h4-7,9-12H,2-3H2,1H3/t4-,5+,6-,7-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University
Curated by ChEMBL
| Assay Description
Inhibition of Sucrase in rat intestinal brush border membranes by D-glucose oxidase-peroxidase method |
J Med Chem 38: 2349-56 (1995)
BindingDB Entry DOI: 10.7270/Q2N878T0 |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM18353

((2R,3R,4R,5S)-2-(hydroxymethyl)-1-methylpiperidine...)Show InChI InChI=1S/C7H15NO4/c1-8-2-5(10)7(12)6(11)4(8)3-9/h4-7,9-12H,2-3H2,1H3/t4-,5+,6-,7-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University
Curated by ChEMBL
| Assay Description
Inhibition of Sucrase in rat intestinal brush border membranes by D-glucose oxidase-peroxidase method |
J Med Chem 38: 2349-56 (1995)
BindingDB Entry DOI: 10.7270/Q2N878T0 |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50375510

(CHEMBL405957)Show InChI InChI=1S/C6H13NO4/c8-1-3-5(10)6(11)4(2-9)7-3/h3-11H,1-2H2/t3-,4-,5-,6-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University
Curated by ChEMBL
| Assay Description
Inhibition of rat isomaltase |
Bioorg Med Chem 16: 2734-40 (2008)
Article DOI: 10.1016/j.bmc.2008.01.032
BindingDB Entry DOI: 10.7270/Q2FB53T0 |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50180584
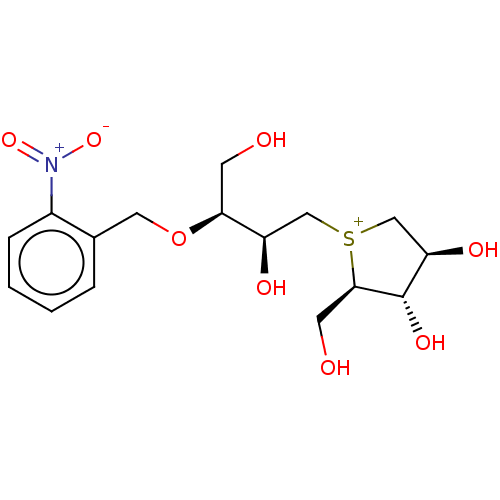
(CHEMBL3814496)Show SMILES [Cl-].OC[C@H](OCc1ccccc1[N+]([O-])=O)[C@H](O)C[S+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C16H24NO8S.ClH/c18-5-14(25-7-10-3-1-2-4-11(10)17(23)24)12(20)8-26-9-13(21)16(22)15(26)6-19;/h1-4,12-16,18-22H,5-9H2;1H/q+1;/p-1/t12-,13-,14+,15-,16+,26?;/m1./s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Kindai University
Curated by ChEMBL
| Assay Description
Inhibition of rat small intestinal sucrase using sucrose as substrate incubated for 30 mins by glucose-oxidase method |
Bioorg Med Chem 24: 3705-15 (2016)
Article DOI: 10.1016/j.bmc.2016.06.013
