 Found 3000 hits Enz. Inhib. hit(s) with Target = 'Stromelysin-1'
Found 3000 hits Enz. Inhib. hit(s) with Target = 'Stromelysin-1' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Stromelysin-1
(Homo sapiens (Human)) | BDBM8465

((2R)-N-hydroxy-2-[(4-methoxybenzene)(pyridin-3-ylm...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C18H23N3O5S/c1-13(2)17(18(22)20-23)21(12-14-5-4-10-19-11-14)27(24,25)16-8-6-15(26-3)7-9-16/h4-11,13,17,23H,12H2,1-3H3,(H,20,22)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Binding affinity to stromelysin-1 catalytic domain expressed in Escherichia coli BL21 (DE3) by isothermal titration colorimetry |
Bioorg Med Chem Lett 20: 280-2 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.114
BindingDB Entry DOI: 10.7270/Q20V8DQN |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM8466
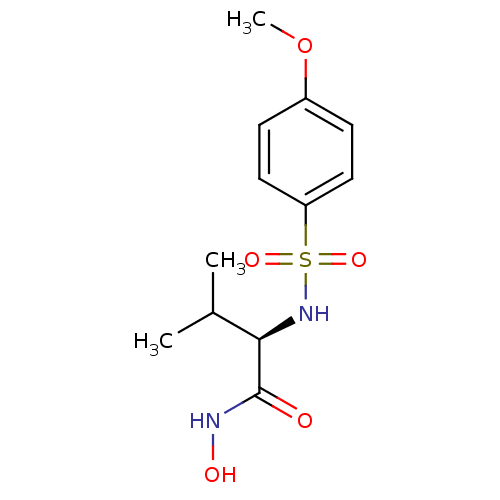
((2R)-N-hydroxy-2-[(4-methoxybenzene)sulfonamido]-3...)Show SMILES COc1ccc(cc1)S(=O)(=O)N[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C12H18N2O5S/c1-8(2)11(12(15)13-16)14-20(17,18)10-6-4-9(19-3)5-7-10/h4-8,11,14,16H,1-3H3,(H,13,15)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Binding affinity to stromelysin-1 catalytic domain expressed in Escherichia coli BL21 (DE3) by isothermal titration colorimetry |
Bioorg Med Chem Lett 20: 280-2 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.114
BindingDB Entry DOI: 10.7270/Q20V8DQN |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM8465

((2R)-N-hydroxy-2-[(4-methoxybenzene)(pyridin-3-ylm...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C18H23N3O5S/c1-13(2)17(18(22)20-23)21(12-14-5-4-10-19-11-14)27(24,25)16-8-6-15(26-3)7-9-16/h4-11,13,17,23H,12H2,1-3H3,(H,20,22)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Binding affinity to stromelysin-1 catalytic domain |
Bioorg Med Chem Lett 20: 280-2 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.114
BindingDB Entry DOI: 10.7270/Q20V8DQN |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50096645
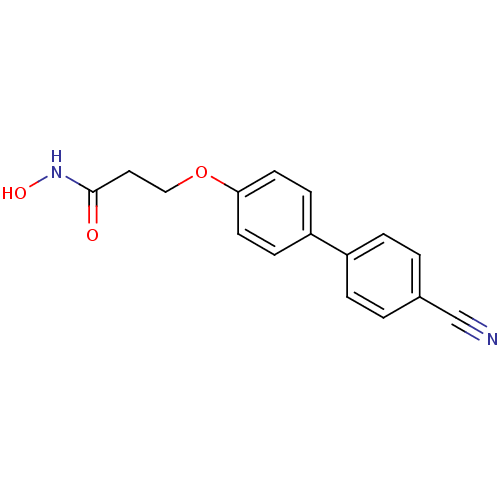
(3-(4'-Cyano-biphenyl-4-yloxy)-N-hydroxy-propionami...)Show InChI InChI=1S/C16H14N2O3/c17-11-12-1-3-13(4-2-12)14-5-7-15(8-6-14)21-10-9-16(19)18-20/h1-8,20H,9-10H2,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Binding affinity to stromelysin-1 catalytic domain |
Bioorg Med Chem Lett 20: 280-2 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.114
BindingDB Entry DOI: 10.7270/Q20V8DQN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50096645

(3-(4'-Cyano-biphenyl-4-yloxy)-N-hydroxy-propionami...)Show InChI InChI=1S/C16H14N2O3/c17-11-12-1-3-13(4-2-12)14-5-7-15(8-6-14)21-10-9-16(19)18-20/h1-8,20H,9-10H2,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Binding affinity to stromelysin-1 catalytic domain expressed in Escherichia coli BL21 (DE3) by isothermal titration colorimetry |
Bioorg Med Chem Lett 20: 280-2 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.114
BindingDB Entry DOI: 10.7270/Q20V8DQN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Stromelysin-1
(Homo sapiens (Human)) | BDBM8466

((2R)-N-hydroxy-2-[(4-methoxybenzene)sulfonamido]-3...)Show SMILES COc1ccc(cc1)S(=O)(=O)N[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C12H18N2O5S/c1-8(2)11(12(15)13-16)14-20(17,18)10-6-4-9(19-3)5-7-10/h4-8,11,14,16H,1-3H3,(H,13,15)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Binding affinity to stromelysin-1 catalytic domain |
Bioorg Med Chem Lett 20: 280-2 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.114
BindingDB Entry DOI: 10.7270/Q20V8DQN |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50305115
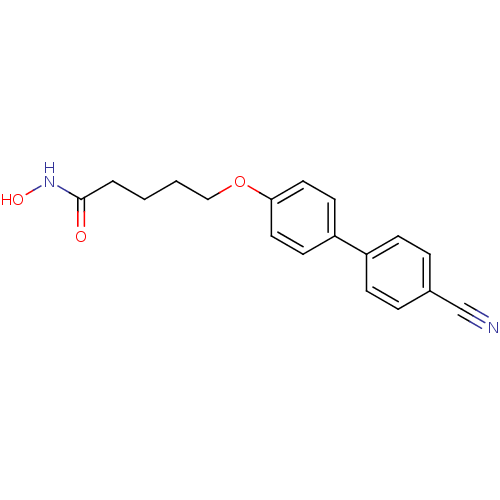
(5-(4'-cyanobiphenyl-4-yloxy)-N-hydroxypentanamide ...)Show InChI InChI=1S/C18H18N2O3/c19-13-14-4-6-15(7-5-14)16-8-10-17(11-9-16)23-12-2-1-3-18(21)20-22/h4-11,22H,1-3,12H2,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Binding affinity to stromelysin-1 catalytic domain |
Bioorg Med Chem Lett 20: 280-2 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.114
BindingDB Entry DOI: 10.7270/Q20V8DQN |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50305115

