 Found 1756 hits of ki data for polymerid = 1622,2113
Found 1756 hits of ki data for polymerid = 1622,2113 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093352
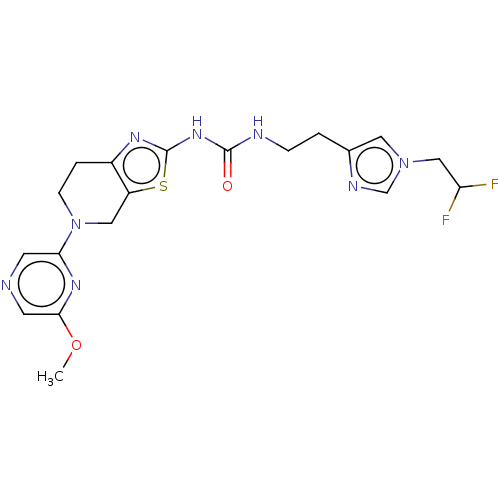
(CHEMBL3586678)Show SMILES COc1cncc(n1)N1CCc2nc(NC(=O)NCCc3cn(CC(F)F)cn3)sc2C1 Show InChI InChI=1S/C19H22F2N8O2S/c1-31-17-7-22-6-16(26-17)29-5-3-13-14(9-29)32-19(25-13)27-18(30)23-4-2-12-8-28(11-24-12)10-15(20)21/h6-8,11,15H,2-5,9-10H2,1H3,(H2,23,25,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093351
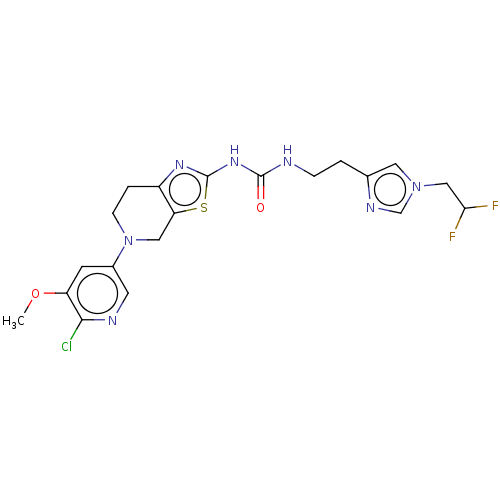
(CHEMBL3585362)Show SMILES COc1cc(cnc1Cl)N1CCc2nc(NC(=O)NCCc3cn(CC(F)F)cn3)sc2C1 Show InChI InChI=1S/C20H22ClF2N7O2S/c1-32-15-6-13(7-25-18(15)21)30-5-3-14-16(9-30)33-20(27-14)28-19(31)24-4-2-12-8-29(11-26-12)10-17(22)23/h6-8,11,17H,2-5,9-10H2,1H3,(H2,24,27,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093355
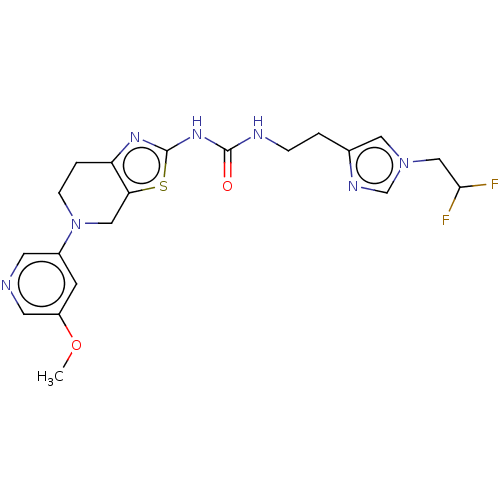
(CHEMBL3586677)Show SMILES COc1cncc(c1)N1CCc2nc(NC(=O)NCCc3cn(CC(F)F)cn3)sc2C1 Show InChI InChI=1S/C20H23F2N7O2S/c1-31-15-6-14(7-23-8-15)29-5-3-16-17(10-29)32-20(26-16)27-19(30)24-4-2-13-9-28(12-25-13)11-18(21)22/h6-9,12,18H,2-5,10-11H2,1H3,(H2,24,26,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093356
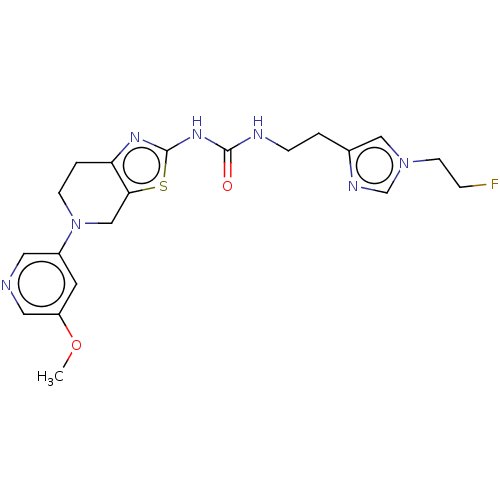
(CHEMBL3586676)Show SMILES COc1cncc(c1)N1CCc2nc(NC(=O)NCCc3cn(CCF)cn3)sc2C1 Show InChI InChI=1S/C20H24FN7O2S/c1-30-16-8-15(9-22-10-16)28-6-3-17-18(12-28)31-20(25-17)26-19(29)23-5-2-14-11-27(7-4-21)13-24-14/h8-11,13H,2-7,12H2,1H3,(H2,23,25,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093354

(CHEMBL3586679)Show SMILES COc1cncc(c1)N1CCc2nc(NC(=O)NCCc3cn(CC(F)(F)F)cn3)sc2C1 Show InChI InChI=1S/C20H22F3N7O2S/c1-32-15-6-14(7-24-8-15)30-5-3-16-17(10-30)33-19(27-16)28-18(31)25-4-2-13-9-29(12-26-13)11-20(21,22)23/h6-9,12H,2-5,10-11H2,1H3,(H2,25,27,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093395
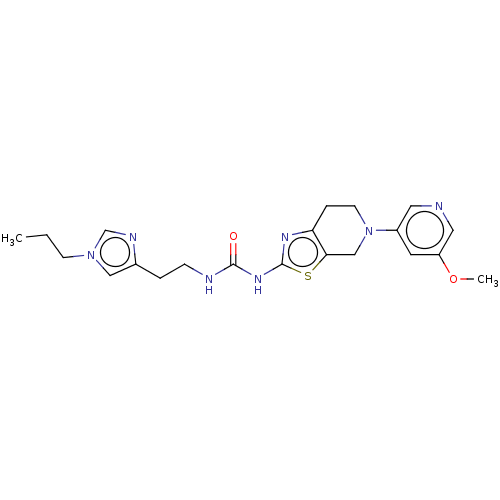
(CHEMBL3586674)Show SMILES CCCn1cnc(CCNC(=O)Nc2nc3CCN(Cc3s2)c2cncc(OC)c2)c1 Show InChI InChI=1S/C21H27N7O2S/c1-3-7-27-12-15(24-14-27)4-6-23-20(29)26-21-25-18-5-8-28(13-19(18)31-21)16-9-17(30-2)11-22-10-16/h9-12,14H,3-8,13H2,1-2H3,(H2,23,25,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093417
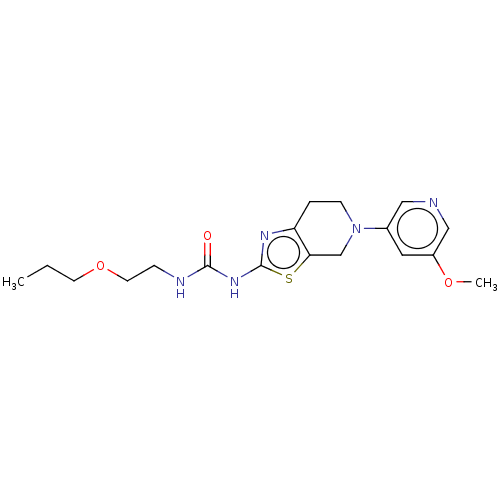
(CHEMBL3586672)Show InChI InChI=1S/C18H25N5O3S/c1-3-7-26-8-5-20-17(24)22-18-21-15-4-6-23(12-16(15)27-18)13-9-14(25-2)11-19-10-13/h9-11H,3-8,12H2,1-2H3,(H2,20,21,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093437
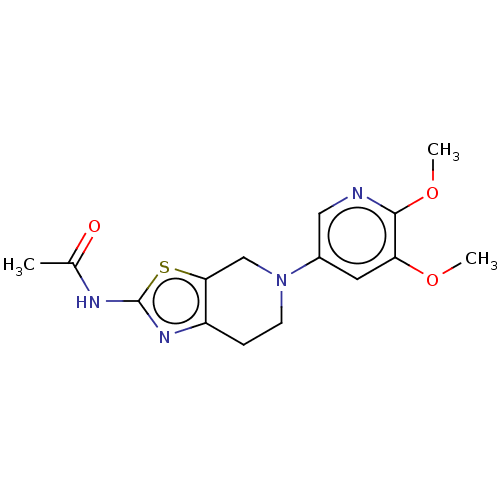
(CHEMBL3586668)Show InChI InChI=1S/C20H32O2/c1-19-7-5-14(21)10-13(19)3-4-15-16(19)6-8-20(2)17(15)9-12-11-22-18(12)20/h12-18,21H,3-11H2,1-2H3/t12-,13+,14-,15-,16+,17+,18+,19+,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149477
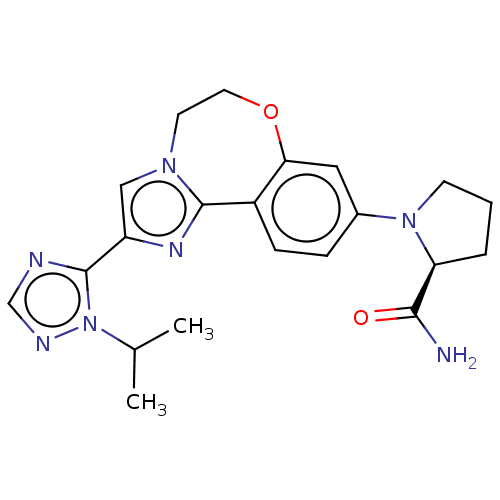
(CHEMBL3770993 | US10851091, U.S. Pat. No. 8,242,10...)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ccc3-c2n1)N1CCC[C@H]1C(N)=O |r| Show InChI InChI=1S/C21H25N7O2/c1-13(2)28-21(23-12-24-28)16-11-26-8-9-30-18-10-14(5-6-15(18)20(26)25-16)27-7-3-4-17(27)19(22)29/h5-6,10-13,17H,3-4,7-9H2,1-2H3,(H2,22,29)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ... |
