

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50570996 (CHEMBL4864797) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human CA2 by stopped-flow assay | Citation and Details Article DOI: 10.1016/j.bmc.2021.116140 BindingDB Entry DOI: 10.7270/Q2TM7FWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50252992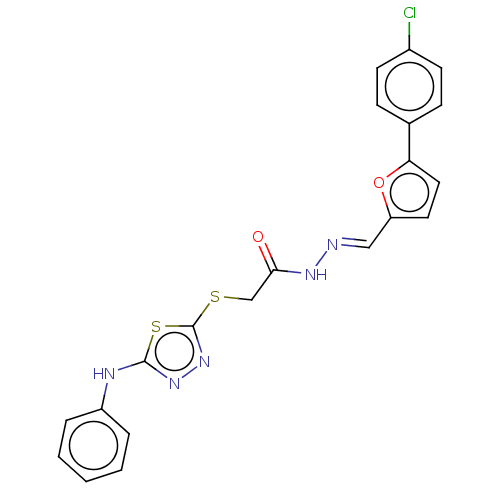 (CHEMBL4100496) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50252998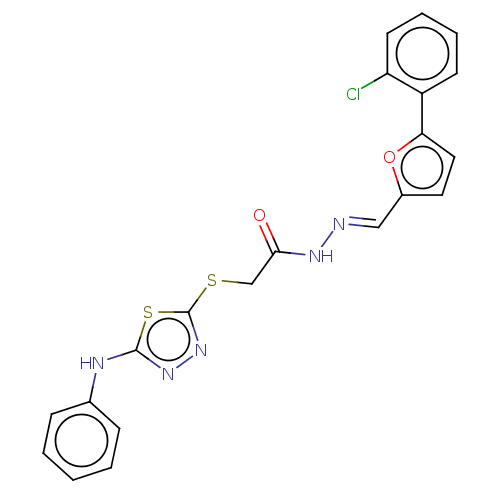 (CHEMBL4105008) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50252992 (CHEMBL4100496) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50252993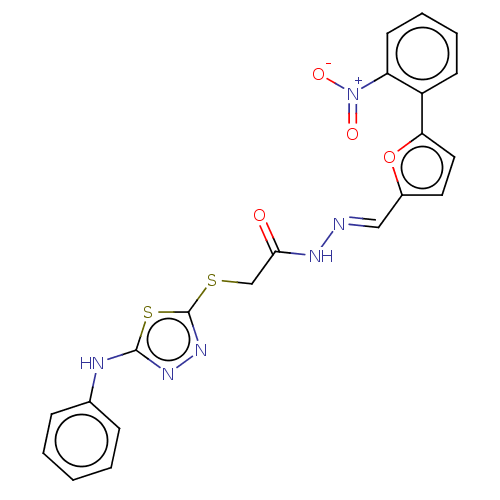 (CHEMBL4073678) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10884 ((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory activity against Human Carbonic anhydrase II (HCA II) | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM10881 (CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 9 after 15 mins by stopped flow CO2 hydration assay | Eur J Med Chem 62: 597-604 (2013) Article DOI: 10.1016/j.ejmech.2013.01.030 BindingDB Entry DOI: 10.7270/Q2X92CNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50252997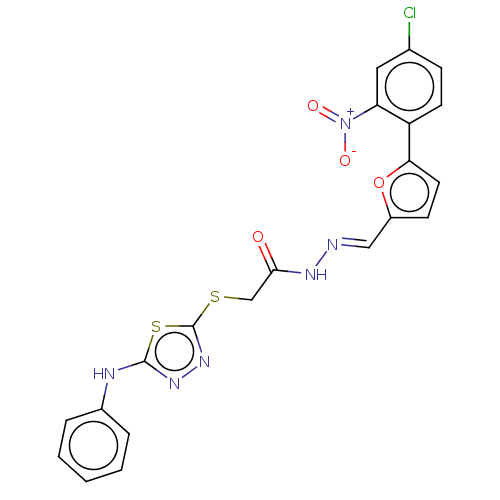 (CHEMBL4098116) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50258992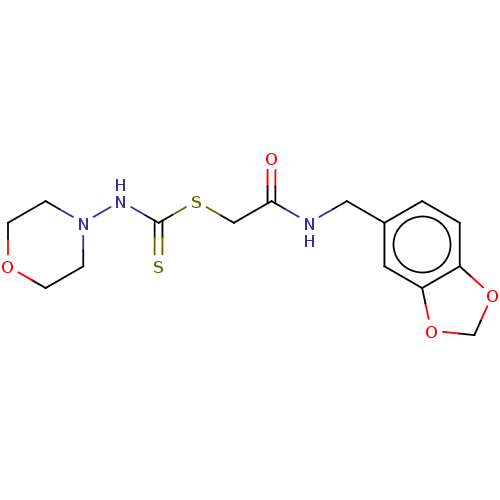 (CHEMBL4081323) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.287 | n/a | n/a | n/a | n/a | n/a | n/a |
Anadolu University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte carbonic anhydrase-2 | Eur J Med Chem 125: 190-196 (2017) Article DOI: 10.1016/j.ejmech.2016.09.035 BindingDB Entry DOI: 10.7270/Q2542R29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50258998 (CHEMBL4099344) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.288 | n/a | n/a | n/a | n/a | n/a | n/a |
Anadolu University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte carbonic anhydrase-1 | Eur J Med Chem 125: 190-196 (2017) Article DOI: 10.1016/j.ejmech.2016.09.035 BindingDB Entry DOI: 10.7270/Q2542R29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50252994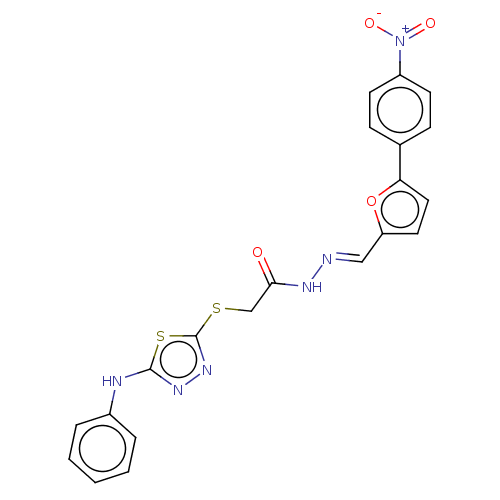 (CHEMBL4082551) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50252996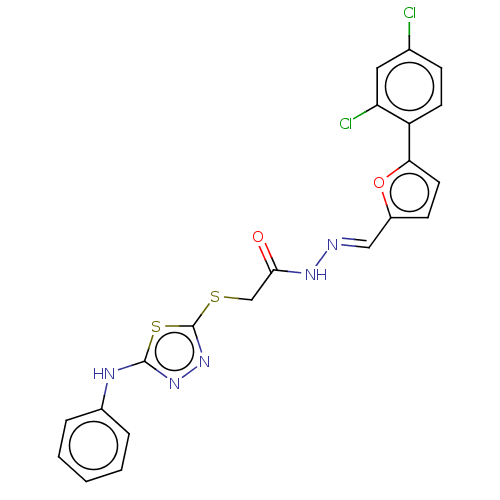 (CHEMBL4097972) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10882 (6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of full length carbonic anhydrase-2 in human erythrocytes | Bioorg Med Chem Lett 23: 3496-9 (2013) Article DOI: 10.1016/j.bmcl.2013.04.048 BindingDB Entry DOI: 10.7270/Q2GH9KFD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50258998 (CHEMBL4099344) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.338 | n/a | n/a | n/a | n/a | n/a | n/a |
Anadolu University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte carbonic anhydrase-2 | Eur J Med Chem 125: 190-196 (2017) Article DOI: 10.1016/j.ejmech.2016.09.035 BindingDB Entry DOI: 10.7270/Q2542R29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50252993 (CHEMBL4073678) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50258992 (CHEMBL4081323) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.346 | n/a | n/a | n/a | n/a | n/a | n/a |
Anadolu University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte carbonic anhydrase-1 | Eur J Med Chem 125: 190-196 (2017) Article DOI: 10.1016/j.ejmech.2016.09.035 BindingDB Entry DOI: 10.7270/Q2542R29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM222054 ((3aR,4S,7R,7aS)-2-(4-((E)-3-(4-bromophenyl)acryloy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.245 | -54.9 | 0.352 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Cumhuriyet University | Assay Description Esterase activity assay was performed based on the method of by Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] as described in pr... | Bioorg Chem 70: 118-125 (2017) Article DOI: 10.1016/j.bioorg.2016.12.001 BindingDB Entry DOI: 10.7270/Q2CF9NZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM222050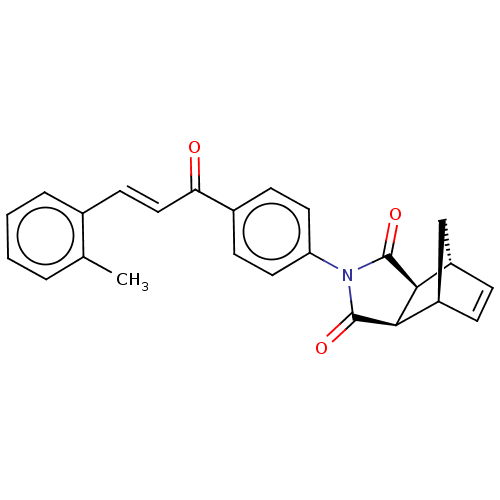 ((3aR,4S,7R,7aS)-2-(4-((E)-3-(o-tolyl)acryloyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.302 | -54.3 | 0.353 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Cumhuriyet University | Assay Description Esterase activity assay was performed based on the method of by Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] as described in pr... | Bioorg Chem 70: 118-125 (2017) Article DOI: 10.1016/j.bioorg.2016.12.001 