(5-(4'-cyanobiphenyl-4-yloxy)-N-hydroxypentanamide ...)Show InChI InChI=1S/C18H18N2O3/c19-13-14-4-6-15(7-5-14)16-8-10-17(11-9-16)23-12-2-1-3-18(21)20-22/h4-11,22H,1-3,12H2,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Binding affinity to stromelysin-1 catalytic domain expressed in Escherichia coli BL21 (DE3) by isothermal titration colorimetry |
Bioorg Med Chem Lett 20: 280-2 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.114
BindingDB Entry DOI: 10.7270/Q20V8DQN |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121943
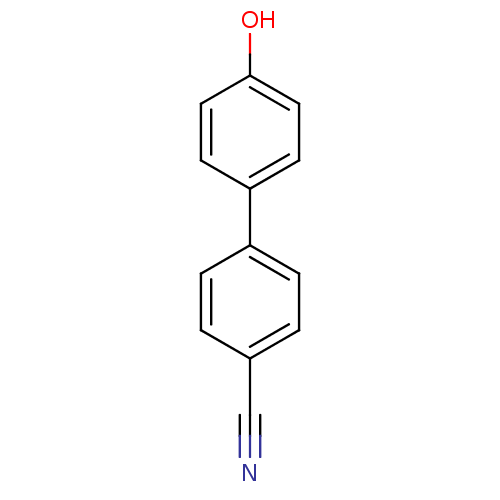
(4'-Hydroxy-biphenyl-4-carbonitrile | CHEMBL114523)Show InChI InChI=1S/C13H9NO/c14-9-10-1-3-11(4-2-10)12-5-7-13(15)8-6-12/h1-8,15H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Dissociation constant for Matrix Metalloprotease-3 (MMP-3) |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121943

(4'-Hydroxy-biphenyl-4-carbonitrile | CHEMBL114523)Show InChI InChI=1S/C13H9NO/c14-9-10-1-3-11(4-2-10)12-5-7-13(15)8-6-12/h1-8,15H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50149241
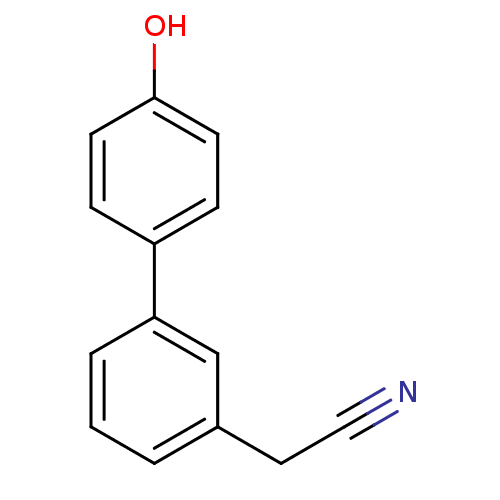
((4'-Hydroxy-biphenyl-3-yl)-acetonitrile | CHEMBL11...)Show InChI InChI=1S/C14H11NO/c15-9-8-11-2-1-3-13(10-11)12-4-6-14(16)7-5-12/h1-7,10,16H,8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Dissociation constant for Matrix Metalloprotease-3 (MMP-3) |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50015152
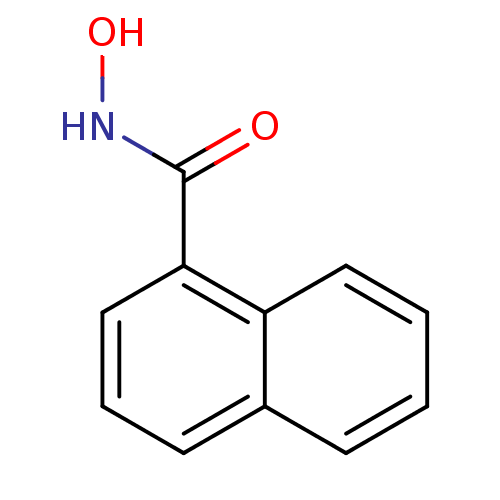
(CHEMBL115468 | N-hydroxy-1-naphthamide | Naphthale...)Show InChI InChI=1S/C11H9NO2/c13-11(12-14)10-7-3-5-8-4-1-2-6-9(8)10/h1-7,14H,(H,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50015152

(CHEMBL115468 | N-hydroxy-1-naphthamide | Naphthale...)Show InChI InChI=1S/C11H9NO2/c13-11(12-14)10-7-3-5-8-4-1-2-6-9(8)10/h1-7,14H,(H,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Dissociation constant for Matrix Metalloprotease-3 (MMP-3) |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121958

(4,4'-Biphenyldiol | 4,4'-Dihydroxybiphenyl | 4,4'-...)Show InChI InChI=1S/C12H10O2/c13-11-5-1-9(2-6-11)10-3-7-12(14)8-4-10/h1-8,13-14H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in the presence of 1-Napthohydroxamate |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121957
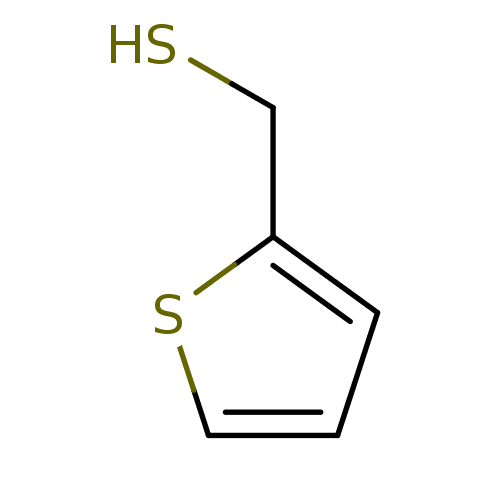
(CHEMBL152603 | Thiophen-2-yl-methanethiol)Show InChI InChI=1S/C5H6S2/c6-4-5-2-1-3-7-5/h1-3,6H,4H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121958