US Patent US10851091 (2020)
BindingDB Entry DOI: 10.7270/Q2WS8X9V |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093399
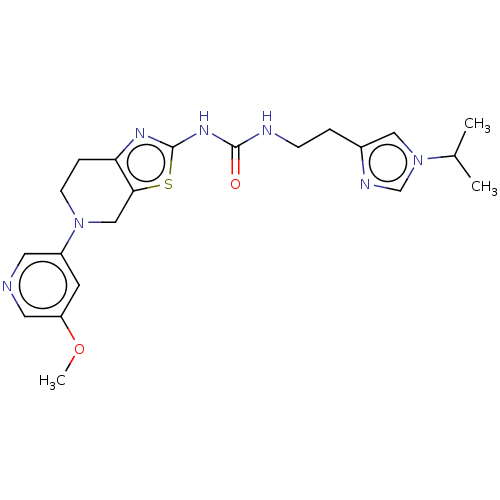
(CHEMBL3586673)Show SMILES COc1cncc(c1)N1CCc2nc(NC(=O)NCCc3cn(cn3)C(C)C)sc2C1 Show InChI InChI=1S/C21H27N7O2S/c1-14(2)28-11-15(24-13-28)4-6-23-20(29)26-21-25-18-5-7-27(12-19(18)31-21)16-8-17(30-3)10-22-9-16/h8-11,13-14H,4-7,12H2,1-3H3,(H2,23,25,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093434
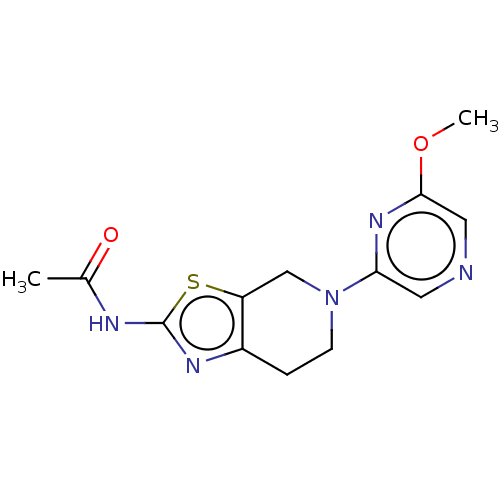
(CHEMBL3586670)Show InChI InChI=1S/C12H11NO8S2/c1-6(14)13-10-4-8(22(16,17)18)2-7-3-9(23(19,20)21)5-11(15)12(7)10/h2-5,15H,1H3,(H,13,14)(H,16,17,18)(H,19,20,21)/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM272979
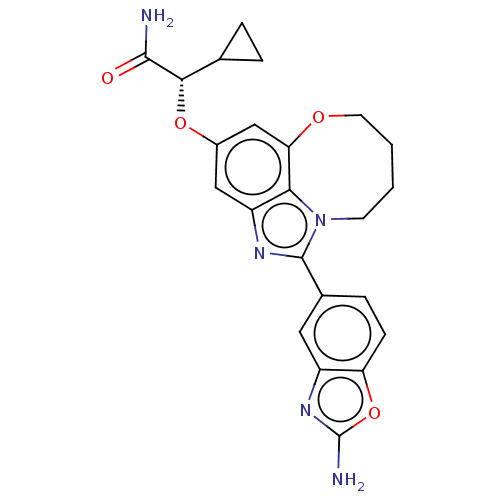
((S)-2-((1-(2- aminobenzo[d]oxazol- 5-yl)-7,8,9,10-...)Show SMILES NC(=O)[C@@H](Oc1cc2OCCCCn3c(nc(c1)c23)-c1ccc2oc(N)nc2c1)C1CC1 |r| Show InChI InChI=1S/C23H23N5O4/c24-21(29)20(12-3-4-12)31-14-10-16-19-18(11-14)30-8-2-1-7-28(19)22(26-16)13-5-6-17-15(9-13)27-23(25)32-17/h5-6,9-12,20H,1-4,7-8H2,(H2,24,29)(H2,25,27)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... |
US Patent US10065970 (2018)
BindingDB Entry DOI: 10.7270/Q27P91FB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM272937
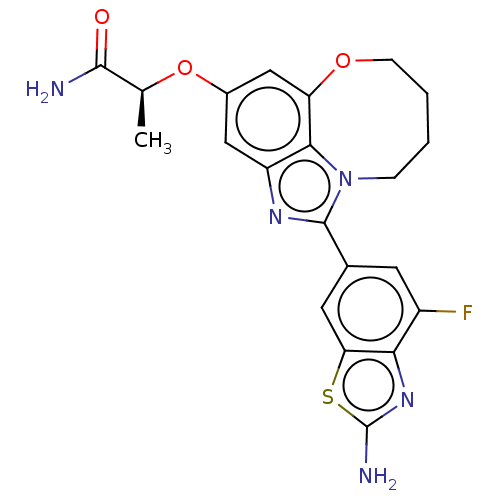
((S)-2-((1-(2-amino-4- fluorobenzo[d]thiazol- 6-yl)...)Show SMILES C[C@H](Oc1cc2OCCCCn3c(nc(c1)c23)-c1cc(F)c2nc(N)sc2c1)C(N)=O |r| Show InChI InChI=1S/C21H20FN5O3S/c1-10(19(23)28)30-12-8-14-18-15(9-12)29-5-3-2-4-27(18)20(25-14)11-6-13(22)17-16(7-11)31-21(24)26-17/h6-10H,2-5H2,1H3,(H2,23,28)(H2,24,26)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... |
US Patent US10065970 (2018)
BindingDB Entry DOI: 10.7270/Q27P91FB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM272967
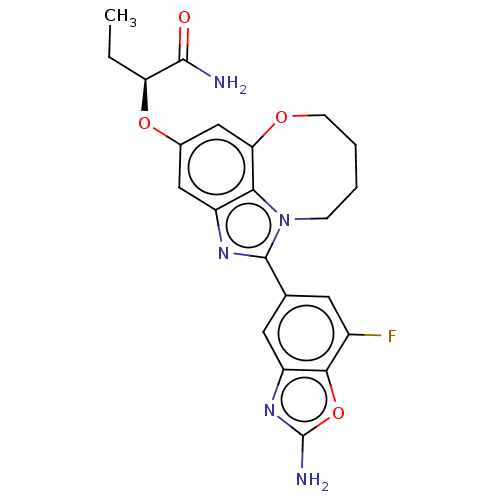
((S)-2-((1-(2-amino-7- fluorobenzo[d]oxazol- 5-yl)-...)Show SMILES CC[C@H](Oc1cc2OCCCCn3c(nc(c1)c23)-c1cc(F)c2oc(N)nc2c1)C(N)=O |r| Show InChI InChI=1S/C22H22FN5O4/c1-2-16(20(24)29)31-12-9-14-18-17(10-12)30-6-4-3-5-28(18)21(26-14)11-7-13(23)19-15(8-11)27-22(25)32-19/h7-10,16H,2-6H2,1H3,(H2,24,29)(H2,25,27)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... |
US Patent US10065970 (2018)
BindingDB Entry DOI: 10.7270/Q27P91FB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM272966
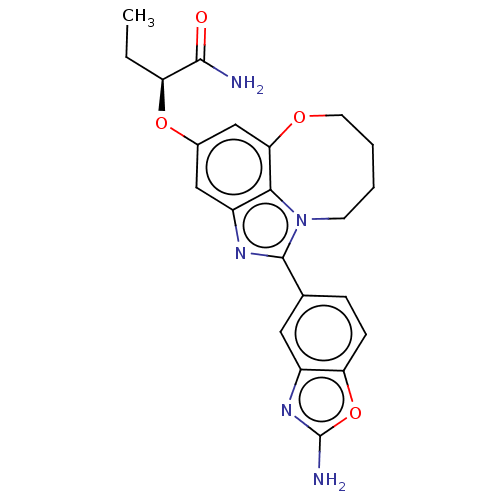
((S)-2-((1-(2- aminobenzo[d]oxazol- 5-yl)-7,8,9,10-...)Show SMILES CC[C@H](Oc1cc2OCCCCn3c(nc(c1)c23)-c1ccc2oc(N)nc2c1)C(N)=O |r| Show InChI InChI=1S/C22H23N5O4/c1-2-16(20(23)28)30-13-10-15-19-18(11-13)29-8-4-3-7-27(19)21(25-15)12-5-6-17-14(9-12)26-22(24)31-17/h5-6,9-11,16H,2-4,7-8H2,1H3,(H2,23,28)(H2,24,26)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... |
US Patent US10065970 (2018)
BindingDB Entry DOI: 10.7270/Q27P91FB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM272989
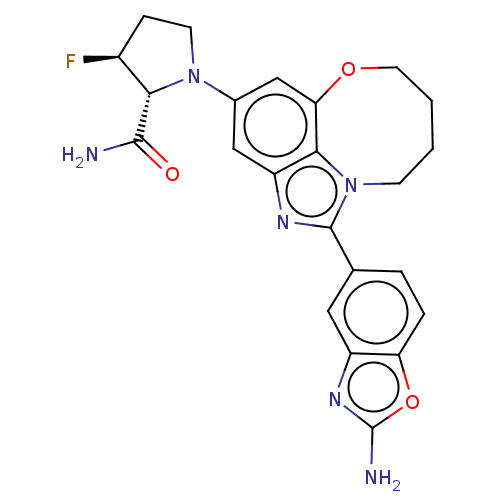