BindingDB Entry DOI: 10.7270/Q241700V |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50048086
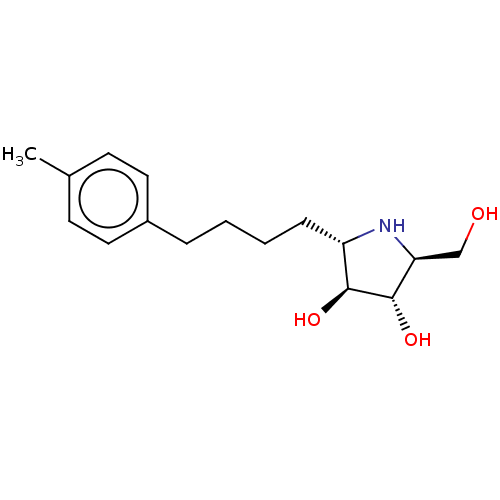
(CHEMBL3311515)Show SMILES Cc1ccc(CCCC[C@@H]2N[C@@H](CO)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C16H25NO3/c1-11-6-8-12(9-7-11)4-2-3-5-13-15(19)16(20)14(10-18)17-13/h6-9,13-20H,2-5,10H2,1H3/t13-,14-,15-,16-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Wistar rat intestinal sucrase assessed as inhibition of D-glucose release after 30 mins by spectrophotometry |
Bioorg Med Chem Lett 24: 3298-301 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.001
BindingDB Entry DOI: 10.7270/Q2T43VRS |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Homo sapiens (Human)) | BDBM50024129

(5-Amino-1-hydroxymethyl-cyclohexane-1,2,3,4-tetrao...)Show SMILES N[C@H]1C[C@](O)(CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C7H15NO5/c8-3-1-7(13,2-9)6(12)5(11)4(3)10/h3-6,9-13H,1-2,8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50263044
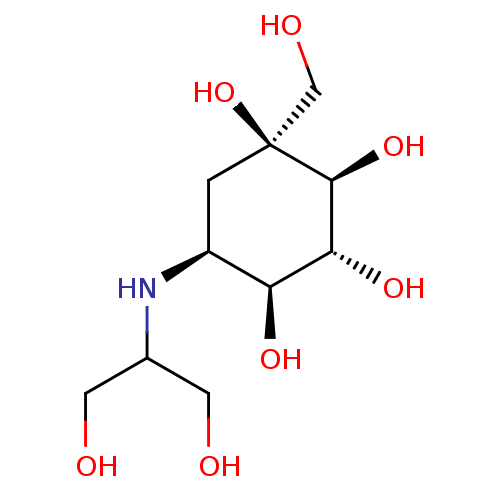
(CHEMBL476960 | Voglibose)Show SMILES OCC(CO)N[C@H]1C[C@](O)(CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H21NO7/c12-2-5(3-13)11-6-1-10(18,4-14)9(17)8(16)7(6)15/h5-9,11-18H,1-4H2/t6-,7-,8+,9-,10-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University
Curated by ChEMBL
| Assay Description
Inhibition of rat intestinal brush border membrane sucrase |
Bioorg Med Chem 16: 7330-6 (2008)
Article DOI: 10.1016/j.bmc.2008.06.026
BindingDB Entry DOI: 10.7270/Q27D2W2B |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50375511
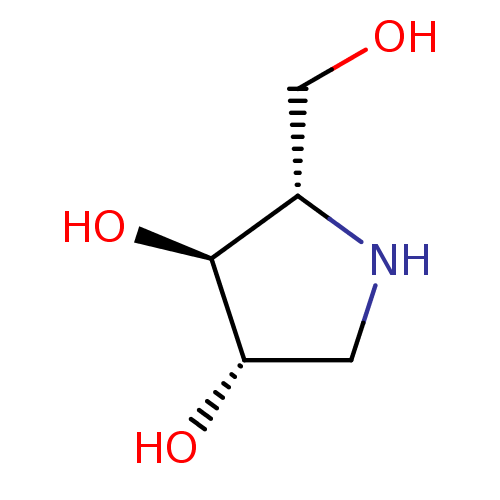
(CHEMBL406973)Show InChI InChI=1S/C5H11NO3/c7-2-3-5(9)4(8)1-6-3/h3-9H,1-2H2/t3-,4-,5-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University
Curated by ChEMBL
| Assay Description
Inhibition of rat isomaltase |
Bioorg Med Chem 16: 2734-40 (2008)
Article DOI: 10.1016/j.bmc.2008.01.032