(4,4'-Biphenyldiol | 4,4'-Dihydroxybiphenyl | 4,4'-...)Show InChI InChI=1S/C12H10O2/c13-11-5-1-9(2-6-11)10-3-7-12(14)8-4-10/h1-8,13-14H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 1.60E+5 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in the presence of acetohydroxamic acid |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121955
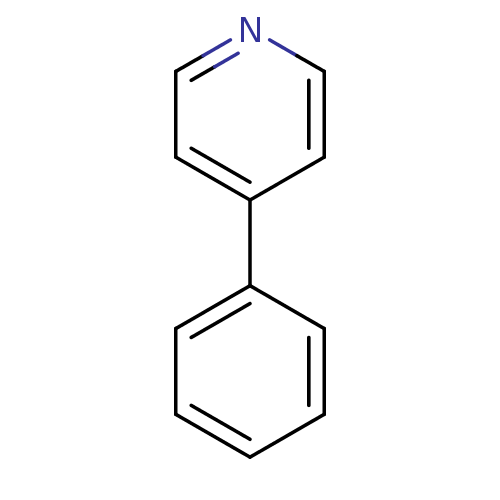
(4-Phenyl-pyridine | CHEMBL109074 | US11634391, Com...)Show InChI InChI=1S/C11H9N/c1-2-4-10(5-3-1)11-6-8-12-9-7-11/h1-9H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 1.70E+5 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in the presence of acetohydroxamic acid |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50149238
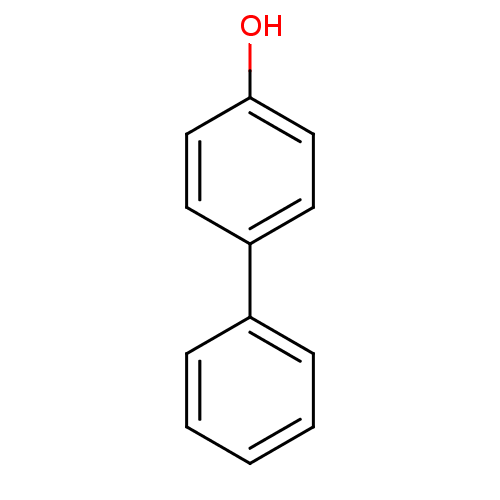
(4-Hydroxybiphenyl | 4-Phenylphenol | 4-biphenylol ...)Show InChI InChI=1S/C12H10O/c13-12-8-6-11(7-9-12)10-4-2-1-3-5-10/h1-9,13H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.80E+5 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human MMP3 catalytic domain (81 to 256 residues) expressed in Escherichia coli BL21 (DE3) pLysS by 15N-HSQC-NMR spectroscopy |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00242
BindingDB Entry DOI: 10.7270/Q2KD22HB |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50149238

(4-Hydroxybiphenyl | 4-Phenylphenol | 4-biphenylol ...)Show InChI InChI=1S/C12H10O/c13-12-8-6-11(7-9-12)10-4-2-1-3-5-10/h1-9,13H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.80E+5 | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Dissociation constant for Matrix Metalloprotease-3 (MMP-3) |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121955

(4-Phenyl-pyridine | CHEMBL109074 | US11634391, Com...)Show InChI InChI=1S/C11H9N/c1-2-4-10(5-3-1)11-6-8-12-9-7-11/h1-9H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 9.00E+5 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in the presence of 1-Napthohydroxamate |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121953
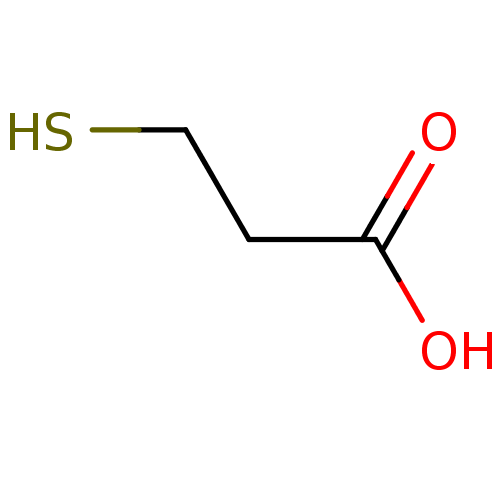
(2-mercaptoethanecarboxylic acid | 3-mercaptopropan...)Show InChI InChI=1S/C3H6O2S/c4-3(5)1-2-6/h6H,1-2H2,(H,4,5) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PubMed
| n/a | n/a | n/a | 3.00E+6 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50015184
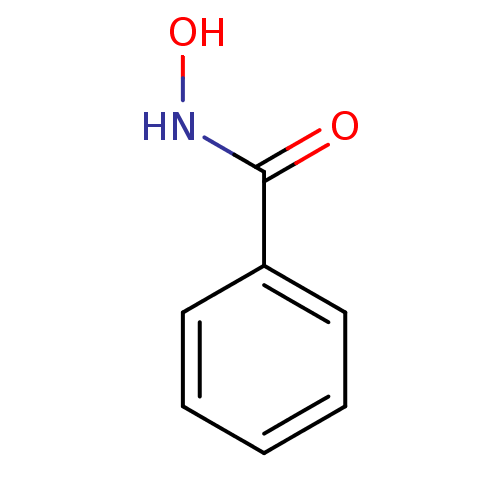
(BENZHYDROXAMIC ACID | BENZOHYDROXAMATE | CHEMBL163...)Show InChI InChI=1S/C7H7NO2/c9-7(8-10)6-4-2-1-3-5-6/h1-5,10H,(H,8,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 7.00E+6 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50099857
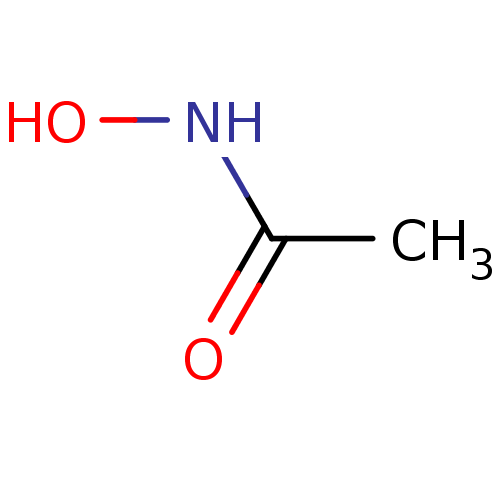
(ACETOHYDROXAMIC ACID (AHA) | AHA | Acethydroxamsae...)Show InChI InChI=1S/C2H5NO2/c1-2(4)3-5/h5H,1H3,(H,3,4) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | 1.70E+7 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human MMP3 catalytic domain (81 to 256 residues) expressed in Escherichia coli BL21 (DE3) pLysS by 15N-HSQC-NMR spectroscopy |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00242
BindingDB Entry DOI: 10.7270/Q2KD22HB |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50099857

