

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Myeloperoxidase (Homo sapiens (Human)) | BDBM217355 (4-benzyl-2-hydroxybenzenecarbohydroxamic acid (HX2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 63 | n/a | n/a | n/a | 5.0 | n/a |
University of Otago Christchurch | Assay Description Binding kinetics were determined by surface plasmon resonance (SPR) using a Biacore S51 (Biacore, Uppsala, Sweden). MPO (50 μg/ml dissolved in 1... | J Biol Chem 288: 36636-47 (2013) Article DOI: 10.1074/jbc.M113.507756 BindingDB Entry DOI: 10.7270/Q20K27DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM217354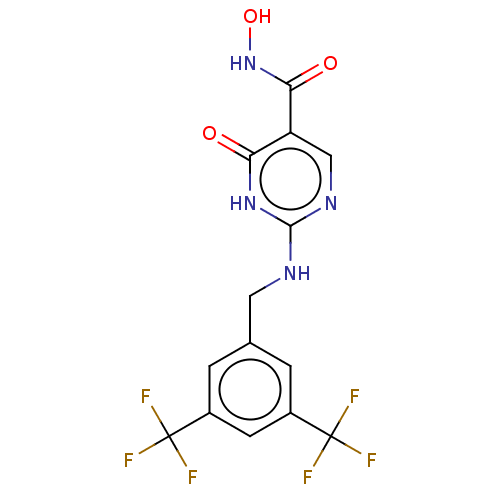 (2-(3,5-bistrifluoromethylbenzylamino)-6-oxo-1H-pyr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | n/a | 15.8 | n/a | n/a | n/a | 5.0 | n/a |
University of Otago Christchurch | Assay Description Binding kinetics were determined by surface plasmon resonance (SPR) using a Biacore S51 (Biacore, Uppsala, Sweden). MPO (50 μg/ml dissolved in 1... | J Biol Chem 288: 36636-47 (2013) Article DOI: 10.1074/jbc.M113.507756 BindingDB Entry DOI: 10.7270/Q20K27DH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM217356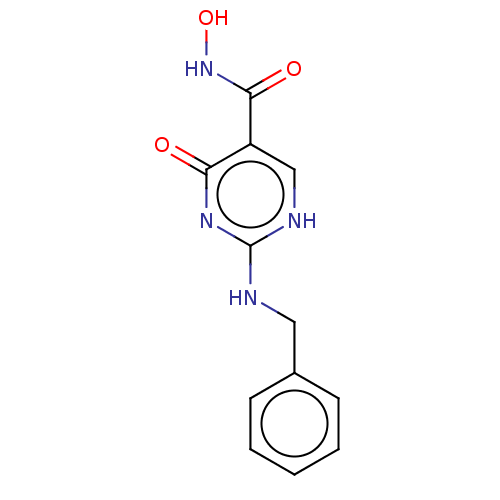 (2-(benzylamino)-6-oxo-3H-pyrimidine-5-carbohydroxa...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | 5.0 | n/a |
University of Otago Christchurch | Assay Description Binding kinetics were determined by surface plasmon resonance (SPR) using a Biacore S51 (Biacore, Uppsala, Sweden). MPO (50 μg/ml dissolved in 1... | J Biol Chem 288: 36636-47 (2013) Article DOI: 10.1074/jbc.M113.507756 BindingDB Entry DOI: 10.7270/Q20K27DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM217355 (4-benzyl-2-hydroxybenzenecarbohydroxamic acid (HX2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25.1 | n/a | n/a | n/a | n/a | 6.5 | n/a |
University of Otago Christchurch | Assay Description Assays were performed at 22 °C with 2 nM MPO and 10 μM hydrogen peroxide (H2O2) in 20 mM NaH2PO4 buffer, pH 6.5 containing 140 mM NaCl, 10 mM ta... | J Biol Chem 288: 36636-47 (2013) Article DOI: 10.1074/jbc.M113.507756 BindingDB Entry DOI: 10.7270/Q20K27DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM217354 (2-(3,5-bistrifluoromethylbenzylamino)-6-oxo-1H-pyr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | 6.5 | n/a |
University of Otago Christchurch | Assay Description Assays were performed at 22 °C with 2 nM MPO and 10 μM hydrogen peroxide (H2O2) in 20 mM NaH2PO4 buffer, pH 6.5 containing 140 mM NaCl, 10 mM ta... | J Biol Chem 288: 36636-47 (2013) Article DOI: 10.1074/jbc.M113.507756 BindingDB Entry DOI: 10.7270/Q20K27DH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50015089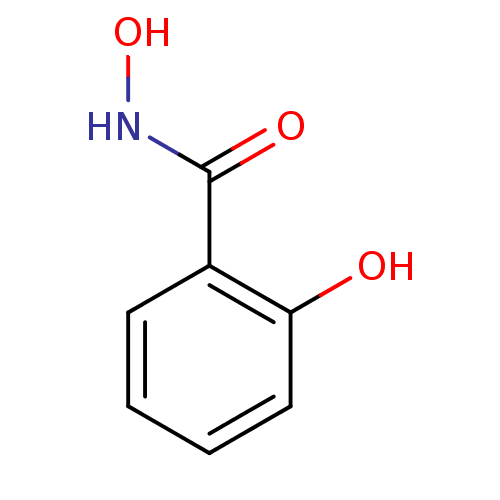 (2,N-Dihydroxy-benzamide | CHEMBL309339 | N,2-dihyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | 6.5 | n/a |
