

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,R541K,D544E,Q553K,L573M] (Human immunodeficiency virus) | BDBM112660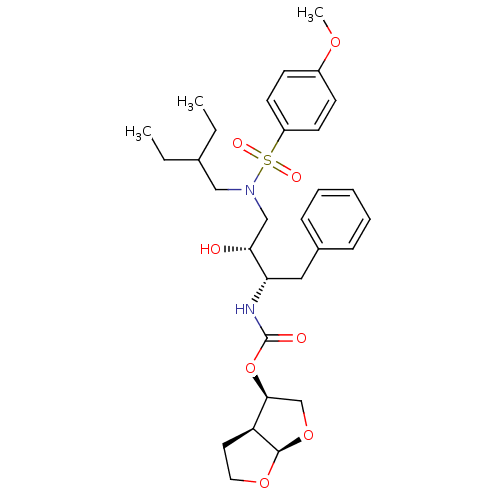 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.000200 | -72.5 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,R541K,D544E,Q553K,L573M] (Human immunodeficiency virus) | BDBM112661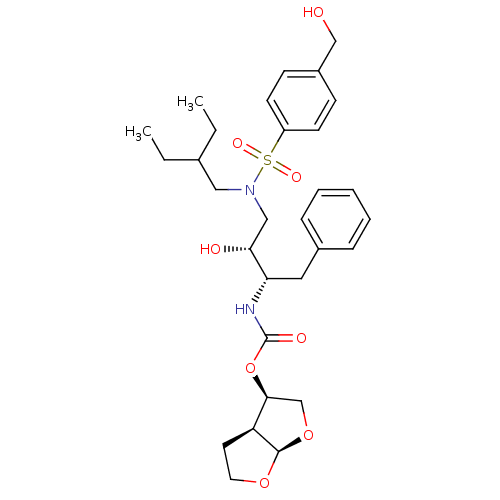 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.000500 | -70.2 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,R541K,D544E,Q553K,L573M] (Human immunodeficiency virus) | BDBM112656 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.000500 | -70.2 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,R541K,D544E,Q553K,L573M] (Human immunodeficiency virus) | BDBM112662 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.000800 | -69.0 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,R541K,D544E,Q553K,L573M] (Human immunodeficiency virus) | BDBM112657 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.000900 | -68.8 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I497V,V499I,E519D,G532V,I538V,R541K,D544E,L547P,Q553K,V566A,L573M] (Human immunodeficiency virus) | BDBM112660 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00100 | -68.5 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,R541K,D544E,Q553K,L573M] (Human immunodeficiency virus) | BDBM112663 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00150 | -67.5 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I497V,V499I,E519D,R541K,D544E,L547P,Q553K,A555V,G557S,I568V,L573M,L574M] (Human immunodeficiency virus) | BDBM112656 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00400 | -65.1 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I497V,V499I,E519D,G532V,I538V,R541K,D544E,L547P,Q553K,V566A,L573M] (Human immunodeficiency virus) | BDBM112655 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00500 | -64.5 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I497V,V499I,E519D,G532V,I538V,R541K,D544E,L547P,Q553K,V566A,L573M] (Human immunodeficiency virus) | BDBM112661 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00500 | -64.5 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,R541K,D544E,Q553K,L573M] (Human immunodeficiency virus) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00500 | -64.5 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,R541K,D544E,Q553K,L573M] (Human immunodeficiency virus) | BDBM112659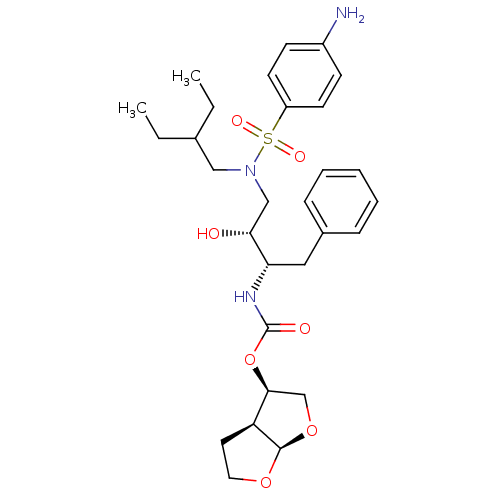 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00500 | -64.5 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,I534V,R541K,D544E,Q553K,A555V,L573M] (Human immunodeficiency