(ACETOHYDROXAMIC ACID (AHA) | AHA | Acethydroxamsae...)Show InChI InChI=1S/C2H5NO2/c1-2(4)3-5/h5H,1H3,(H,3,4) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | 1.70E+7 | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Dissociation constant for Matrix Metalloprotease-3 (MMP-3) |
J Med Chem 47: 3463-82 (2004)
Article DOI: 10.1021/jm040031v
BindingDB Entry DOI: 10.7270/Q2NC61ZJ |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50099857

(ACETOHYDROXAMIC ACID (AHA) | AHA | Acethydroxamsae...)Show InChI InChI=1S/C2H5NO2/c1-2(4)3-5/h5H,1H3,(H,3,4) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PubMed
| n/a | n/a | n/a | 1.70E+7 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50056900
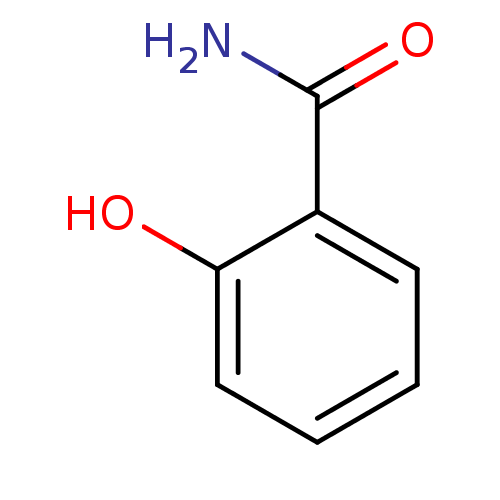
(2-Carbamoylphenol | 2-Carboxamidophenol | 2-Hydrox...)Show InChI InChI=1S/C7H7NO2/c8-7(10)5-3-1-2-4-6(5)9/h1-4,9H,(H2,8,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | >2.50E+7 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121960

(4,4,4-Trifluoro-1-phenyl-butane-1,3-dione | 4,4,4-...)Show InChI InChI=1S/C10H7F3O2/c11-10(12,13)9(15)6-8(14)7-4-2-1-3-5-7/h1-5H,6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | n/a | >2.50E+7 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121952
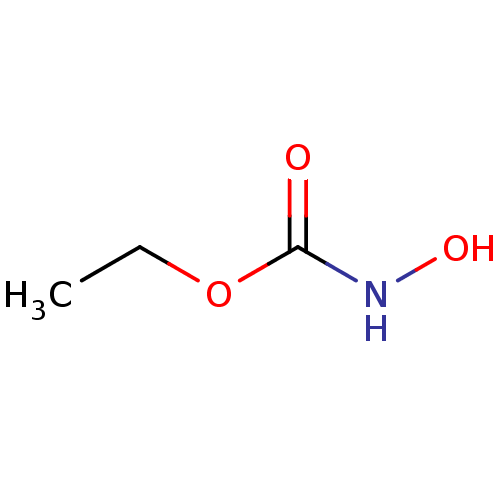
(CHEMBL153081 | Ethyl hydroxycarbamate)Show InChI InChI=1S/C3H7NO3/c1-2-7-3(5)4-6/h6H,2H2,1H3,(H,4,5) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | n/a | >2.50E+7 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50017811
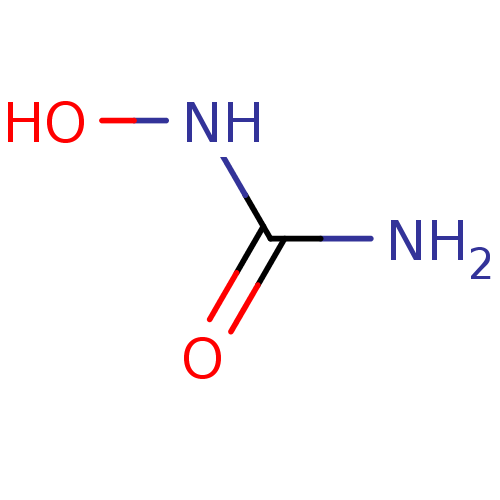
(CHEMBL467 | HU | US10155732, Compound HU | hydroxy...)Show InChI InChI=1S/CH4N2O2/c2-1(4)3-5/h5H,(H3,2,3,4) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PubMed
| n/a | n/a | n/a | >2.50E+7 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50026891