BindingDB Entry DOI: 10.7270/Q2CF9NZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM222046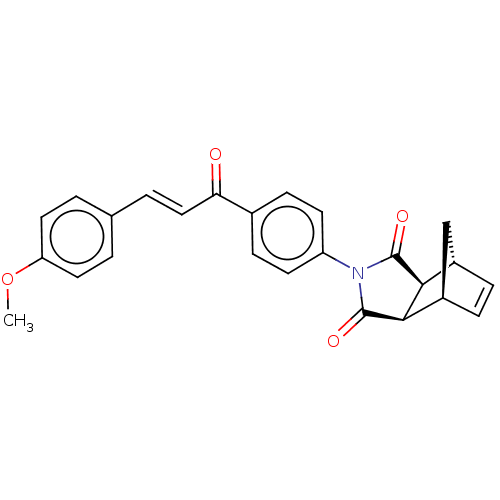 ((3aR,4S,7R,7aS)-2-(4-((E)-3-(4-methoxyphenyl)acryl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.277 | -54.5 | 0.356 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Cumhuriyet University | Assay Description Esterase activity assay was performed based on the method of by Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] as described in pr... | Bioorg Chem 70: 118-125 (2017) Article DOI: 10.1016/j.bioorg.2016.12.001 BindingDB Entry DOI: 10.7270/Q2CF9NZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM222051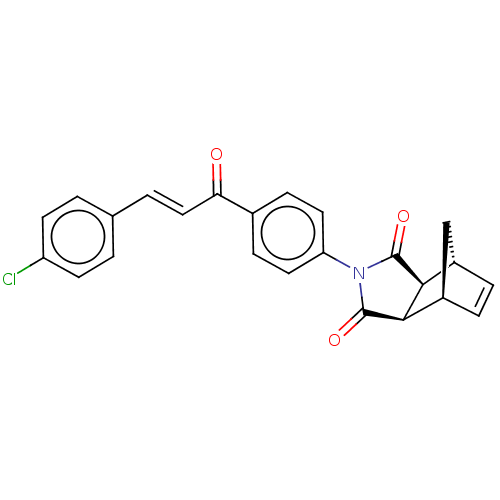 ((3aR,4S,7R,7aS)-2-(4-((E)-3-(4-chlorophenyl)acrylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.258 | -54.7 | 0.373 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Cumhuriyet University | Assay Description Esterase activity assay was performed based on the method of by Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] as described in pr... | Bioorg Chem 70: 118-125 (2017) Article DOI: 10.1016/j.bioorg.2016.12.001 BindingDB Entry DOI: 10.7270/Q2CF9NZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM222055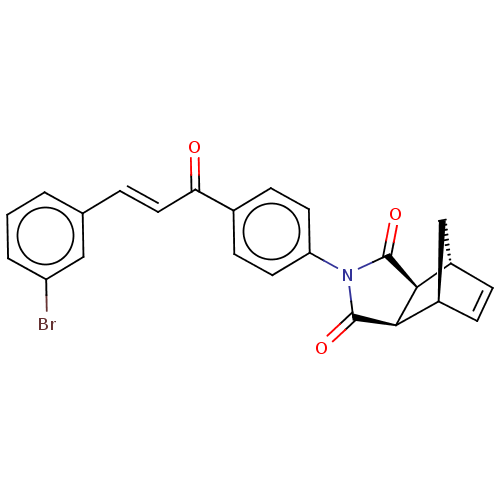 ((3aR,4S,7R,7aS)-2-(4-((E)-3-(3-bromophenyl)acryloy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.343 | -54.0 | 0.382 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Cumhuriyet University | Assay Description Esterase activity assay was performed based on the method of by Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] as described in pr... | Bioorg Chem 70: 118-125 (2017) Article DOI: 10.1016/j.bioorg.2016.12.001 BindingDB Entry DOI: 10.7270/Q2CF9NZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50252996 (CHEMBL4097972) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50515806 (CHEMBL4533252) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of Protein kinase C alpha expressed in Sf-9 cells | Eur J Med Chem 162: 679-734 (2019) Article DOI: 10.1016/j.ejmech.2018.11.017 BindingDB Entry DOI: 10.7270/Q21839DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM222048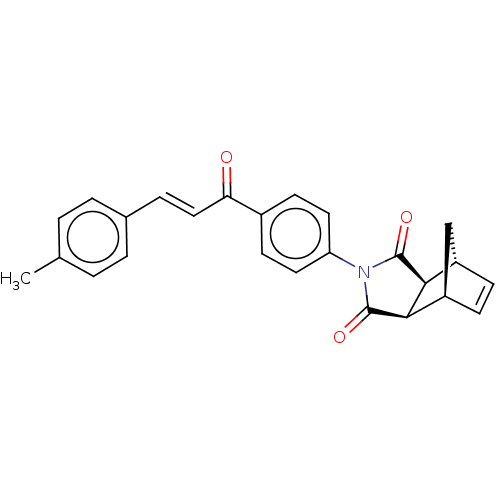 ((3aR,4S,7R,7aS)-2-(4-((E)-3-(p-tolyl)acryloyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | -53.6 | 0.406 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Cumhuriyet University | Assay Description Esterase activity assay was performed based on the method of by Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] as described in pr... | Bioorg Chem 70: 118-125 (2017) Article DOI: 10.1016/j.bioorg.2016.12.001 BindingDB Entry DOI: 10.7270/Q2CF9NZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50252995 (CHEMBL4075535) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM222056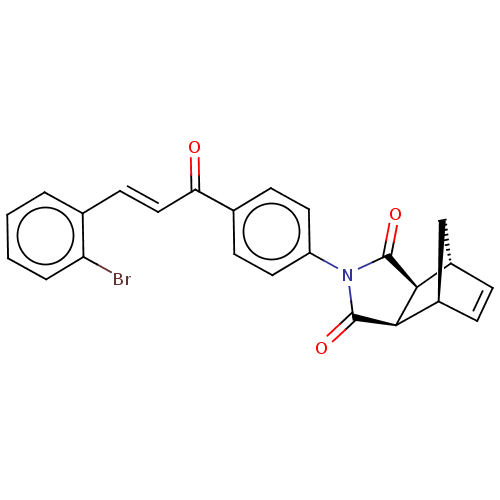 ((3aR,4S,7R,7aS)-2-(4-((E)-3-(2-bromophenyl)acryloy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.379 | -53.8 | 0.415 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Cumhuriyet University | Assay Description Esterase activity assay was performed based on the method of by Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] as described in pr... | Bioorg Chem 70: 118-125 (2017) Article DOI: 10.1016/j.bioorg.2016.12.001 BindingDB Entry DOI: 10.7270/Q2CF9NZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM222052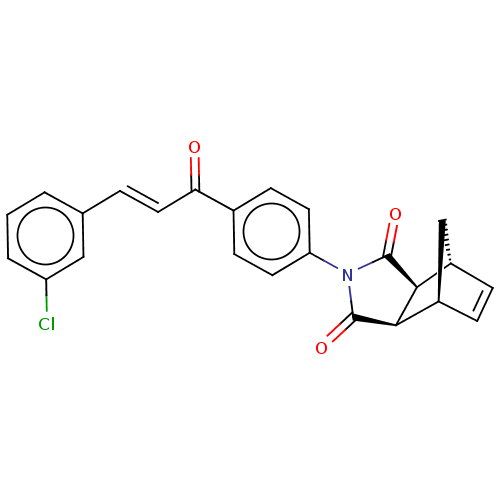 ((3aR,4S,7R,7aS)-2-(4-((E)-3-(3-chlorophenyl)acrylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.361 | -53.9 | 0.419 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Cumhuriyet University | Assay Description Esterase activity assay was performed based on the method of by Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] as described in pr... | Bioorg Chem 70: 118-125 (2017) Article DOI: 10.1016/j.bioorg.2016.12.001 BindingDB Entry DOI: 10.7270/Q2CF9NZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM222047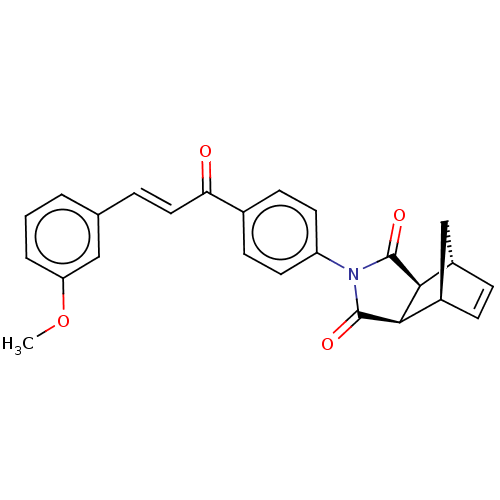 ((3aR,4S,7R,7aS)-2-(4-((E)-3-(3-methoxyphenyl)acryl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.337 | -54.1 | 0.435 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Cumhuriyet University | Assay Description Esterase activity assay was performed based on the method of by Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] as described in pr... | Bioorg Chem 70: 118-125 (2017) Article DOI: 10.1016/j.bioorg.2016.12.001 BindingDB Entry DOI: 