((2R,3S)-1-(1-(2- aminobenzo[d]oxazol- 5-yl)-7,8,9,...)Show SMILES NC(=O)[C@@H]1[C@@H](F)CCN1c1cc2OCCCCn3c(nc(c1)c23)-c1ccc2oc(N)nc2c1 |r| Show InChI InChI=1S/C23H23FN6O3/c24-14-5-7-29(19(14)21(25)31)13-10-16-20-18(11-13)32-8-2-1-6-30(20)22(27-16)12-3-4-17-15(9-12)28-23(26)33-17/h3-4,9-11,14,19H,1-2,5-8H2,(H2,25,31)(H2,26,28)/t14-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... |
US Patent US10065970 (2018)
BindingDB Entry DOI: 10.7270/Q27P91FB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM272991
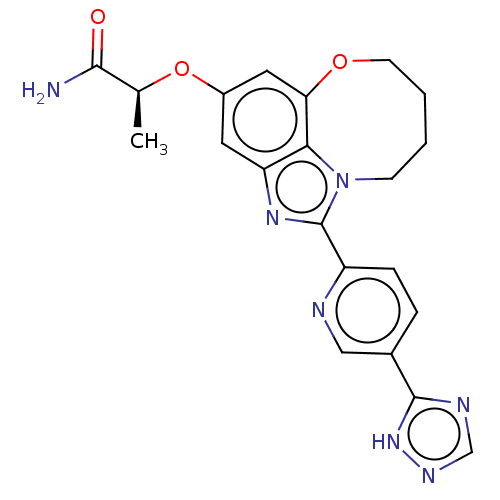
((S)-2-((1-(5-(1H-1,2,4- triazol-5-yl)pyridin-2- yl...)Show SMILES C[C@H](Oc1cc2OCCCCn3c(nc(c1)c23)-c1ccc(cn1)-c1ncn[nH]1)C(N)=O |r| Show InChI InChI=1S/C21H21N7O3/c1-12(19(22)29)31-14-8-16-18-17(9-14)30-7-3-2-6-28(18)21(26-16)15-5-4-13(10-23-15)20-24-11-25-27-20/h4-5,8-12H,2-3,6-7H2,1H3,(H2,22,29)(H,24,25,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... |
US Patent US10065970 (2018)
BindingDB Entry DOI: 10.7270/Q27P91FB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM273000
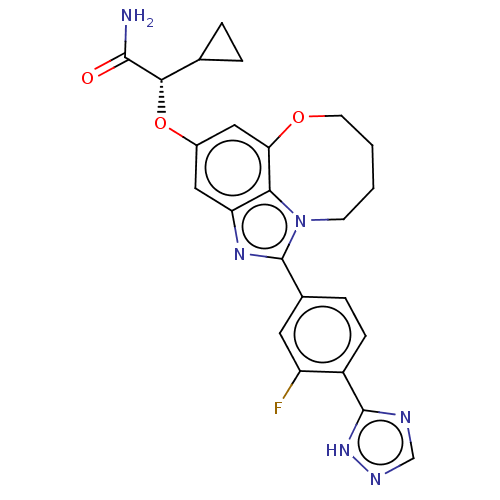
((S)-2-cyclopropyl-2- ((1-(3-fluoro-4-(1H- 1,2,4-tr...)Show SMILES NC(=O)[C@@H](Oc1cc2OCCCCn3c(nc(c1)c23)-c1ccc(-c2ncn[nH]2)c(F)c1)C1CC1 |r| Show InChI InChI=1S/C24H23FN6O3/c25-17-9-14(5-6-16(17)23-27-12-28-30-23)24-29-18-10-15(34-21(22(26)32)13-3-4-13)11-19-20(18)31(24)7-1-2-8-33-19/h5-6,9-13,21H,1-4,7-8H2,(H2,26,32)(H,27,28,30)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... |
US Patent US10065970 (2018)
BindingDB Entry DOI: 10.7270/Q27P91FB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM273002
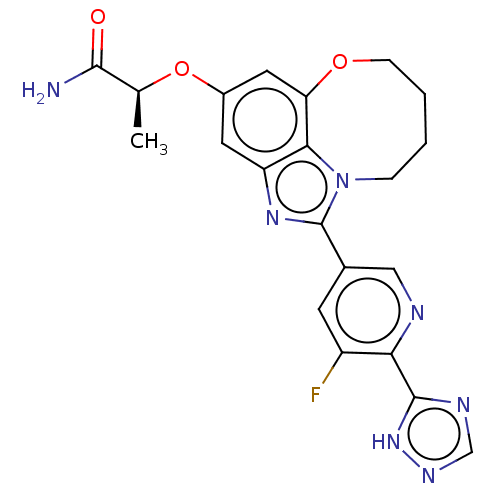
((S)-2-((1-(5-fluoro-6- (1H-1,2,4-triazol-5- yl)pyr...)Show SMILES C[C@H](Oc1cc2OCCCCn3c(nc(c1)c23)-c1cnc(-c2ncn[nH]2)c(F)c1)C(N)=O |r| Show InChI InChI=1S/C21H20FN7O3/c1-11(19(23)30)32-13-7-15-18-16(8-13)31-5-3-2-4-29(18)21(27-15)12-6-14(22)17(24-9-12)20-25-10-26-28-20/h6-11H,2-5H2,1H3,(H2,23,30)(H,25,26,28)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... |
US Patent US10065970 (2018)
BindingDB Entry DOI: 10.7270/Q27P91FB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM273010
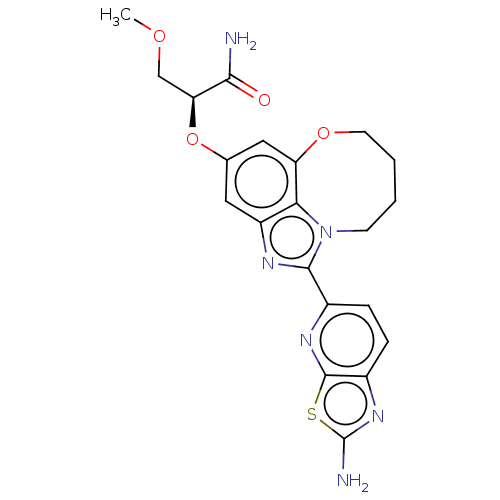
((S)-2-((1-(2- aminothiazolo[5,4- b]pyridin-5-yl)- ...)Show SMILES COC[C@H](Oc1cc2OCCCCn3c(nc(c1)c23)-c1ccc2nc(N)sc2n1)C(N)=O |r| Show InChI InChI=1S/C21H22N6O4S/c1-29-10-16(18(22)28)31-11-8-14-17-15(9-11)30-7-3-2-6-27(17)19(24-14)12-4-5-13-20(25-12)32-21(23)26-13/h4-5,8-9,16H,2-3,6-7,10H2,1H3,(H2,22,28)(H2,23,26)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... |
US Patent US10065970 (2018)
BindingDB Entry DOI: 10.7270/Q27P91FB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM295675
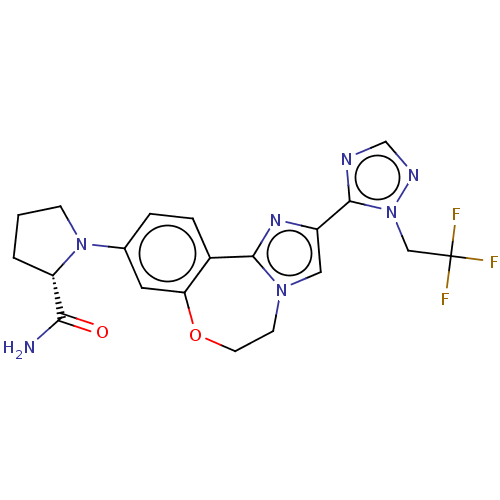
(US10851091, U.S. Pat. No. 8,242,104 No. 469 | US82...)Show SMILES NC(=O)[C@@H]1CCCN1c1ccc2-c3nc(cn3CCOc2c1)-c1ncnn1CC(F)(F)F |r| Show InChI InChI=1S/C20H20F3N7O2/c21-20(22,23)10-30-19(25-11-26-30)14-9-28-6-7-32-16-8-12(3-4-13(16)18(28)27-14)29-5-1-2-15(29)17(24)31/h3-4,8-9,11,15H,1-2,5-7,10H2,(H2,24,31)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ... |