(1-Pyridin-2-yl-ethanone | CHEMBL11945)Show InChI InChI=1S/C7H7NO/c1-6(9)7-4-2-3-5-8-7/h2-5H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | n/a | >2.50E+7 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM26193
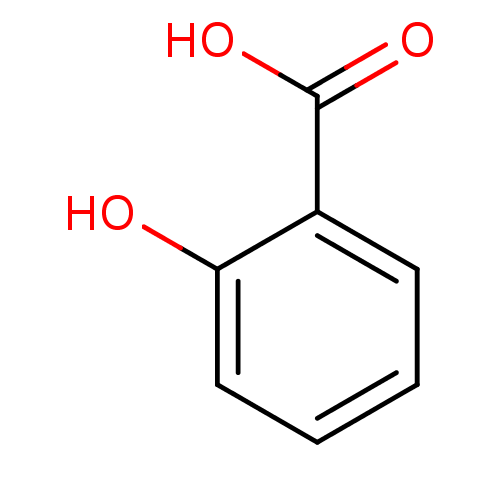
(2-Hydroxybenzoate, I | 2-hydroxybenzoic acid | CHE...)Show InChI InChI=1S/C7H6O3/c8-6-4-2-1-3-5(6)7(9)10/h1-4,8H,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | >2.50E+7 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121956
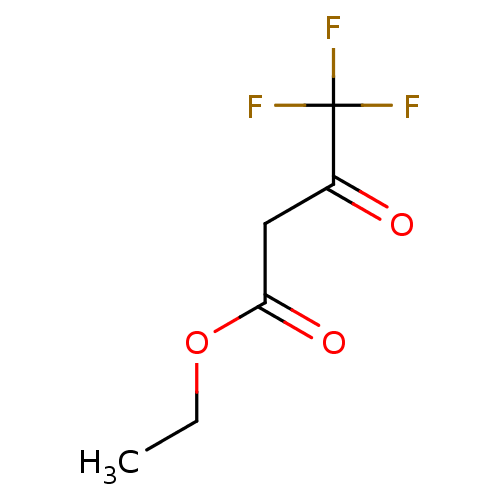
(4,4,4-Trifluoro-3-oxo-butyric acid ethyl ester | C...)Show InChI InChI=1S/C6H7F3O3/c1-2-12-5(11)3-4(10)6(7,8)9/h2-3H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | n/a | >2.50E+7 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50082556
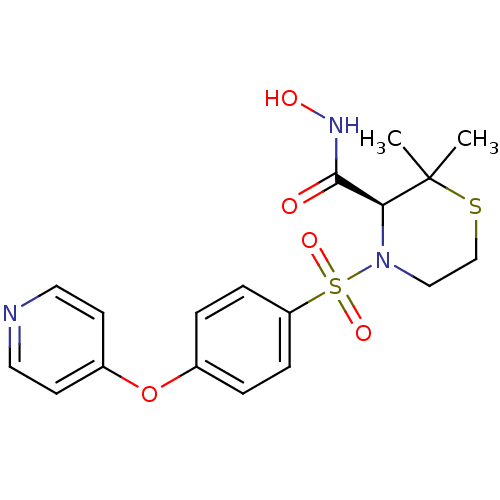
((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloprotease-3 |
J Med Chem 42: 4547-62 (1999)
BindingDB Entry DOI: 10.7270/Q2D79C32 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-3 (MMP-3). |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM11875
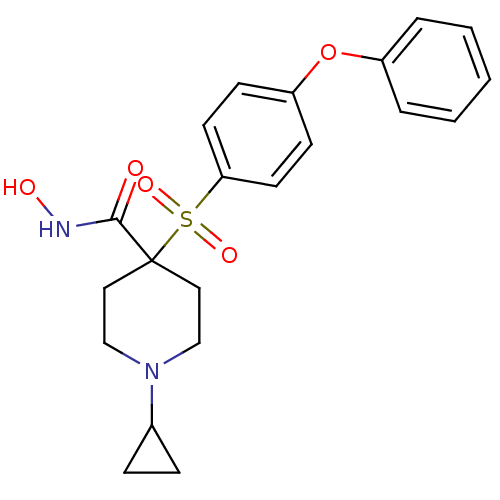
(1-Cyclopropyl-N-hydroxy-4-{[4-(phenoxyphenyl]-sulf...)Show SMILES ONC(=O)C1(CCN(CC1)C1CC1)S(=O)(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C21H24N2O5S/c24-20(22-25)21(12-14-23(15-13-21)16-6-7-16)29(26,27)19-10-8-18(9-11-19)28-17-4-2-1-3-5-17/h1-5,8-11,16,25H,6-7,12-15H2,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Inhibitors were assayed against purified hMMP-1, hMMP-2, hMMP-3, hMMP-8, hMMP-9, and hMMP-13 using an enzyme assay based on cleavage of the quenched ... |
J Med Chem 48: 6713-30 (2005)
Article DOI: 10.1021/jm0500875
BindingDB Entry DOI: 10.7270/Q2N58JMZ |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM11874
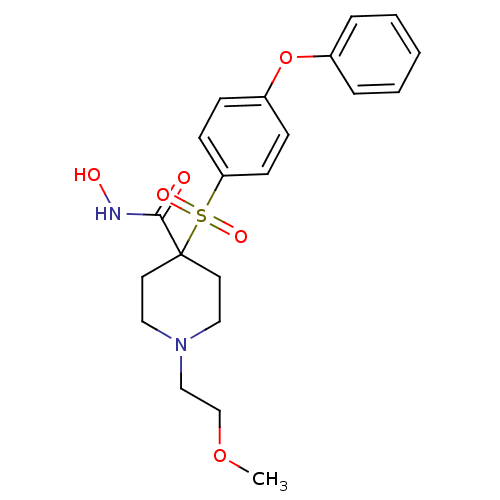
(N-Hydroxy-1-(2-methoxyethyl)-4-{[4-(phenoxyphenyl]...)Show SMILES COCCN1CCC(CC1)(C(=O)NO)S(=O)(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C21H26N2O6S/c1-28-16-15-23-13-11-21(12-14-23,20(24)22-25)30(26,27)19-9-7-18(8-10-19)29-17-5-3-2-4-6-17/h2-10,25H,11-16H2,1H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Inhibitors were assayed against purified hMMP-1, hMMP-2, hMMP-3, hMMP-8, hMMP-9, and hMMP-13 using an enzyme assay based on cleavage of the quenched ... |
J Med Chem 48: 6713-30 (2005)
Article DOI: 10.1021/jm0500875
BindingDB Entry DOI: 10.7270/Q2N58JMZ |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50143729