10.7270/Q2CF9NZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM222053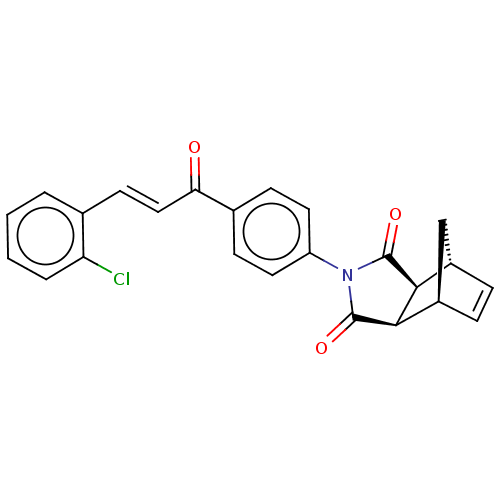 ((3aR,4S,7R,7aS)-2-(4-((E)-3-(2-chlorophenyl)acrylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.377 | -53.8 | 0.444 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Cumhuriyet University | Assay Description Esterase activity assay was performed based on the method of by Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] as described in pr... | Bioorg Chem 70: 118-125 (2017) Article DOI: 10.1016/j.bioorg.2016.12.001 BindingDB Entry DOI: 10.7270/Q2CF9NZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM222049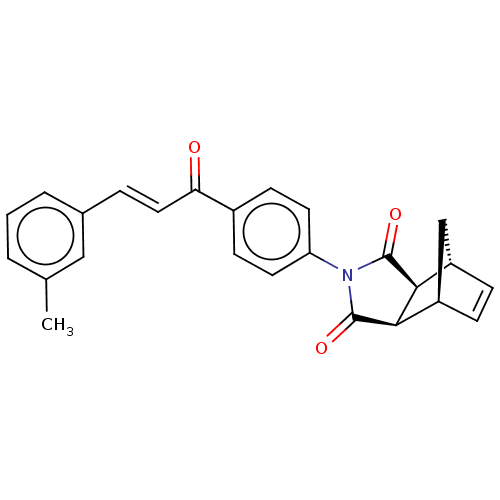 ((3aR,4S,7R,7aS)-2-(4-((E)-3-(m-tolyl)acryloyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.404 | -53.6 | 0.449 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Cumhuriyet University | Assay Description Esterase activity assay was performed based on the method of by Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] as described in pr... | Bioorg Chem 70: 118-125 (2017) Article DOI: 10.1016/j.bioorg.2016.12.001 BindingDB Entry DOI: 10.7270/Q2CF9NZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50253005 (CHEMBL4076887) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50537511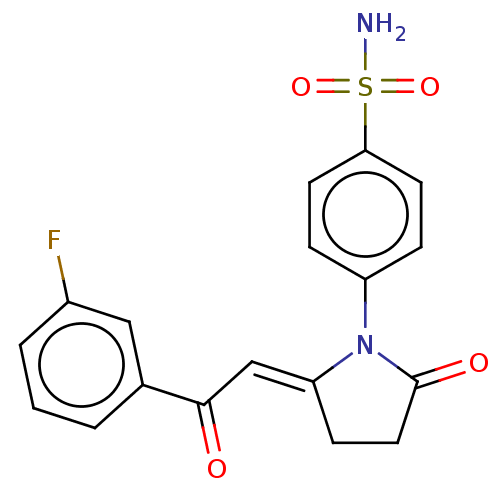 (CHEMBL4633178) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of Protein kinase C eta expressed in Sf-9 cells | Eur J Med Chem 162: 679-734 (2019) Article DOI: 10.1016/j.ejmech.2018.11.017 BindingDB Entry DOI: 10.7270/Q21839DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50537510 (CHEMBL4644942) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology Curated by ChEMBL | Assay Description Inhibition of Protein kinase C eta expressed in Sf-9 cells | Eur J Med Chem 162: 679-734 (2019) Article DOI: 10.1016/j.ejmech.2018.11.017 BindingDB Entry DOI: 10.7270/Q21839DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM222054 ((3aR,4S,7R,7aS)-2-(4-((E)-3-(4-bromophenyl)acryloy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.442 | -53.4 | 0.466 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Cumhuriyet University | Assay Description Esterase activity assay was performed based on the method of by Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] as described in pr... | Bioorg Chem 70: 118-125 (2017) Article DOI: 10.1016/j.bioorg.2016.12.001 BindingDB Entry DOI: 10.7270/Q2CF9NZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50253004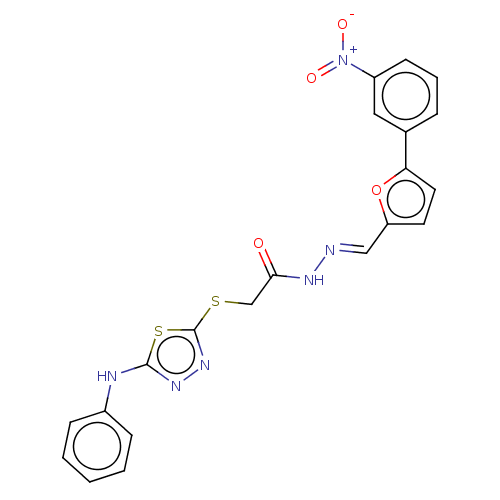 (CHEMBL4072122) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50430551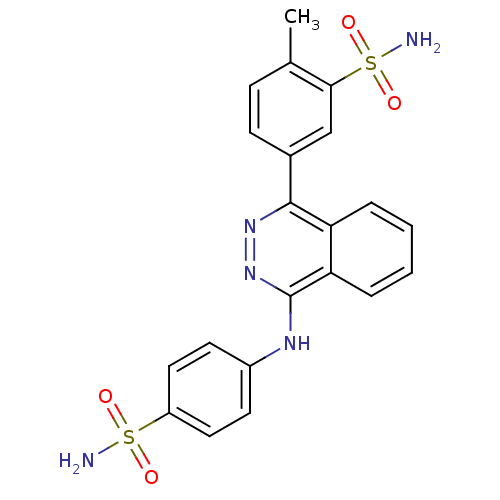 (CHEMBL2336905) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 9 after 15 mins by stopped flow CO2 hydration assay | Eur J Med Chem 62: 597-604 (2013) Article DOI: 10.1016/j.ejmech.2013.01.030 BindingDB Entry DOI: 10.7270/Q2X92CNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 0.343 | -54.0 | 0.485 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Cumhuriyet University | Assay Description Esterase activity assay was performed based on the method of by Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] as described in pr... | Bioorg Chem 70: 118-125 (2017) Article DOI: 10.1016/j.bioorg.2016.12.001 BindingDB Entry DOI: 10.7270/Q2CF9NZB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM222058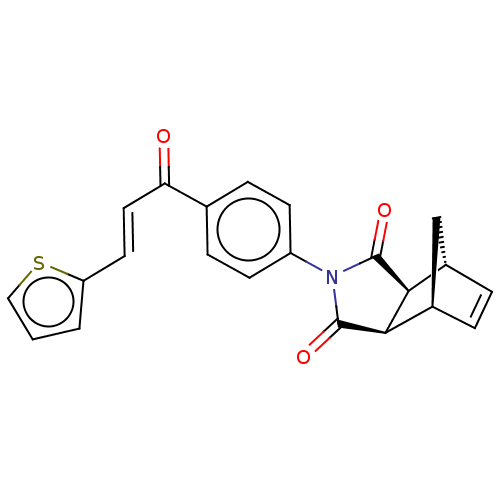 ((3aR,4S,7R,7aS)-2-(4-((E)-3-(thiophen-2-yl)acryloy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.412 | -53.6 | 0.488 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Cumhuriyet University | Assay Description Esterase activity assay was performed based on the method of by Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] as described in pr... | Bioorg Chem 70: 118-125 (2017) Article DOI: 10.1016/j.bioorg.2016.12.001 BindingDB Entry DOI: 10.7270/Q2CF9NZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM222051 ((3aR,4S,7R,7aS)-2-(4-((E)-3-(4-chlorophenyl)acrylo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.475 | -53.2 | 0.506 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Cumhuriyet University | Assay Description Esterase activity assay was performed based on the method of by Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] as described in pr... | Bioorg Chem 70: 118-125 (2017) Article DOI: 10.1016/j.bioorg.2016.12.001 BindingDB Entry DOI: 10.7270/Q2CF9NZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50258995 (CHEMBL4060713) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.518 | n/a | n/a | n/a | n/a | n/a | n/a |