virus) | BDBM112654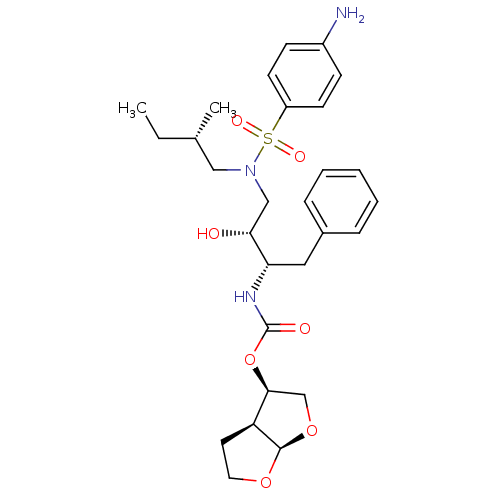 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00600 | -64.1 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,I534V,R541K,D544E,Q553K,A555V,L573M] (Human immunodeficiency virus) | BDBM112662 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00600 | -64.1 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,I534V,R541K,D544E,Q553K,A555V,L573M] (Human immunodeficiency virus) | BDBM112660 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0100 | -62.8 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I497V,V499I,E519D,G532V,I538V,R541K,D544E,L547P,Q553K,V566A,L573M] (Human immunodeficiency virus) | BDBM112658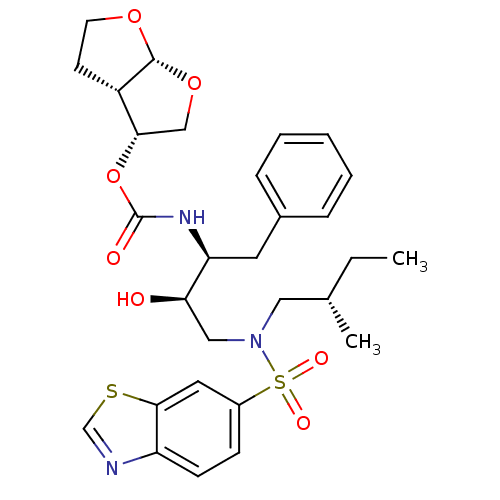 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0120 | -62.3 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I497V,V499I,E519D,R541K,D544E,L547P,Q553K,A555V,G557S,I568V,L573M,L574M] (Human immunodeficiency virus) | BDBM112654 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0130 | -62.1 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,I534V,R541K,D544E,Q553K,A555V,L573M] (Human immunodeficiency virus) | BDBM112656 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0130 | -62.1 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,R541K,D544E,Q553K,L573M] (Human immunodeficiency virus) | BDBM112654 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0150 | -61.8 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I497V,V499I,E519D,G532V,I538V,R541K,D544E,L547P,Q553K,V566A,L573M] (Human immunodeficiency virus) | BDBM112654 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0160 | -61.6 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,R541K,D544E,Q553K,L573M] (Human immunodeficiency virus) | BDBM112658 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0190 | -61.2 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,R541K,D544E,Q553K,L573M] (Human immunodeficiency virus) | BDBM112655 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0200 | -61.1 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I497V,V499I,E519D,R541K,D544E,L547P,Q553K,A555V,G557S,I568V,L573M,L574M] (Human immunodeficiency virus) | BDBM112655 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0210 | -60.9 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I497V,V499I,E519D,R541K,D544E,L547P,Q553K,A555V,G557S,I568V,L573M,L574M] (Human immunodeficiency virus) | BDBM112659 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0220 | -60.8 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I497V,V499I,E519D,G532V,I538V,R541K,D544E,L547P,Q553K,V566A,L573M] (Human immunodeficiency virus) | BDBM112656 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0220 | -60.8 