US Patent US10851091 (2020)
BindingDB Entry DOI: 10.7270/Q2WS8X9V |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM272830
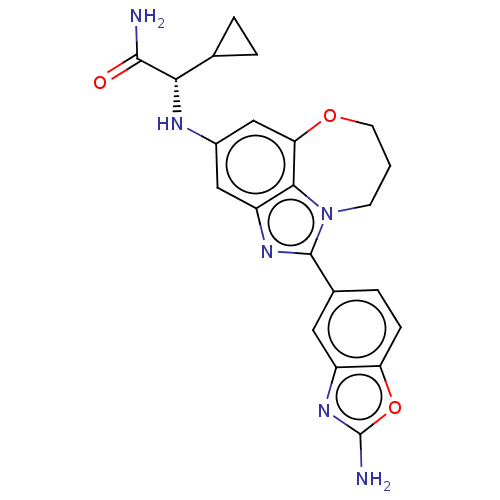
((S)-2-((1-(2- aminobenzo[d]oxazol- 5-yl)-8,9-dihyd...)Show SMILES NC(=O)[C@@H](Nc1cc2OCCCn3c(nc(c1)c23)-c1ccc2oc(N)nc2c1)C1CC1 |r| Show InChI InChI=1S/C22H22N6O3/c23-20(29)18(11-2-3-11)25-13-9-15-19-17(10-13)30-7-1-6-28(19)21(26-15)12-4-5-16-14(8-12)27-22(24)31-16/h4-5,8-11,18,25H,1-3,6-7H2,(H2,23,29)(H2,24,27)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... |
US Patent US10065970 (2018)
BindingDB Entry DOI: 10.7270/Q27P91FB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM272899
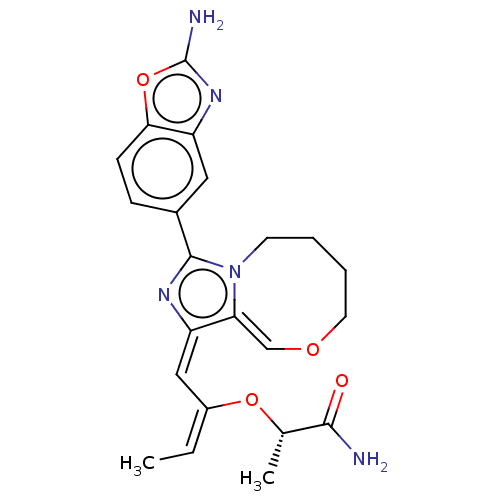
((S)-2-((1-(2- aminobenzo[d]oxazol- 5-yl)-7,8,9,10-...)Show SMILES C\C=C(\O[C@@H](C)C(N)=O)/C=c1/nc(-c2ccc3oc(N)nc3c2)n2CCCCO/C=c/1\2 |r,t:31| Show InChI InChI=1S/C22H25N5O4/c1-3-15(30-13(2)20(23)28)11-16-18-12-29-9-5-4-8-27(18)21(25-16)14-6-7-19-17(10-14)26-22(24)31-19/h3,6-7,10-13H,4-5,8-9H2,1-2H3,(H2,23,28)(H2,24,26)/b15-3+,16-11+,18-12-/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... |
US Patent US10065970 (2018)
BindingDB Entry DOI: 10.7270/Q27P91FB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM272903
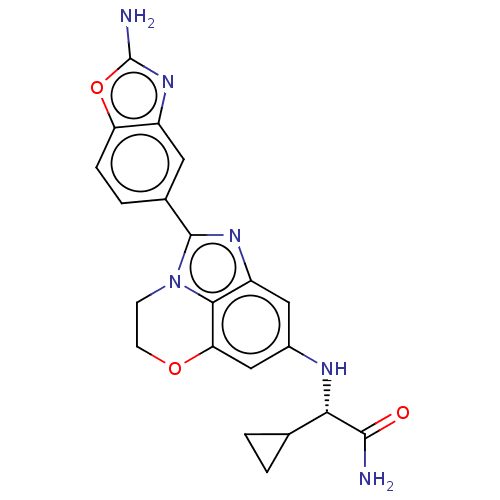
((S)-2-((2-(2- aminobenzo[d]oxazol- 5-yl)-3,4-dihyd...)Show SMILES NC(=O)[C@@H](Nc1cc2OCCn3c(nc(c1)c23)-c1ccc2oc(N)nc2c1)C1CC1 |r| Show InChI InChI=1S/C21H20N6O3/c22-19(28)17(10-1-2-10)24-12-8-14-18-16(9-12)29-6-5-27(18)20(25-14)11-3-4-15-13(7-11)26-21(23)30-15/h3-4,7-10,17,24H,1-2,5-6H2,(H2,22,28)(H2,23,26)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... |
US Patent US10065970 (2018)
BindingDB Entry DOI: 10.7270/Q27P91FB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM272909
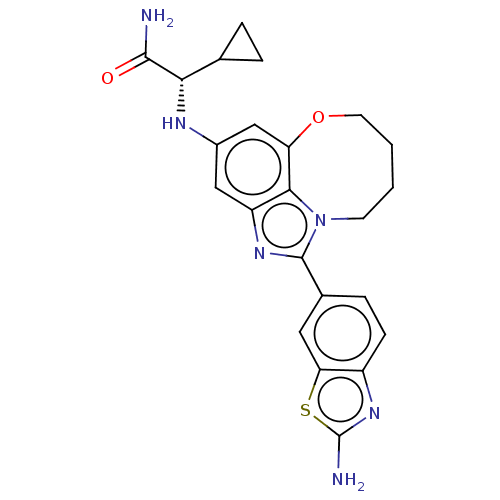
((S)-2-((1-(2- aminobenzo[d]thiazol- 6-yl)-7,8,9,10...)Show SMILES NC(=O)[C@@H](Nc1cc2OCCCCn3c(nc(c1)c23)-c1ccc2nc(N)sc2c1)C1CC1 |r| Show InChI InChI=1S/C23H24N6O2S/c24-21(30)19(12-3-4-12)26-14-10-16-20-17(11-14)31-8-2-1-7-29(20)22(27-16)13-5-6-15-18(9-13)32-23(25)28-15/h5-6,9-12,19,26H,1-4,7-8H2,(H2,24,30)(H2,25,28)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... |
US Patent US10065970 (2018)
BindingDB Entry DOI: 10.7270/Q27P91FB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM272936
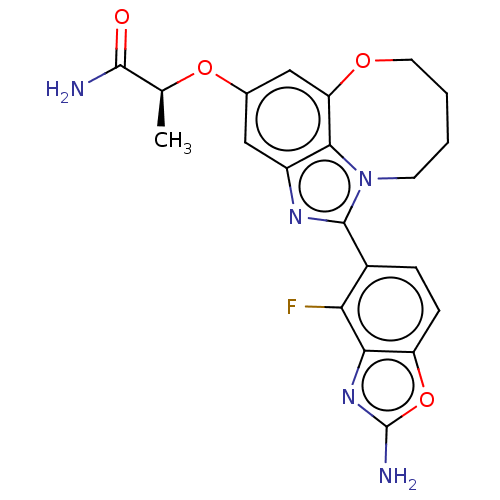
((S)-2-((1-(2-amino-4- fluorobenzo[d]oxazol- 5-yl)-...)Show SMILES C[C@H](Oc1cc2OCCCCn3c(nc(c1)c23)-c1ccc2oc(N)nc2c1F)C(N)=O |r| Show InChI InChI=1S/C21H20FN5O4/c1-10(19(23)28)30-11-8-13-18-15(9-11)29-7-3-2-6-27(18)20(25-13)12-4-5-14-17(16(12)22)26-21(24)31-14/h4-5,8-10H,2-3,6-7H2,1H3,(H2,23,28)(H2,24,26)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... |
US Patent US10065970 (2018)
BindingDB Entry DOI: 10.7270/Q27P91FB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM272975
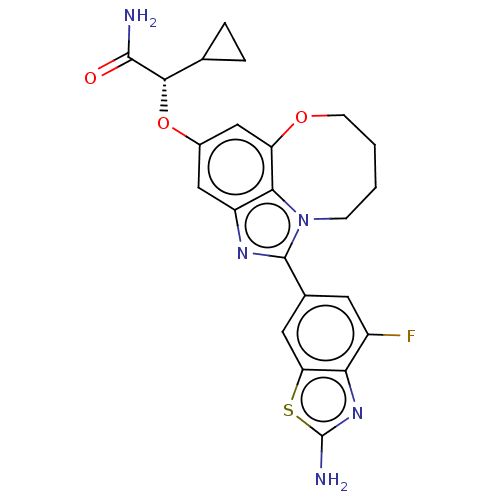