Anadolu University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte carbonic anhydrase-1 | Eur J Med Chem 125: 190-196 (2017) Article DOI: 10.1016/j.ejmech.2016.09.035 BindingDB Entry DOI: 10.7270/Q2542R29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50252991 (CHEMBL4064130) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-2 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50258993 (CHEMBL4062231) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.535 | n/a | n/a | n/a | n/a | n/a | n/a |
Anadolu University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte carbonic anhydrase-1 | Eur J Med Chem 125: 190-196 (2017) Article DOI: 10.1016/j.ejmech.2016.09.035 BindingDB Entry DOI: 10.7270/Q2542R29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM222050 ((3aR,4S,7R,7aS)-2-(4-((E)-3-(o-tolyl)acryloyl)phen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.529 | -52.9 | 0.537 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Cumhuriyet University | Assay Description Esterase activity assay was performed based on the method of by Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] as described in pr... | Bioorg Chem 70: 118-125 (2017) Article DOI: 10.1016/j.bioorg.2016.12.001 BindingDB Entry DOI: 10.7270/Q2CF9NZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50041029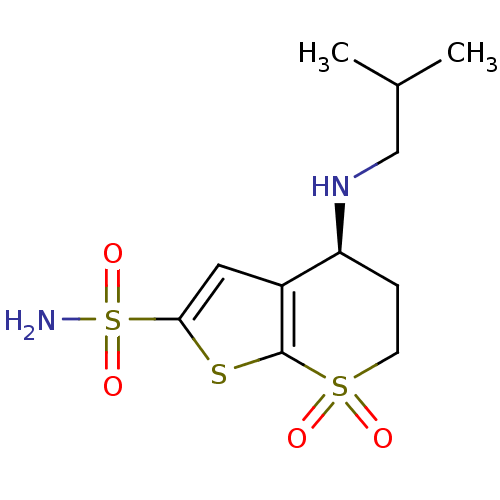 ((S)-4-Isobutylamino-7,7-dioxo-4,5,6,7-tetrahydro-7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the inhibitory activity against Human Carbonic anhydrase II (HCA II) | J Med Chem 37: 1035-54 (1994) BindingDB Entry DOI: 10.7270/Q26Q1XWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM222059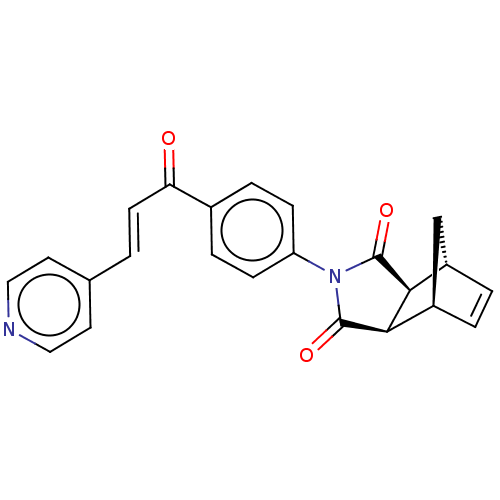 ((3aR,4S,7R,7aS)-2-(4-((E)-3-(pyridin-4-yl)acryloyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.490 | -53.1 | 0.551 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Cumhuriyet University | Assay Description Esterase activity assay was performed based on the method of by Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] as described in pr... | Bioorg Chem 70: 118-125 (2017) Article DOI: 10.1016/j.bioorg.2016.12.001 BindingDB