((2R,3R,4R,5R)-3,4,5-Trihydroxy-1-(4-phenoxy-benzen...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@H](O)CN1S(=O)(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C18H20N2O8S/c21-14-10-20(15(18(24)19-25)17(23)16(14)22)29(26,27)13-8-6-12(7-9-13)28-11-4-2-1-3-5-11/h1-9,14-17,21-23,25H,10H2,(H,19,24)/t14-,15-,16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloprotease 3 |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50289127
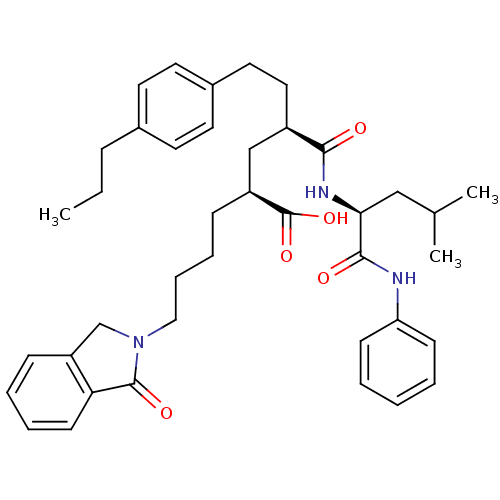
((2S,4R)-4-(3-Methyl-1-phenylcarbamoyl-butylcarbamo...)Show SMILES CCCc1ccc(CC[C@H](C[C@H](CCCCN2Cc3ccccc3C2=O)C(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C40H51N3O5/c1-4-12-29-18-20-30(21-19-29)22-23-31(37(44)42-36(25-28(2)3)38(45)41-34-15-6-5-7-16-34)26-32(40(47)48)13-10-11-24-43-27-33-14-8-9-17-35(33)39(43)46/h5-9,14-21,28,31-32,36H,4,10-13,22-27H2,1-3H3,(H,41,45)(H,42,44)(H,47,48)/t31-,32+,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-3(MMP-3). |
Bioorg Med Chem Lett 6: 803-806 (1996)
Article DOI: 10.1016/0960-894X(96)00109-6
BindingDB Entry DOI: 10.7270/Q2HM58FH |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50141575
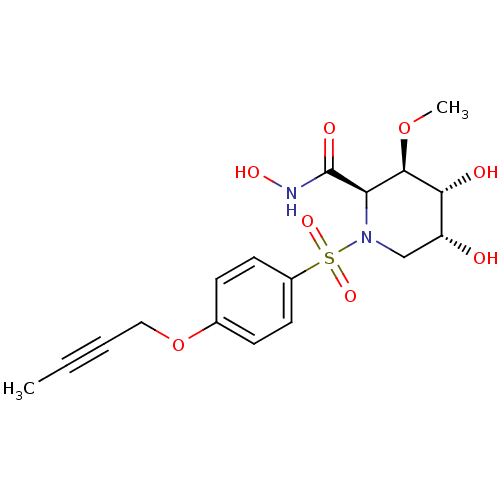
((2R,3R,4R,5R)-1-(4-But-2-ynyloxy-benzenesulfonyl)-...)Show SMILES CO[C@H]1[C@H](O)[C@H](O)CN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(OCC#CC)cc1 Show InChI InChI=1S/C17H22N2O8S/c1-3-4-9-27-11-5-7-12(8-6-11)28(24,25)19-10-13(20)15(21)16(26-2)14(19)17(22)18-23/h5-8,13-16,20-21,23H,9-10H2,1-2H3,(H,18,22)/t13-,14-,15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon K.K.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human matrix metalloproteinase-3 |
Bioorg Med Chem Lett 14: 1569-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.091
BindingDB Entry DOI: 10.7270/Q2KS6QZB |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50109621
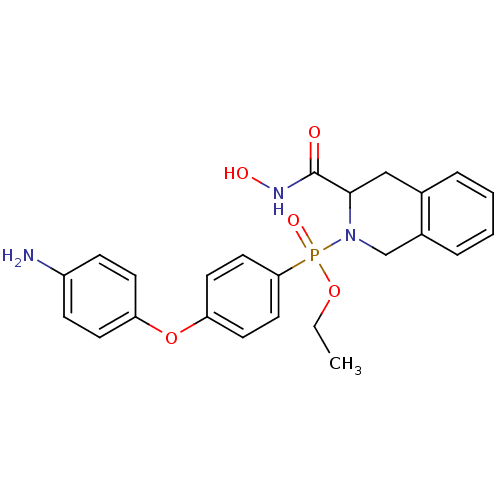
(CHEMBL425316 | [4-(4-Amino-phenoxy)-phenyl]-(3-hyd...)Show SMILES CCOP(=O)(N1Cc2ccccc2CC1C(=O)NO)c1ccc(Oc2ccc(N)cc2)cc1 Show InChI InChI=1S/C24H26N3O5P/c1-2-31-33(30,22-13-11-21(12-14-22)32-20-9-7-19(25)8-10-20)27-16-18-6-4-3-5-17(18)15-23(27)24(28)26-29/h3-14,23,29H,2,15-16,25H2,1H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K.
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-3 (MMP-3)(stromelysin-1). |
J Med Chem 45: 919-29 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8G9Q |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50131385

((2R,3R,4R,5S)-3,4,5-Trihydroxy-1-(4-phenoxy-benzen...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1S(=O)(=O)c1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C18H20N2O8S/c21-14-10-20(15(18(24)19-25)17(23)16(14)22)29(26,27)13-8-6-12(7-9-13)28-11-4-2-1-3-5-11/h1-9,14-17,21-23,25H,10H2,(H,19,24)/t14-,15+,16+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloprotease 3 |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50131385