((S)-2-((1-(2-amino-4- fluorobenzo[d]thiazol- 6-yl)...)Show SMILES NC(=O)[C@@H](Oc1cc2OCCCCn3c(nc(c1)c23)-c1cc(F)c2nc(N)sc2c1)C1CC1 |r| Show InChI InChI=1S/C23H22FN5O3S/c24-14-7-12(8-17-18(14)28-23(26)33-17)22-27-15-9-13(32-20(21(25)30)11-3-4-11)10-16-19(15)29(22)5-1-2-6-31-16/h7-11,20H,1-6H2,(H2,25,30)(H2,26,28)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... |
US Patent US10065970 (2018)
BindingDB Entry DOI: 10.7270/Q27P91FB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM272938
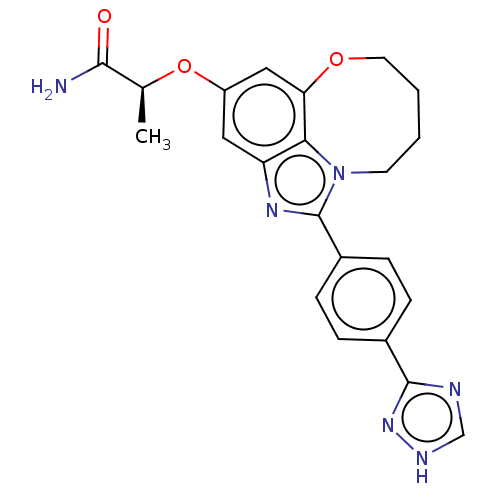
((S)-2-((1-(4-(1H- 1,2,4-triazol-3- yl)phenyl)-7,8,...)Show SMILES C[C@H](Oc1cc2OCCCCn3c(nc(c1)c23)-c1ccc(cc1)-c1nc[nH]n1)C(N)=O |r| Show InChI InChI=1S/C22H22N6O3/c1-13(20(23)29)31-16-10-17-19-18(11-16)30-9-3-2-8-28(19)22(26-17)15-6-4-14(5-7-15)21-24-12-25-27-21/h4-7,10-13H,2-3,8-9H2,1H3,(H2,23,29)(H,24,25,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... |
US Patent US10065970 (2018)
BindingDB Entry DOI: 10.7270/Q27P91FB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM272939
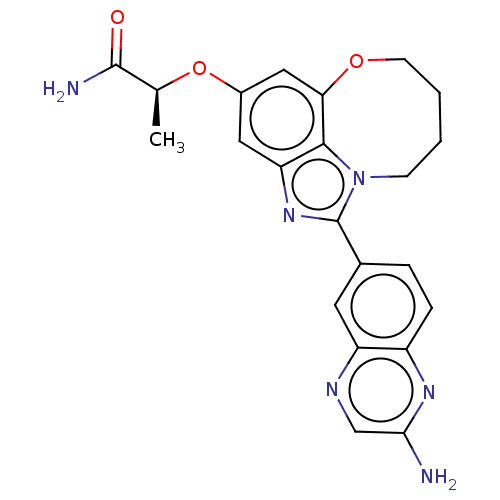
((S)-2-((1-(2- aminoquinoxalin-6- yl)-7,8,9,10- tet...)Show SMILES C[C@H](Oc1cc2OCCCCn3c(nc(c1)c23)-c1ccc2nc(N)cnc2c1)C(N)=O |r| Show InChI InChI=1S/C22H22N6O3/c1-12(21(24)29)31-14-9-17-20-18(10-14)30-7-3-2-6-28(20)22(27-17)13-4-5-15-16(8-13)25-11-19(23)26-15/h4-5,8-12H,2-3,6-7H2,1H3,(H2,23,26)(H2,24,29)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... |
US Patent US10065970 (2018)
BindingDB Entry DOI: 10.7270/Q27P91FB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM272941
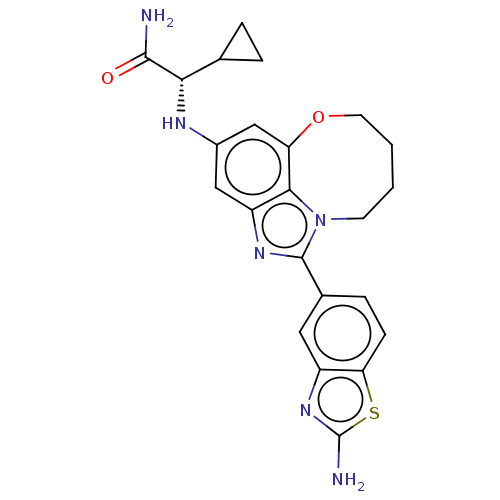
((S)-2-((1-(2- Aminobenzo[d]thiazol- 5-yl)-7,8,9,10...)Show SMILES NC(=O)[C@@H](Nc1cc2OCCCCn3c(nc(c1)c23)-c1ccc2sc(N)nc2c1)C1CC1 |r| Show InChI InChI=1S/C23H24N6O2S/c24-21(30)19(12-3-4-12)26-14-10-16-20-17(11-14)31-8-2-1-7-29(20)22(27-16)13-5-6-18-15(9-13)28-23(25)32-18/h5-6,9-12,19,26H,1-4,7-8H2,(H2,24,30)(H2,25,28)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... |
US Patent US10065970 (2018)
BindingDB Entry DOI: 10.7270/Q27P91FB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM272984
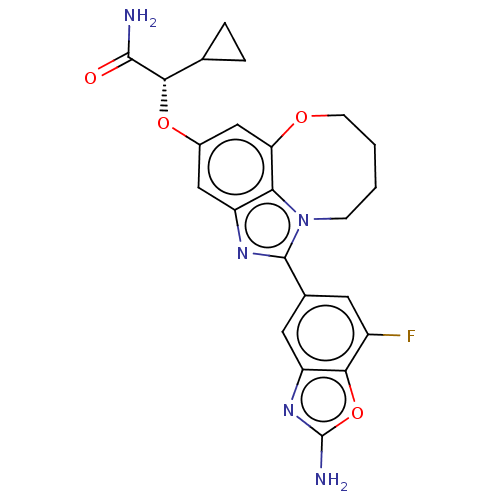
((S)-2-((1-(2-amino-7- fluorobenzo[d]oxazol- 5-yl)-...)Show SMILES NC(=O)[C@@H](Oc1cc2OCCCCn3c(nc(c1)c23)-c1cc(F)c2oc(N)nc2c1)C1CC1 |r| Show InChI InChI=1S/C23H22FN5O4/c24-14-7-12(8-16-20(14)33-23(26)28-16)22-27-15-9-13(32-19(21(25)30)11-3-4-11)10-17-18(15)29(22)5-1-2-6-31-17/h7-11,19H,1-6H2,(H2,25,30)(H2,26,28)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... |
US Patent US10065970 (2018)
BindingDB Entry DOI: 10.7270/Q27P91FB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM272944
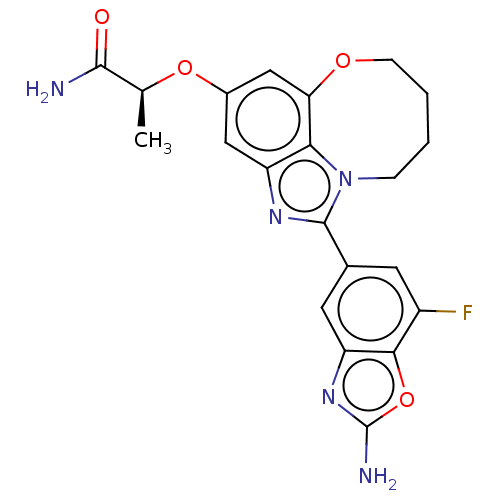
((S)-2-((1-(2-amino-7- fluorobenzo[d]oxazol- 5-yl)-...)Show SMILES C[C@H](Oc1cc2OCCCCn3c(nc(c1)c23)-c1cc(F)c2oc(N)nc2c1)C(N)=O |r| Show InChI InChI=1S/C21H20FN5O4/c1-10(19(23)28)30-12-8-14-17-16(9-12)29-5-3-2-4-27(17)20(25-14)11-6-13(22)18-15(7-11)26-21(24)31-18/h6-10H,2-5H2,1H3,(H2,23,28)(H2,24,26)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... |
US Patent US10065970 (2018)
BindingDB Entry DOI: 10.7270/Q27P91FB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM272945
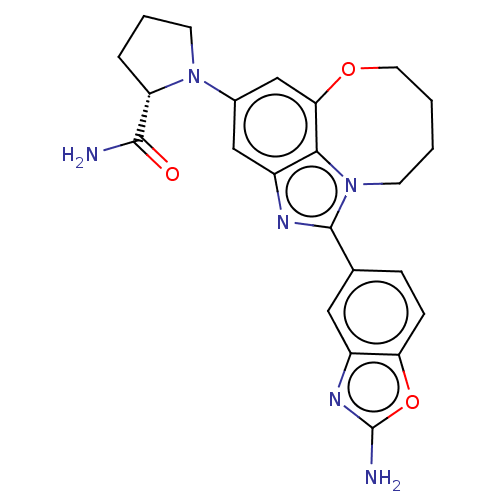