BindingDB Entry DOI: 10.7270/Q2FB53T0 |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
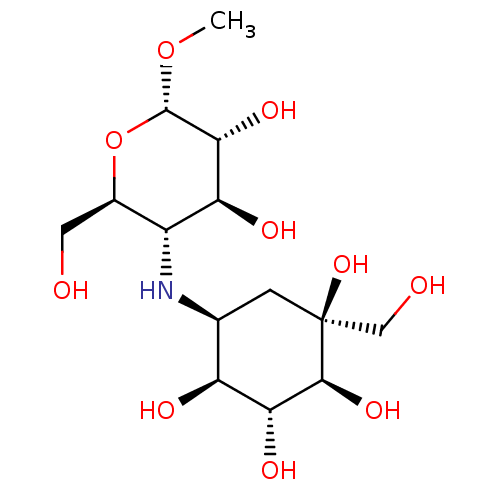
(Homo sapiens (Human)) | BDBM50405393
(CHEMBL2051981)Show SMILES CO[C@H]1O[C@H](CO)[C@@H](N[C@H]2C[C@](O)(CO)[C@@H](O)[C@H](O)[C@H]2O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C14H27NO10/c1-24-13-11(21)9(19)7(6(3-16)25-13)15-5-2-14(23,4-17)12(22)10(20)8(5)18/h5-13,15-23H,2-4H2,1H3/t5-,6+,7+,8-,9-,10+,11+,12-,13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Alpha-D-glucosidase inhibitory activity and enzyme inhibition in vitro against porcine sucrase |
J Med Chem 29: 1038-46 (1986)
BindingDB Entry DOI: 10.7270/Q27P8ZZV |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
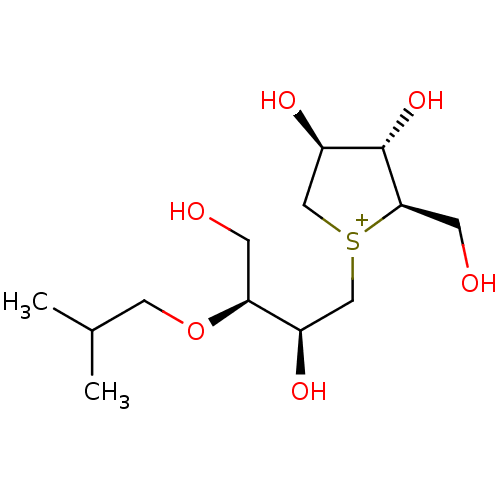
(Rattus norvegicus (Rat)) | BDBM50180582
(CHEMBL3814988)Show SMILES [Cl-].CC(C)CO[C@@H](CO)[C@H](O)C[S+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C13H27O6S.ClH/c1-8(2)5-19-11(3-14)9(16)6-20-7-10(17)13(18)12(20)4-15;/h8-18H,3-7H2,1-2H3;1H/q+1;/p-1/t9-,10-,11+,12-,13+,20?;/m1./s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Kindai University
Curated by ChEMBL
| Assay Description
Inhibition of rat small intestinal sucrase using sucrose as substrate incubated for 30 mins by glucose-oxidase method |
Bioorg Med Chem 24: 3705-15 (2016)
Article DOI: 10.1016/j.bmc.2016.06.013
BindingDB Entry DOI: 10.7270/Q241700V |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
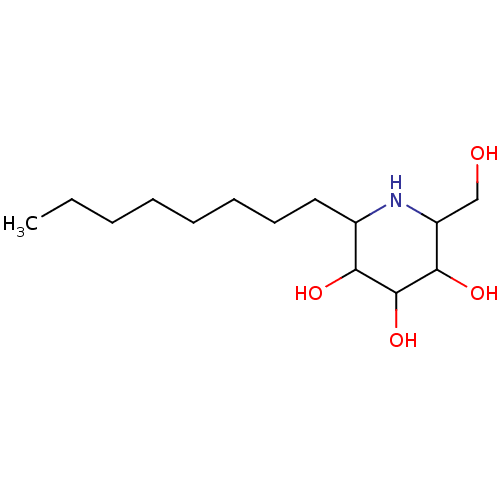
(Rattus norvegicus (Rat)) | BDBM50156356
(2-Hydroxymethyl-6-octyl-piperidine-3,4,5-triol | C...)Show InChI InChI=1S/C14H29NO4/c1-2-3-4-5-6-7-8-10-12(17)14(19)13(18)11(9-16)15-10/h10-19H,2-9H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans
Curated by ChEMBL
| Assay Description
Inhibition of rat intestinal isomaltase using disaccharide |
Bioorg Med Chem Lett 14: 5991-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.086