((2R,3R,4R,5S)-3,4,5-Trihydroxy-1-(4-phenoxy-benzen...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1S(=O)(=O)c1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C18H20N2O8S/c21-14-10-20(15(18(24)19-25)17(23)16(14)22)29(26,27)13-8-6-12(7-9-13)28-11-4-2-1-3-5-11/h1-9,14-17,21-23,25H,10H2,(H,19,24)/t14-,15+,16+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human matrix metalloproteinase-3 |
Bioorg Med Chem Lett 13: 2737-40 (2003)
BindingDB Entry DOI: 10.7270/Q2PC31SG |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50109635
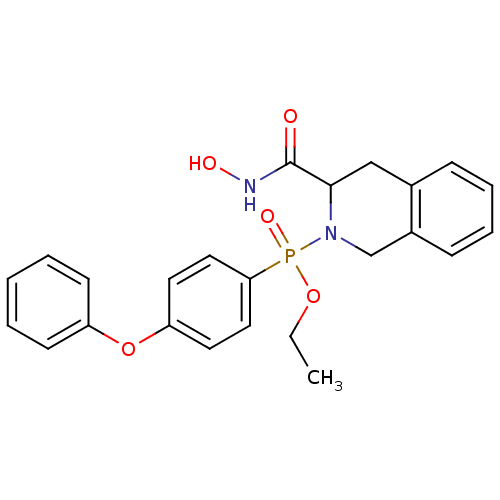
((3-Hydroxycarbamoyl-3,4-dihydro-1H-isoquinolin-2-y...)Show SMILES CCOP(=O)(N1Cc2ccccc2CC1C(=O)NO)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C24H25N2O5P/c1-2-30-32(29,22-14-12-21(13-15-22)31-20-10-4-3-5-11-20)26-17-19-9-7-6-8-18(19)16-23(26)24(27)25-28/h3-15,23,28H,2,16-17H2,1H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Organon K.K.
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-3 (MMP-3)(stromelysin-1). |
J Med Chem 45: 919-29 (2002)
BindingDB Entry DOI: 10.7270/Q2XK8G9Q |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50283701
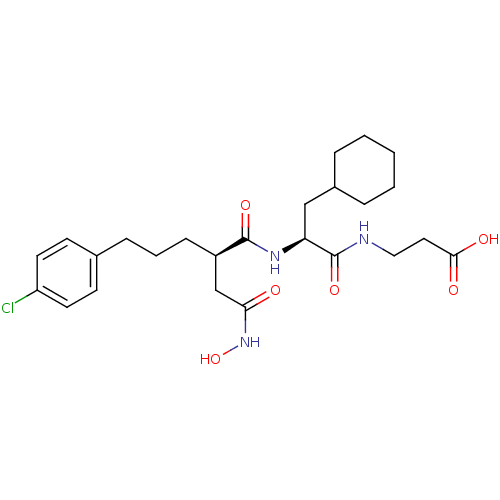
(3-{(S)-2-[(R)-5-(4-Chloro-phenyl)-2-hydroxycarbamo...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCC(O)=O Show InChI InChI=1S/C25H36ClN3O6/c26-20-11-9-17(10-12-20)7-4-8-19(16-22(30)29-35)24(33)28-21(15-18-5-2-1-3-6-18)25(34)27-14-13-23(31)32/h9-12,18-19,21,35H,1-8,13-16H2,(H,27,34)(H,28,33)(H,29,30)(H,31,32)/t19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the stromelysin enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50101516
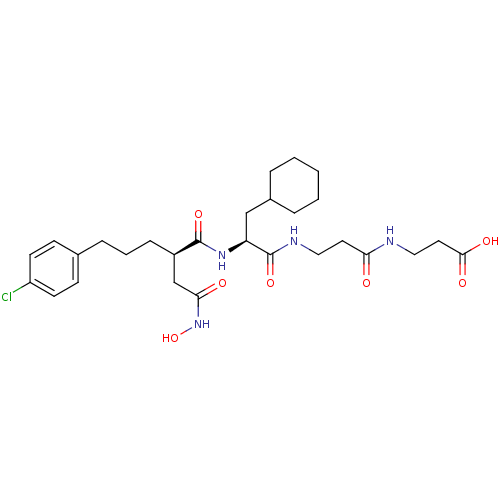
(3-(3-{2-[5-(4-Chloro-phenyl)-2-hydroxycarbamoylmet...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCC(=O)NCCC(O)=O Show InChI InChI=1S/C28H41ClN4O7/c29-22-11-9-19(10-12-22)7-4-8-21(18-25(35)33-40)27(38)32-23(17-20-5-2-1-3-6-20)28(39)31-15-13-24(34)30-16-14-26(36)37/h9-12,20-21,23,40H,1-8,13-18H2,(H,30,34)(H,31,39)(H,32,38)(H,33,35)(H,36,37)/t21-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-3 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50183784
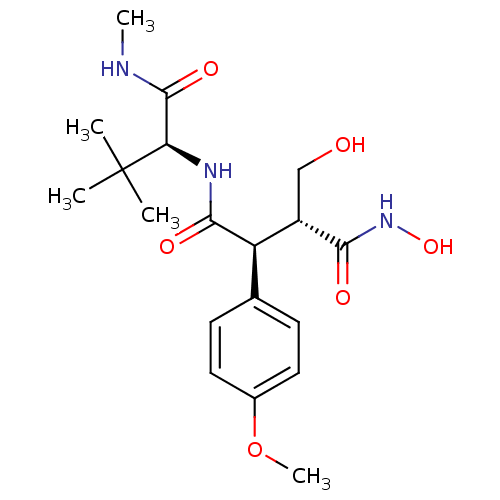
((2S,3R)-N1-((S)-3,3-dimethyl-1-(methylamino)-1-oxo...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@@H]([C@H](CO)C(=O)NO)c1ccc(OC)cc1)C(C)(C)C |r| Show InChI InChI=1S/C19H29N3O6/c1-19(2,3)15(18(26)20-4)21-17(25)14(13(10-23)16(24)22-27)11-6-8-12(28-5)9-7-11/h6-9,13-15,23,27H,10H2,1-5H3,(H,20,26)(H,21,25)(H,22,24)/t13-,14+,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical Research Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory potency against Matrix metalloprotease-3 (MMP-3) |
J Med Chem 45: 2289-93 (2002)
Article DOI: 10.1021/jm0110993
BindingDB Entry DOI: 10.7270/Q2HQ42NC |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50183784