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I497V,V499I,E519D,R541K,D544E,L547P,Q553K,A555V,G557S,I568V,L573M,L574M] (Human immunodeficiency virus) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0240 | -60.6 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,I534V,R541K,D544E,Q553K,A555V,L573M] (Human immunodeficiency virus) | BDBM112658 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0250 | -60.5 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I497V,V499I,E519D,G532V,I538V,R541K,D544E,L547P,Q553K,V566A,L573M] (Human immunodeficiency virus) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0250 | -60.5 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,I534V,R541K,D544E,Q553K,A555V,L573M] (Human immunodeficiency virus) | BDBM112661 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0260 | -60.4 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I497V,V499I,E519D,G532V,I538V,R541K,D544E,L547P,Q553K,V566A,L573M] (Human immunodeficiency virus) | BDBM112657 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0290 | -60.1 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I497V,V499I,E519D,G532V,I538V,R541K,D544E,L547P,Q553K,V566A,L573M] (Human immunodeficiency virus) | BDBM112659 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0340 | -59.8 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I497V,V499I,E519D,G532V,I538V,R541K,D544E,L547P,Q553K,V566A,L573M] (Human immunodeficiency virus) | BDBM112662 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0340 | -59.8 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,I534V,R541K,D544E,Q553K,A555V,L573M] (Human immunodeficiency virus) | BDBM112657 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0350 | -59.7 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I497V,V499I,E519D,R541K,D544E,L547P,Q553K,A555V,G557S,I568V,L573M,L574M] (Human immunodeficiency virus) | BDBM112658 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0410 | -59.3 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I497V,V499I,E519D,R541K,D544E,L547P,Q553K,A555V,G557S,I568V,L573M,L574M] (Human immunodeficiency virus) | BDBM112663 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0520 | -58.7 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,I534V,R541K,D544E,Q553K,A555V,L573M] (Human immunodeficiency virus) | BDBM112659 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0620 | -58.3 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I497V,V499I,E519D,R541K,D544E,L547P,Q553K,A555V,G557S,I568V,L573M,L574M] (Human immunodeficiency virus) | BDBM112661 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0700 | -58.0 