BindingDB Entry DOI: 10.7270/Q27P904W |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50180583
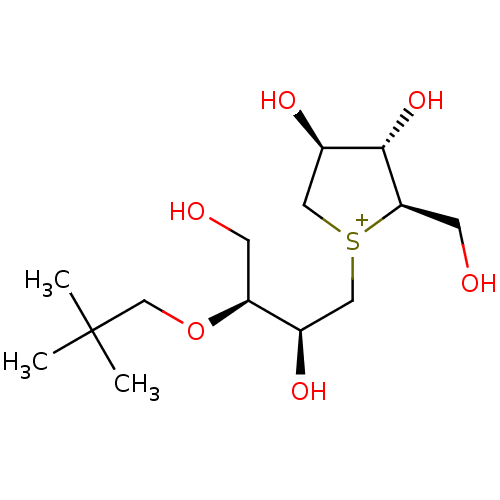
(CHEMBL3815109)Show SMILES [Cl-].CC(C)(C)CO[C@@H](CO)[C@H](O)C[S+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C14H29O6S.ClH/c1-14(2,3)8-20-11(4-15)9(17)6-21-7-10(18)13(19)12(21)5-16;/h9-13,15-19H,4-8H2,1-3H3;1H/q+1;/p-1/t9-,10-,11+,12-,13+,21?;/m1./s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Kindai University
Curated by ChEMBL
| Assay Description
Inhibition of rat small intestinal sucrase using sucrose as substrate incubated for 30 mins by glucose-oxidase method |
Bioorg Med Chem 24: 3705-15 (2016)
Article DOI: 10.1016/j.bmc.2016.06.013
BindingDB Entry DOI: 10.7270/Q241700V |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50242818
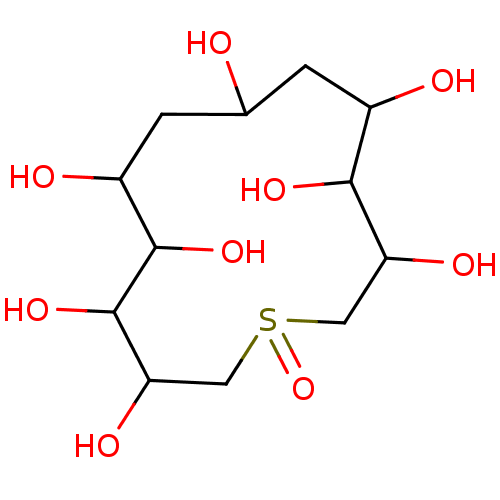
(1-Oxo-1lambda-4-thia-cyclotridecan-3,4,5,6,8,10,11...)Show InChI InChI=1S/C12H24O9S/c13-5-1-6(14)10(18)8(16)3-22(21)4-9(17)12(20)11(19)7(15)2-5/h5-20H,1-4H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
Fuji-Sangyo Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of rat intestinal isomaltase |
J Nat Prod 71: 981-4 (2008)
Article DOI: 10.1021/np070604h
BindingDB Entry DOI: 10.7270/Q2XS5W9M |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50573735
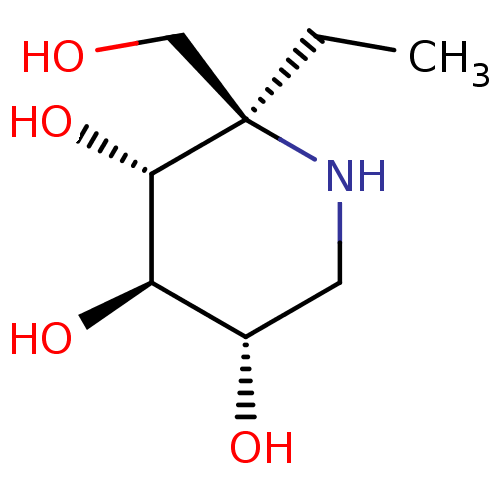
(CHEMBL4860203)Show SMILES Cl.CC[C@]1(CO)NC[C@H](O)[C@@H](O)[C@@H]1O |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rat intestinal sucrase using p-nitrophenyl glycoside as substrate assessed as release of p-nitrophenol measured by spectrometric assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113716
BindingDB Entry DOI: 10.7270/Q27W6H1P |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50375510

(CHEMBL405957)Show InChI InChI=1S/C6H13NO4/c8-1-3-5(10)6(11)4(2-9)7-3/h3-11H,1-2H2/t3-,4-,5-,6-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University