Entry DOI: 10.7270/Q2CF9NZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM222057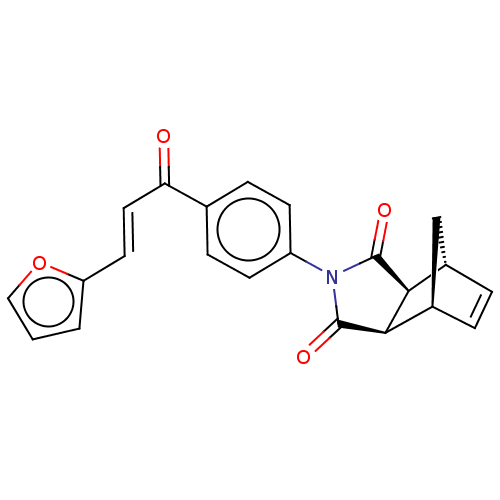 ((3aR,4S,7R,7aS)-2-(4-((E)-3-(furan-2-yl)acryloyl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.390 | -53.7 | 0.555 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Cumhuriyet University | Assay Description Esterase activity assay was performed based on the method of by Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] as described in pr... | Bioorg Chem 70: 118-125 (2017) Article DOI: 10.1016/j.bioorg.2016.12.001 BindingDB Entry DOI: 10.7270/Q2CF9NZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50258997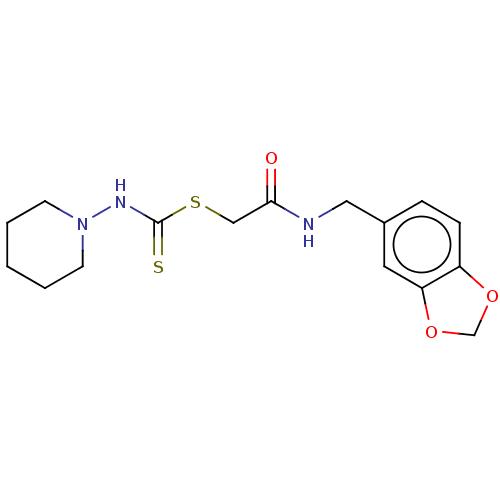 (CHEMBL4089189) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.556 | n/a | n/a | n/a | n/a | n/a | n/a |
Anadolu University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte carbonic anhydrase-1 | Eur J Med Chem 125: 190-196 (2017) Article DOI: 10.1016/j.ejmech.2016.09.035 BindingDB Entry DOI: 10.7270/Q2542R29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50253004 (CHEMBL4072122) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50252991 (CHEMBL4064130) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Anadolu University, 26470 Eski?ehir, Turkey. Electronic address: mdaltintop@anadolu.edu.tr. Curated by ChEMBL | Assay Description Inhibition of carbonic anhydrase-1 in human erythrocyte membranes assessed as reduction in H+ release using CO2 as substrate by bromine thymol blue i... | Bioorg Med Chem 25: 3547-3554 (2017) Article DOI: 10.1016/j.bmc.2017.05.005 BindingDB Entry DOI: 10.7270/Q28G8P41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM222056 ((3aR,4S,7R,7aS)-2-(4-((E)-3-(2-bromophenyl)acryloy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.405 | -53.6 | 0.573 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Cumhuriyet University | Assay Description Esterase activity assay was performed based on the method of by Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] as described in pr... | Bioorg Chem 70: 118-125 (2017) Article DOI: 10.1016/j.bioorg.2016.12.001 BindingDB Entry DOI: 10.7270/Q2CF9NZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 38851 total ) | Next | Last >> |