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I497V,V499I,E519D,R541K,D544E,L547P,Q553K,A555V,G557S,I568V,L573M,L574M] (Human immunodeficiency virus) | BDBM112657 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0990 | -57.1 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,I534V,R541K,D544E,Q553K,A555V,L573M] (Human immunodeficiency virus) | BDBM112655 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.103 | -57.0 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I497V,V499I,E519D,R541K,D544E,L547P,Q553K,A555V,G557S,I568V,L573M,L574M] (Human immunodeficiency virus) | BDBM112662 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.104 | -57.0 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,I534V,R541K,D544E,Q553K,A555V,L573M] (Human immunodeficiency virus) | BDBM112663 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.108 | -56.9 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I497V,V499I,E519D,G532V,I538V,R541K,D544E,L547P,Q553K,V566A,L573M] (Human immunodeficiency virus) | BDBM112663 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.126 | -56.5 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I497V,V499I,E519D,R541K,D544E,L547P,Q553K,A555V,G557S,I568V,L573M,L574M] (Human immunodeficiency virus) | BDBM112660 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.134 | -56.4 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [484-582,Q491K,I494L,I497V,V499I,E519D,I534V,R541K,D544E,Q553K,A555V,L573M] (Human immunodeficiency virus) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.245 | -54.9 | n/a | n/a | n/a | n/a | n/a | 4.7 | 25 |
University of Massachusetts Medical School, Worcester, MA 01605, USA | Assay Description The reaction mixture contained 2 µL of protease and 2 µL of inhibitor (or DMSO as a control) and was incubated for 20-30 min at room temper... | Chem Biol 20: 1116-24 (2013) Article DOI: 10.1016/j.chembiol.2013.07.014 BindingDB Entry DOI: 10.7270/Q2HQ3XKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM36648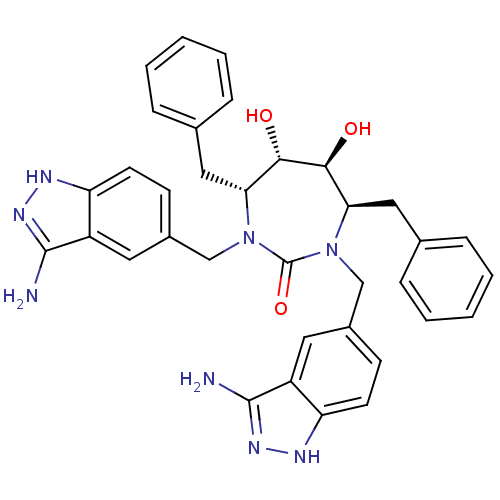 (3-alkylaminoindazole cyclic urea, (H)) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM36647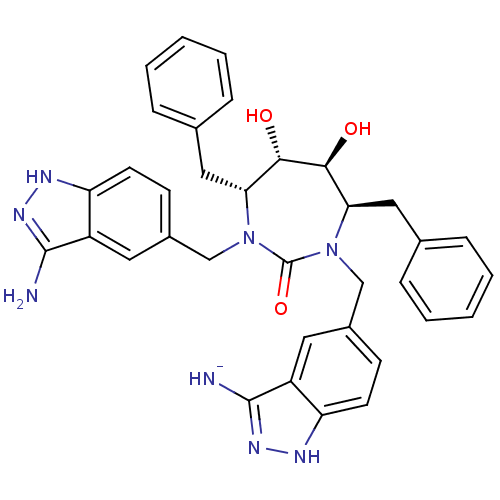 (3-Aminoindazole, 2) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM36656 (Cyclobutylmethyl cyclic urea) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM161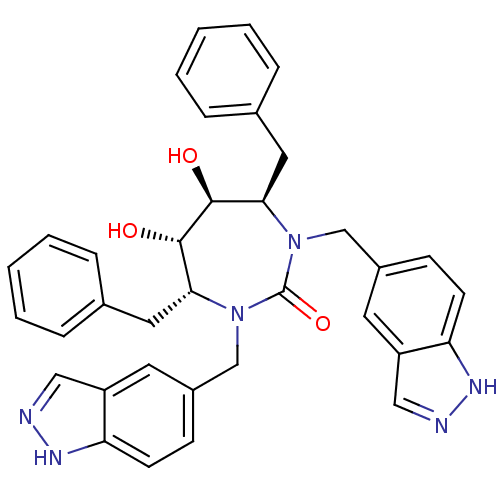 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis(1...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM36649 (3-alkylaminoindazole cyclic urea, (Me)) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM36655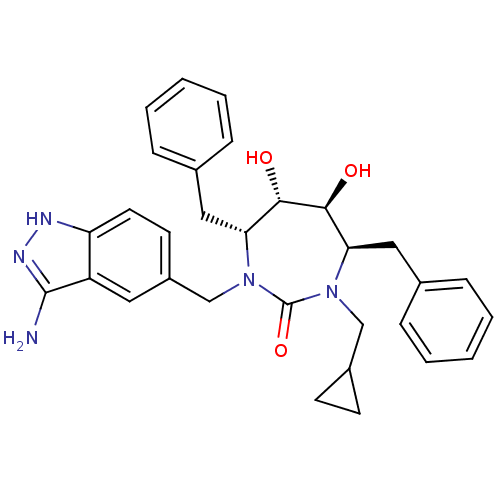 (Cyclopropylmethyl cyclic urea) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 721 total ) | Next | Last >> |