Curated by ChEMBL
| Assay Description
Inhibition of rat sucrase |
Bioorg Med Chem 16: 2734-40 (2008)
Article DOI: 10.1016/j.bmc.2008.01.032
BindingDB Entry DOI: 10.7270/Q2FB53T0 |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50612995

(CHEMBL5276586)Show SMILES Cl.[H][C@@]1(OC(OC[C@H]1O)c1ccccc1[N+]([O-])=O)[C@H](O)C[S@@+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50242271
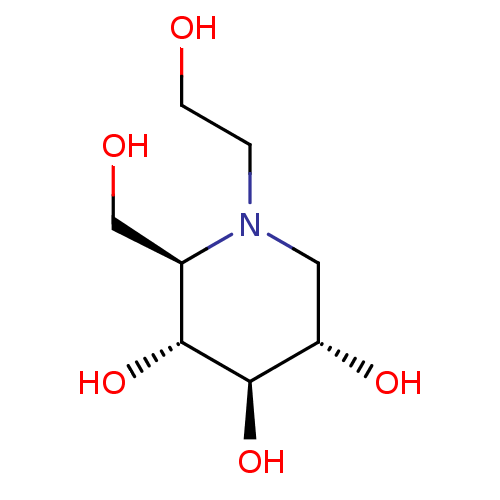
((2R,3R,4R,5S)-1-(2-hydroxyethyl)-2-(hydroxymethyl)...)Show SMILES OCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C8H17NO5/c10-2-1-9-3-6(12)8(14)7(13)5(9)4-11/h5-8,10-14H,1-4H2/t5-,6+,7-,8-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University
Curated by ChEMBL
| Assay Description
Inhibition of rat intestinal brush border membrane sucrase |
Bioorg Med Chem 16: 7330-6 (2008)
Article DOI: 10.1016/j.bmc.2008.06.026
BindingDB Entry DOI: 10.7270/Q27D2W2B |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50333457
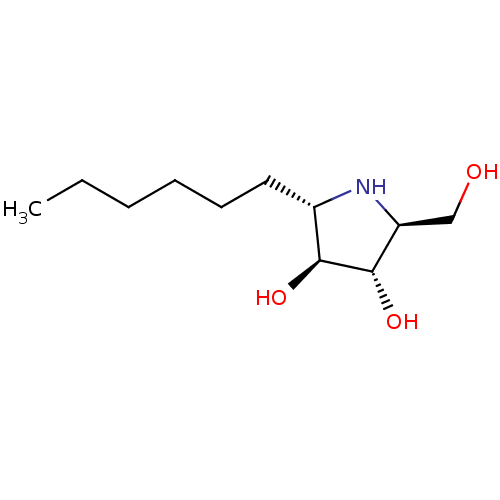
((2S,3S,4S,5S)-2-hexyl-5-(hydroxymethyl)pyrrolidine...)Show SMILES CCCCCC[C@@H]1N[C@@H](CO)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H23NO3/c1-2-3-4-5-6-8-10(14)11(15)9(7-13)12-8/h8-15H,2-7H2,1H3/t8-,9-,10-,11-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Wistar rat small intestine sucrase after 30 mins |
Bioorg Med Chem Lett 21: 738-41 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.112
BindingDB Entry DOI: 10.7270/Q2D21XVS |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50333457

((2S,3S,4S,5S)-2-hexyl-5-(hydroxymethyl)pyrrolidine...)Show SMILES CCCCCC[C@@H]1N[C@@H](CO)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H23NO3/c1-2-3-4-5-6-8-10(14)11(15)9(7-13)12-8/h8-15H,2-7H2,1H3/t8-,9-,10-,11-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama
Curated by ChEMBL
| Assay Description