((S)-1-(1-(2- aminobenzo[d]oxazol- 5-yl)-7,8,9,10- ...)Show SMILES NC(=O)[C@@H]1CCCN1c1cc2OCCCCn3c(nc(c1)c23)-c1ccc2oc(N)nc2c1 |r| Show InChI InChI=1S/C23H24N6O3/c24-21(30)17-4-3-8-28(17)14-11-16-20-19(12-14)31-9-2-1-7-29(20)22(26-16)13-5-6-18-15(10-13)27-23(25)32-18/h5-6,10-12,17H,1-4,7-9H2,(H2,24,30)(H2,25,27)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... |
US Patent US10065970 (2018)
BindingDB Entry DOI: 10.7270/Q27P91FB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM272949
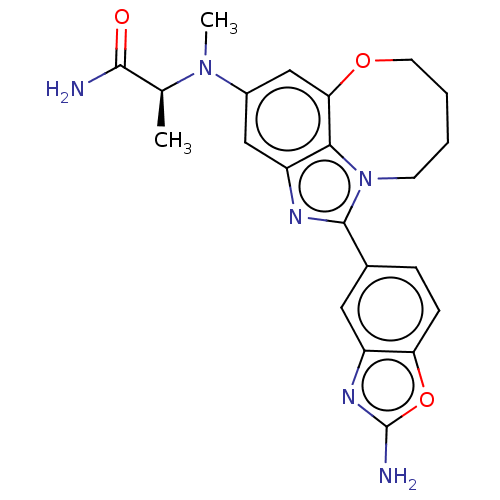
((S)-2-((1-(2- aminobenzo[d]oxazol- 5-yl)-7,8,9,10-...)Show SMILES C[C@H](N(C)c1cc2OCCCCn3c(nc(c1)c23)-c1ccc2oc(N)nc2c1)C(N)=O |r| Show InChI InChI=1S/C22H24N6O3/c1-12(20(23)29)27(2)14-10-16-19-18(11-14)30-8-4-3-7-28(19)21(25-16)13-5-6-17-15(9-13)26-22(24)31-17/h5-6,9-12H,3-4,7-8H2,1-2H3,(H2,23,29)(H2,24,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... |
US Patent US10065970 (2018)
BindingDB Entry DOI: 10.7270/Q27P91FB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM272956
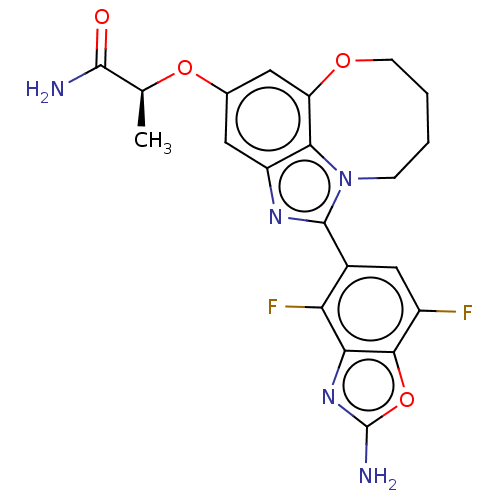
((S)-2-((1-(2-amino-4,7- difluorobenzo[d]oxazol- 5-...)Show SMILES C[C@H](Oc1cc2OCCCCn3c(nc(c1)c23)-c1cc(F)c2oc(N)nc2c1F)C(N)=O |r| Show InChI InChI=1S/C21H19F2N5O4/c1-9(19(24)29)31-10-6-13-17-14(7-10)30-5-3-2-4-28(17)20(26-13)11-8-12(22)18-16(15(11)23)27-21(25)32-18/h6-9H,2-5H2,1H3,(H2,24,29)(H2,25,27)/t9-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... |
US Patent US10065970 (2018)
BindingDB Entry DOI: 10.7270/Q27P91FB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM272959
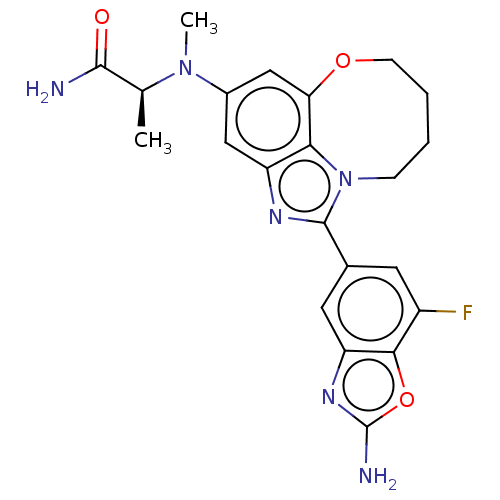
((S)-2-((1-(2-amino-7- fluorobenzo[d]oxazol- 5-yl)-...)Show SMILES C[C@H](N(C)c1cc2OCCCCn3c(nc(c1)c23)-c1cc(F)c2oc(N)nc2c1)C(N)=O |r| Show InChI InChI=1S/C22H23FN6O3/c1-11(20(24)30)28(2)13-9-15-18-17(10-13)31-6-4-3-5-29(18)21(26-15)12-7-14(23)19-16(8-12)27-22(25)32-19/h7-11H,3-6H2,1-2H3,(H2,24,30)(H2,25,27)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... |
US Patent US10065970 (2018)
BindingDB Entry DOI: 10.7270/Q27P91FB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM272960
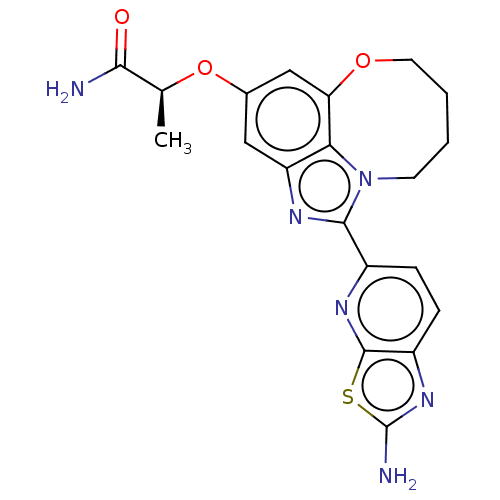
((S)-2-((1-(2- aminothiazolo[5,4- b]pyridin-5-yl)- ...)Show SMILES C[C@H](Oc1cc2OCCCCn3c(nc(c1)c23)-c1ccc2nc(N)sc2n1)C(N)=O |r| Show InChI InChI=1S/C20H20N6O3S/c1-10(17(21)27)29-11-8-14-16-15(9-11)28-7-3-2-6-26(16)18(23-14)12-4-5-13-19(24-12)30-20(22)25-13/h4-5,8-10H,2-3,6-7H2,1H3,(H2,21,27)(H2,22,25)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... |
US Patent US10065970 (2018)
BindingDB Entry DOI: 10.7270/Q27P91FB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM272964
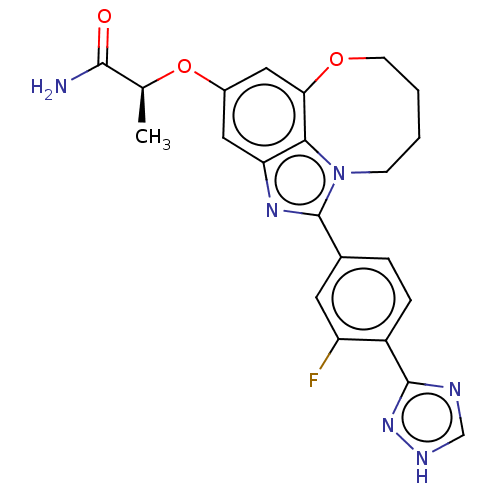
((S)-2-((1-(3-fluoro-4- (1H-1,2,4-triazol-3- yl)phe...)Show SMILES C[C@H](Oc1cc2OCCCCn3c(nc(c1)c23)-c1ccc(-c2nc[nH]n2)c(F)c1)C(N)=O |r| Show InChI InChI=1S/C22H21FN6O3/c1-12(20(24)30)32-14-9-17-19-18(10-14)31-7-3-2-6-29(19)22(27-17)13-4-5-15(16(23)8-13)21-25-11-26-28-21/h4-5,8-12H,2-3,6-7H2,1H3,(H2,24,30)(H,25,26,28)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... |