((2S,3R)-N1-((S)-3,3-dimethyl-1-(methylamino)-1-oxo...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@@H]([C@H](CO)C(=O)NO)c1ccc(OC)cc1)C(C)(C)C |r| Show InChI InChI=1S/C19H29N3O6/c1-19(2,3)15(18(26)20-4)21-17(25)14(13(10-23)16(24)22-27)11-6-8-12(28-5)9-7-11/h6-9,13-15,23,27H,10H2,1-5H3,(H,20,26)(H,21,25)(H,22,24)/t13-,14+,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant MMP3 |
Bioorg Med Chem Lett 16: 2632-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.042
BindingDB Entry DOI: 10.7270/Q2JS9Q12 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50283708
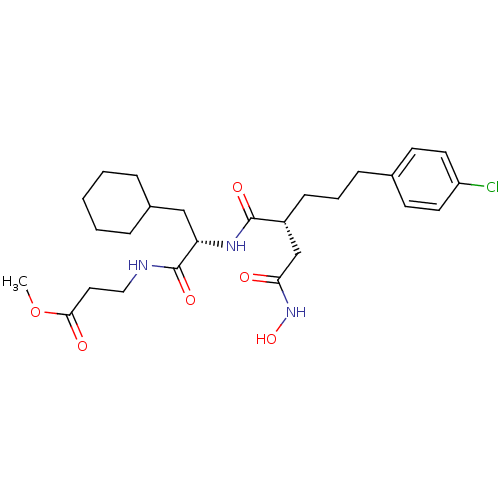
(3-{(S)-2-[(R)-5-(4-Chloro-phenyl)-2-hydroxycarbamo...)Show SMILES COC(=O)CCNC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](CCCc1ccc(Cl)cc1)CC(=O)NO Show InChI InChI=1S/C26H38ClN3O6/c1-36-24(32)14-15-28-26(34)22(16-19-6-3-2-4-7-19)29-25(33)20(17-23(31)30-35)9-5-8-18-10-12-21(27)13-11-18/h10-13,19-20,22,35H,2-9,14-17H2,1H3,(H,28,34)(H,29,33)(H,30,31)/t20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of the stromelysin enzyme. |
Bioorg Med Chem Lett 4: 2741-2746 (1994)
Article DOI: 10.1016/S0960-894X(01)80587-4
BindingDB Entry DOI: 10.7270/Q2P84BT0 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50101505
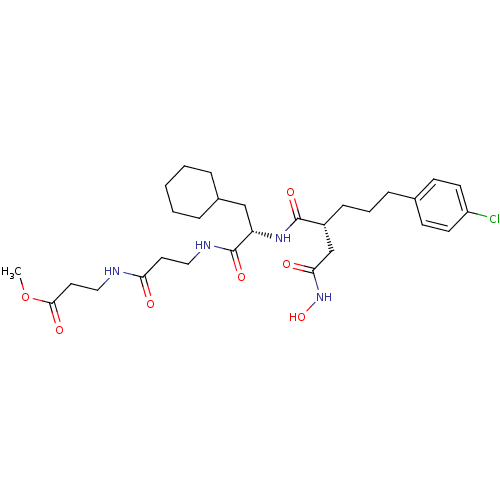
(3-(3-{2-[5-(4-Chloro-phenyl)-2-hydroxycarbamoylmet...)Show SMILES COC(=O)CCNC(=O)CCNC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](CCCc1ccc(Cl)cc1)CC(=O)NO Show InChI InChI=1S/C29H43ClN4O7/c1-41-27(37)15-17-31-25(35)14-16-32-29(39)24(18-21-6-3-2-4-7-21)33-28(38)22(19-26(36)34-40)9-5-8-20-10-12-23(30)13-11-20/h10-13,21-22,24,40H,2-9,14-19H2,1H3,(H,31,35)(H,32,39)(H,33,38)(H,34,36)/t22-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-3 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50101518
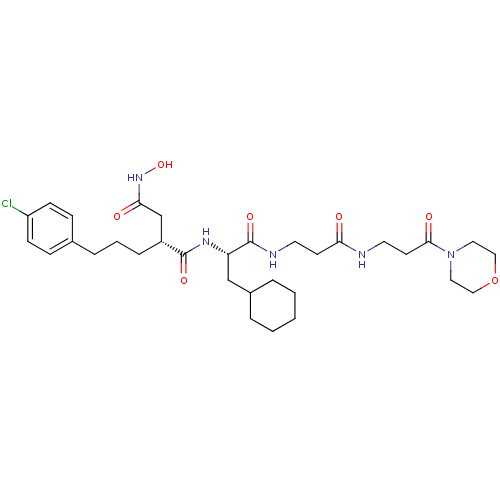
(2-[3-(4-Chloro-phenyl)-propyl]-N*1*-{2-cyclohexyl-...)Show SMILES ONC(=O)C[C@@H](CCCc1ccc(Cl)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCC(=O)NCCC(=O)N1CCOCC1 Show InChI InChI=1S/C32H48ClN5O7/c33-26-11-9-23(10-12-26)7-4-8-25(22-29(40)37-44)31(42)36-27(21-24-5-2-1-3-6-24)32(43)35-15-13-28(39)34-16-14-30(41)38-17-19-45-20-18-38/h9-12,24-25,27,44H,1-8,13-22H2,(H,34,39)(H,35,43)(H,36,42)(H,37,40)/t25-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibitory constant against matrix metalloproteinase-3 |
J Med Chem 44: 2333-43 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KFR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data