Inhibition of rat intestinal sucrase using sucrose as substrate |
J Med Chem 55: 10347-62 (2012)
Article DOI: 10.1021/jm301304e
BindingDB Entry DOI: 10.7270/Q2K35VTX |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50048088
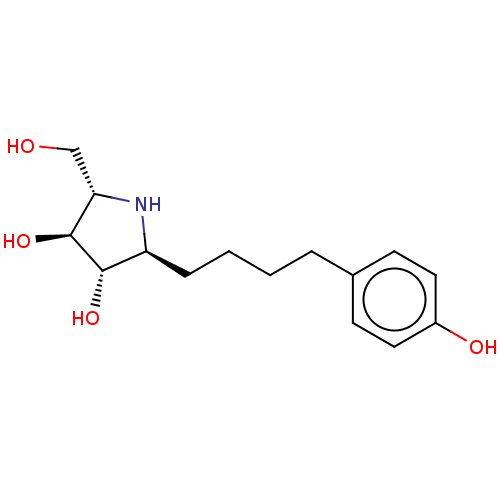
(CHEMBL3311517)Show SMILES OC[C@@H]1N[C@@H](CCCCc2ccc(O)cc2)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H23NO4/c17-9-13-15(20)14(19)12(16-13)4-2-1-3-10-5-7-11(18)8-6-10/h5-8,12-20H,1-4,9H2/t12-,13-,14-,15-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Wistar rat intestinal sucrase assessed as inhibition of D-glucose release after 30 mins by spectrophotometry |
Bioorg Med Chem Lett 24: 3298-301 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.001
BindingDB Entry DOI: 10.7270/Q2T43VRS |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50573741
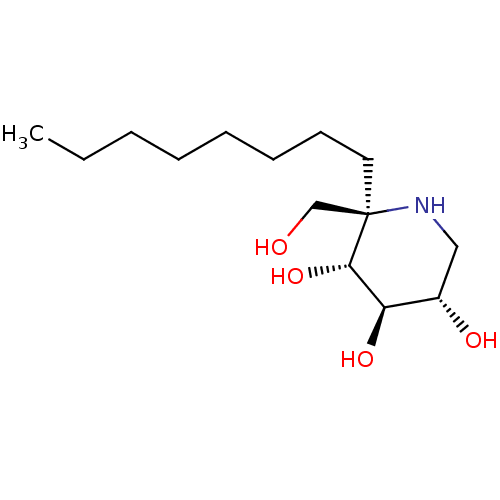
(CHEMBL4873807)Show SMILES Cl.CCCCCCCC[C@]1(CO)NC[C@H](O)[C@@H](O)[C@@H]1O |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rat intestinal sucrase using p-nitrophenyl glycoside as substrate assessed as release of p-nitrophenol measured by spectrometric assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113716
BindingDB Entry DOI: 10.7270/Q27W6H1P |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50180520
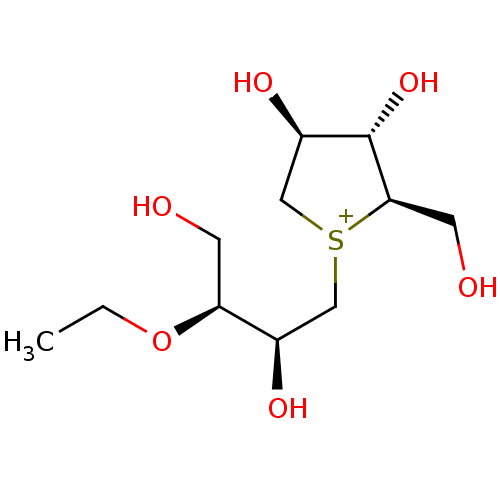
(CHEMBL3814838)Show SMILES [Cl-].CCO[C@@H](CO)[C@H](O)C[S+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C11H23O6S.ClH/c1-2-17-9(3-12)7(14)5-18-6-8(15)11(16)10(18)4-13;/h7-16H,2-6H2,1H3;1H/q+1;/p-1/t7-,8-,9+,10-,11+,18?;/m1./s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Kindai University
Curated by ChEMBL
| Assay Description
Inhibition of rat small intestinal sucrase using sucrose as substrate incubated for 30 mins by glucose-oxidase method |
Bioorg Med Chem 24: 3705-15 (2016)
Article DOI: 10.1016/j.bmc.2016.06.013