US Patent US10065970 (2018)
BindingDB Entry DOI: 10.7270/Q27P91FB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM272963
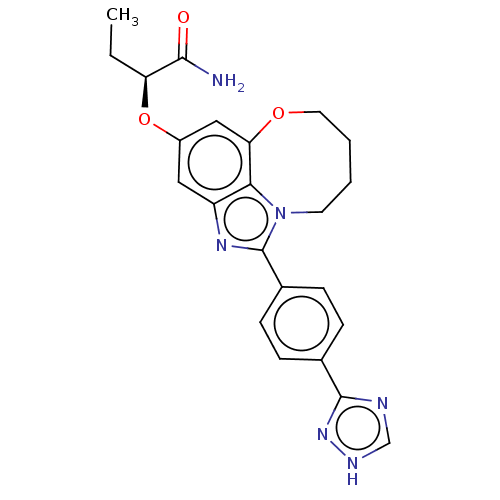
((S)-2-((1-(4-(1H-1,2,4- triazol-3-yl)phenyl)- 7,8,...)Show SMILES CC[C@H](Oc1cc2OCCCCn3c(nc(c1)c23)-c1ccc(cc1)-c1nc[nH]n1)C(N)=O |r| Show InChI InChI=1S/C23H24N6O3/c1-2-18(21(24)30)32-16-11-17-20-19(12-16)31-10-4-3-9-29(20)23(27-17)15-7-5-14(6-8-15)22-25-13-26-28-22/h5-8,11-13,18H,2-4,9-10H2,1H3,(H2,24,30)(H,25,26,28)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... |
US Patent US10065970 (2018)
BindingDB Entry DOI: 10.7270/Q27P91FB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM272988
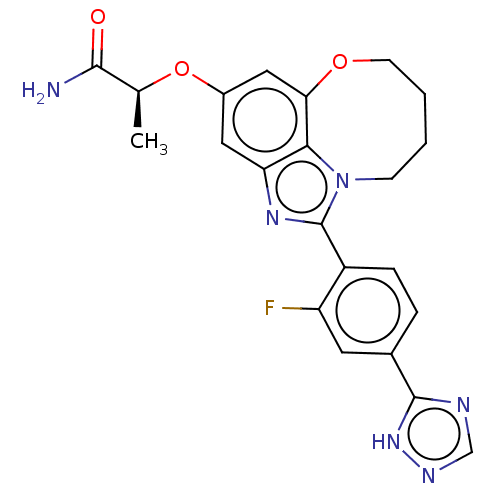
((S)-2-((1-(2-fluoro-4- (1H-1,2,4-triazol-5- yl)phe...)Show SMILES C[C@H](Oc1cc2OCCCCn3c(nc(c1)c23)-c1ccc(cc1F)-c1ncn[nH]1)C(N)=O |r| Show InChI InChI=1S/C22H21FN6O3/c1-12(20(24)30)32-14-9-17-19-18(10-14)31-7-3-2-6-29(19)22(27-17)15-5-4-13(8-16(15)23)21-25-11-26-28-21/h4-5,8-12H,2-3,6-7H2,1H3,(H2,24,30)(H,25,26,28)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... |
US Patent US10065970 (2018)
BindingDB Entry DOI: 10.7270/Q27P91FB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM295679
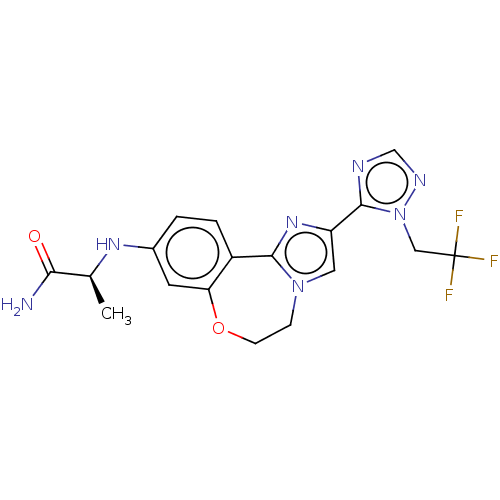
(US10851091, U.S. Pat. No. 8,242,104 No. 529 | US82...)Show SMILES C[C@H](Nc1ccc2-c3nc(cn3CCOc2c1)-c1ncnn1CC(F)(F)F)C(N)=O |r| Show InChI InChI=1S/C18H18F3N7O2/c1-10(15(22)29)25-11-2-3-12-14(6-11)30-5-4-27-7-13(26-16(12)27)17-23-9-24-28(17)8-18(19,20)21/h2-3,6-7,9-10,25H,4-5,8H2,1H3,(H2,22,29)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ... |
US Patent US10851091 (2020)
BindingDB Entry DOI: 10.7270/Q2WS8X9V |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM272987
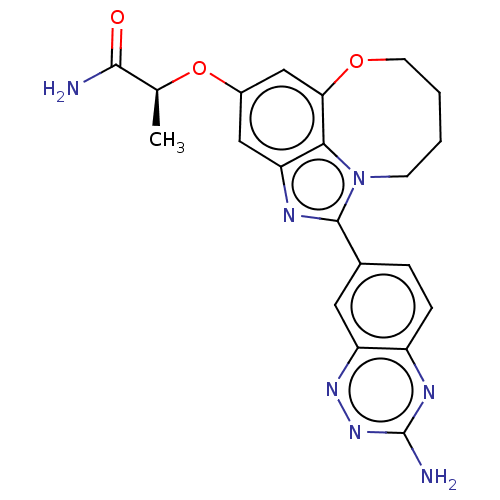
((S)-2-((1-(3- aminobenzo[e][1,2,4]tri- azin-7-yl)-...)Show SMILES C[C@H](Oc1cc2OCCCCn3c(nc(c1)c23)-c1ccc2nc(N)nnc2c1)C(N)=O |r| Show InChI InChI=1S/C21H21N7O3/c1-11(19(22)29)31-13-9-16-18-17(10-13)30-7-3-2-6-28(18)20(24-16)12-4-5-14-15(8-12)26-27-21(23)25-14/h4-5,8-11H,2-3,6-7H2,1H3,(H2,22,29)(H2,23,25,27)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... |
US Patent US10065970 (2018)
BindingDB Entry DOI: 10.7270/Q27P91FB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM272942
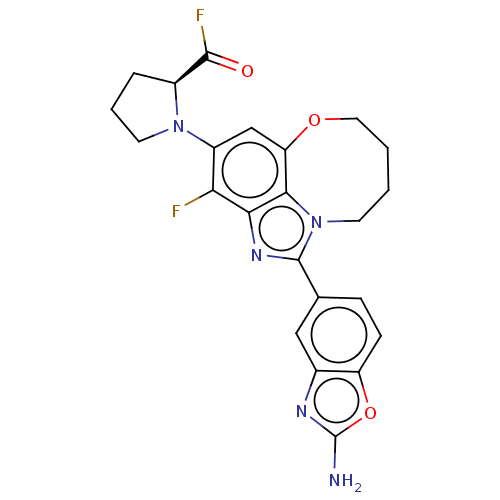
((S)-1-(1-(2- aminobenzo[d]oxazol- 5-yl)-3-fluoro-7...)Show SMILES Nc1nc2cc(ccc2o1)-c1nc2c(F)c(cc3OCCCCn1c23)N1CCC[C@H]1C(F)=O |r| Show InChI InChI=1S/C23H21F2N5O3/c24-18-15(29-8-3-4-14(29)21(25)31)11-17-20-19(18)28-22(30(20)7-1-2-9-32-17)12-5-6-16-13(10-12)27-23(26)33-16/h5-6,10-11,14H,1-4,7-9H2,(H2,26,27)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... |
US Patent US10065970 (2018)
BindingDB Entry DOI: 10.7270/Q27P91FB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093436
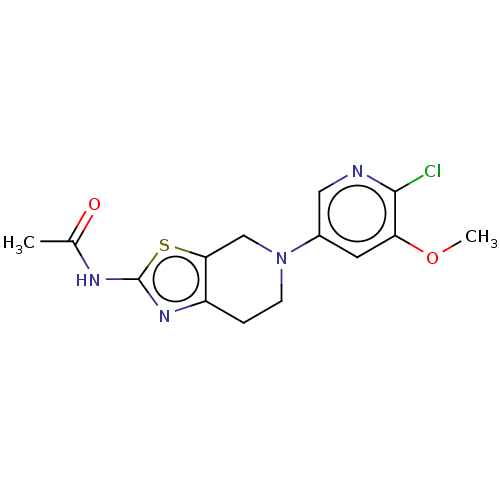
(CHEMBL3586669)Show InChI InChI=1S/C22H18O12/c23-13-5-1-11(9-15(13)25)3-7-17(27)33-19(21(29)30)20(22(31)32)34-18(28)8-4-12-2-6-14(24)16(26)10-12/h1-10,19-20,23-26H,(H,29,30)(H,31,32)/p-2/b7-3+,8-4+/t19-,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50093353
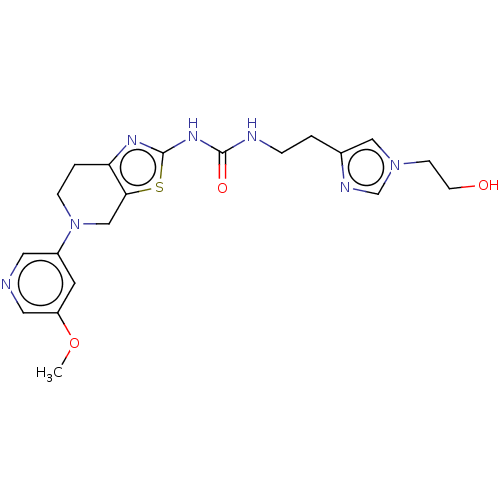
(CHEMBL3586680)Show SMILES COc1cncc(c1)N1CCc2nc(NC(=O)NCCc3cn(CCO)cn3)sc2C1 Show InChI InChI=1S/C20H25N7O3S/c1-30-16-8-15(9-21-10-16)27-5-3-17-18(12-27)31-20(24-17)25-19(29)22-4-2-14-11-26(6-7-28)13-23-14/h8-11,13,28H,2-7,12H2,1H3,(H2,22,24,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using [33P]ATP and PIP2 incubated for 15 mins by liquid scintillation counting method |
J Med Chem 58: 5684-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00498
BindingDB Entry DOI: 10.7270/Q2Z89F53 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM272889
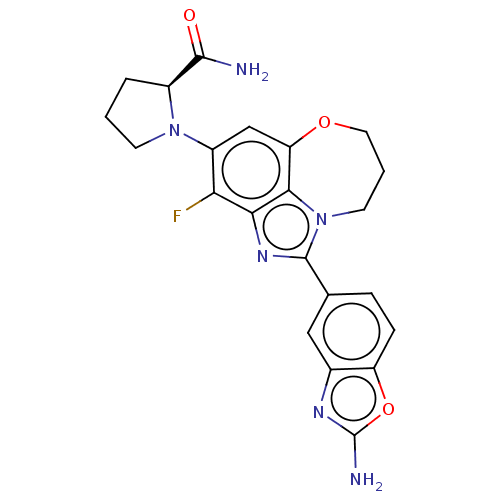
((S)-1-(1-(2- aminobenzo[d]oxazol- 5-yl)-3-fluoro-8...)Show SMILES NC(=O)[C@@H]1CCCN1c1cc2OCCCn3c(nc(c1F)c23)-c1ccc2oc(N)nc2c1 |r| Show InChI InChI=1S/C22H21FN6O3/c23-17-14(28-6-1-3-13(28)20(24)30)10-16-19-18(17)27-21(29(19)7-2-8-31-16)11-4-5-15-12(9-11)26-22(25)32-15/h4-5,9-10,13H,1-3,6-8H2,(H2,24,30)(H2,25,26)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... |
US Patent US10065970 (2018)
BindingDB Entry DOI: 10.7270/Q27P91FB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602306
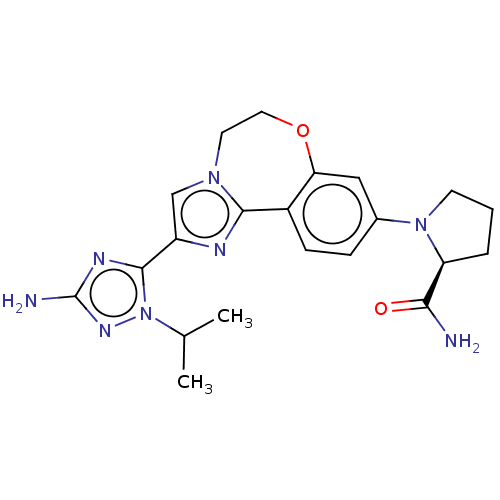
(CHEMBL5208487)Show SMILES CC(C)n1nc(N)nc1-c1cn2CCOc3cc(ccc3-c2n1)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM272976
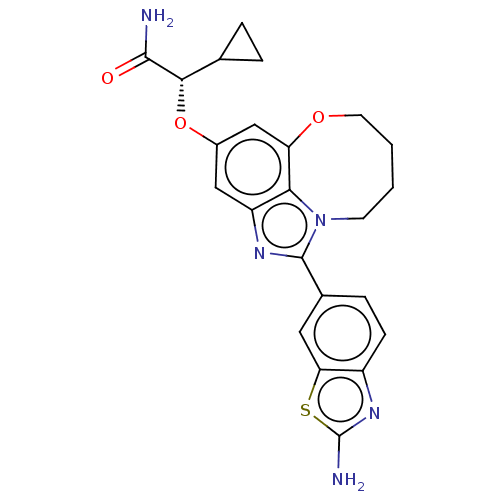
((S)-2-((1-(2- aminobenzo[d]thiazol- 6-yl)-7,8,9,10...)Show SMILES NC(=O)[C@@H](Oc1cc2OCCCCn3c(nc(c1)c23)-c1ccc2nc(N)sc2c1)C1CC1 |r| Show InChI InChI=1S/C23H23N5O3S/c24-21(29)20(12-3-4-12)31-14-10-16-19-17(11-14)30-8-2-1-7-28(19)22(26-16)13-5-6-15-18(9-13)32-23(25)27-15/h5-6,9-12,20H,1-4,7-8H2,(H2,24,29)(H2,25,27)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... |
US Patent US10065970 (2018)
BindingDB Entry DOI: 10.7270/Q27P91FB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM272943
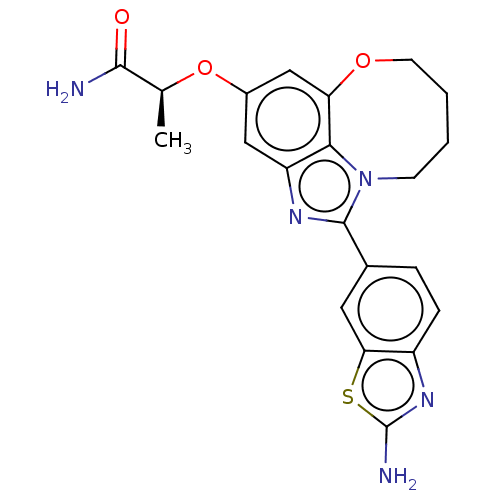
((S)-2-((1-(2- aminobenzo[d]thiazol- 6-yl)-7,8,9,10...)Show SMILES C[C@H](Oc1cc2OCCCCn3c(nc(c1)c23)-c1ccc2nc(N)sc2c1)C(N)=O |r| Show InChI InChI=1S/C21H21N5O3S/c1-11(19(22)27)29-13-9-15-18-16(10-13)28-7-3-2-6-26(18)20(24-15)12-4-5-14-17(8-12)30-21(23)25-14/h4-5,8-11H,2-3,6-7H2,1H3,(H2,22,27)(H2,23,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform... |
US Patent US10065970 (2018)
BindingDB Entry DOI: 10.7270/Q27P91FB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50149477

(CHEMBL3770993 | US10851091, U.S. Pat. No. 8,242,10...)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ccc3-c2n1)N1CCC[C@H]1C(N)=O |r| Show InChI InChI=1S/C21H25N7O2/c1-13(2)28-21(23-12-24-28)16-11-26-8-9-30-18-10-14(5-6-15(18)20(26)25-16)27-7-3-4-17(27)19(22)29/h5-6,10-13,17H,3-4,7-9H2,1-2H3,(H2,22,29)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K alpha (unknown origin) using PIP2 as substrate by fluorescence polarization assay |
J Med Chem 59: 985-1002 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01483
BindingDB Entry DOI: 10.7270/Q2QF8VRV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data