BindingDB Entry DOI: 10.7270/Q241700V |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM18363
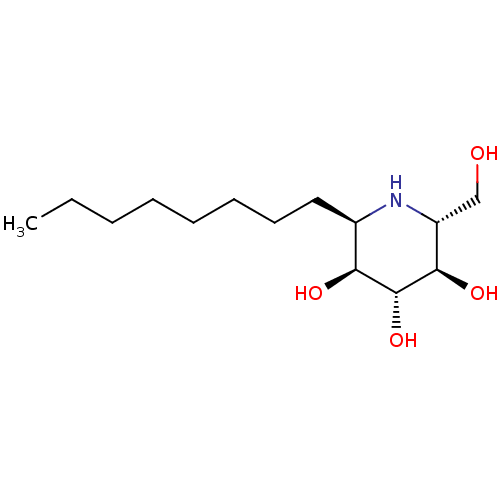
((2R,3R,4R,5S,6R)-2-(hydroxymethyl)-6-octylpiperidi...)Show SMILES CCCCCCCC[C@H]1N[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C14H29NO4/c1-2-3-4-5-6-7-8-10-12(17)14(19)13(18)11(9-16)15-10/h10-19H,2-9H2,1H3/t10-,11-,12+,13-,14-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama
Curated by ChEMBL
| Assay Description
Inhibition of rat intestinal isomaltase using isomoltose as substrate |
J Med Chem 55: 10347-62 (2012)
Article DOI: 10.1021/jm301304e
BindingDB Entry DOI: 10.7270/Q2K35VTX |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50342937

((1S,2R,3S,4S)-1-((2S,3S)-3-ethoxy-2,4-dihydroxybut...)Show SMILES CCO[C@@H](CO)[C@H](O)C[S@@+]1C[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C11H23O6S/c1-2-17-9(3-12)7(14)5-18-6-8(15)11(16)10(18)4-13/h7-16H,2-6H2,1H3/q+1/t7-,8-,9+,10-,11+,18-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University
Curated by ChEMBL
| Assay Description
Inhibition of rat small intestinal sucrase after 30 mins by glucose-oxidase method |
Bioorg Med Chem Lett 21: 3159-62 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.109
BindingDB Entry DOI: 10.7270/Q2DF6RJC |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50048087
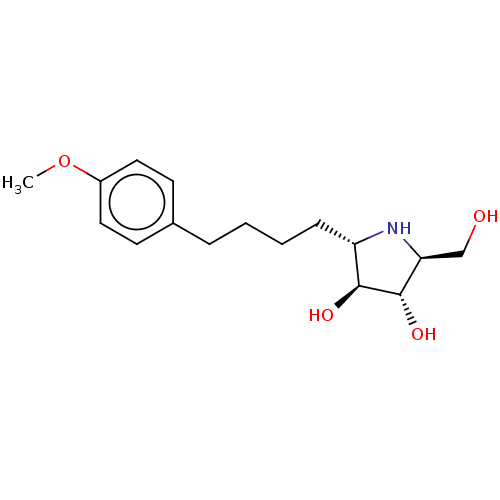
(CHEMBL3311516)Show SMILES COc1ccc(CCCC[C@@H]2N[C@@H](CO)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C16H25NO4/c1-21-12-8-6-11(7-9-12)4-2-3-5-13-15(19)16(20)14(10-18)17-13/h6-9,13-20H,2-5,10H2,1H3/t13-,14-,15-,16-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Wistar rat intestinal sucrase assessed as inhibition of D-glucose release after 30 mins by spectrophotometry |
Bioorg Med Chem Lett 24: 3298-301 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.001
BindingDB Entry DOI: 10.7270/Q2T43VRS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data