University of Otago Christchurch | Assay Description Assays were performed at 22 °C with 2 nM MPO and 10 μM hydrogen peroxide (H2O2) in 20 mM NaH2PO4 buffer, pH 6.5 containing 140 mM NaCl, 10 mM ta... | J Biol Chem 288: 36636-47 (2013) Article DOI: 10.1074/jbc.M113.507756 BindingDB Entry DOI: 10.7270/Q20K27DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM217356 (2-(benzylamino)-6-oxo-3H-pyrimidine-5-carbohydroxa...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | 6.5 | n/a |
University of Otago Christchurch | Assay Description Assays were performed at 22 °C with 2 nM MPO and 10 μM hydrogen peroxide (H2O2) in 20 mM NaH2PO4 buffer, pH 6.5 containing 140 mM NaCl, 10 mM ta... | J Biol Chem 288: 36636-47 (2013) Article DOI: 10.1074/jbc.M113.507756 BindingDB Entry DOI: 10.7270/Q20K27DH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM119872 (3‐(2‐ethoxypropyl)‐2‐sulfa...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 413 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Worldwide Research and Development | Assay Description Assay mixtures (100 µL) contained 50 mM NaPi (pH 7.4), 150 mM NaCl, 1 mM DTPA, 2% DMSO, the indicated concentrations of H2O2, and Amplex Red. Th... | Biochemistry 52: 9187-201 (2013) Article DOI: 10.1021/bi401354d BindingDB Entry DOI: 10.7270/Q2K35SBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM119874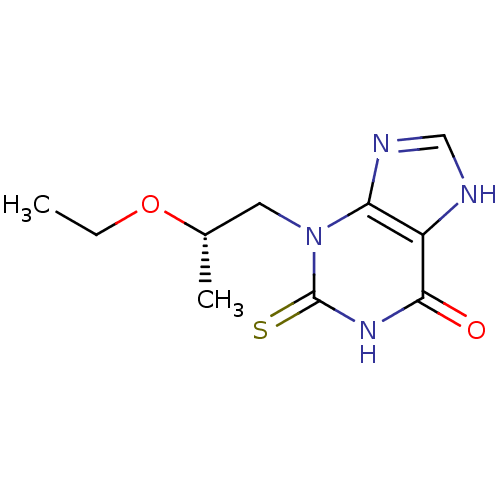 (3‐[(2S)‐2‐ethoxypropyl]‐2&...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 429 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Worldwide Research and Development | Assay Description Assay mixtures (100 µL) contained 50 mM NaPi (pH 7.4), 150 mM NaCl, 1 mM DTPA, 2% DMSO, the indicated concentrations of H2O2, and Amplex Red. Th... | Biochemistry 52: 9187-201 (2013) Article DOI: 10.1021/bi401354d BindingDB Entry DOI: 10.7270/Q2K35SBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM119873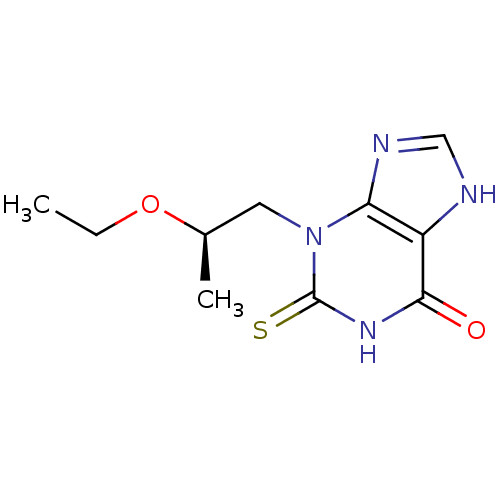 (3‐[(2R)‐2‐ethoxypropyl]‐2&...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 546 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Worldwide Research and Development | Assay Description Assay mixtures (100 µL) contained 50 mM NaPi (pH 7.4), 150 mM NaCl, 1 mM DTPA, 2% DMSO, the indicated concentrations of H2O2, and Amplex Red. Th... | Biochemistry 52: 9187-201 (2013) Article DOI: 10.1021/bi401354d BindingDB Entry DOI: 10.7270/Q2K35SBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM92470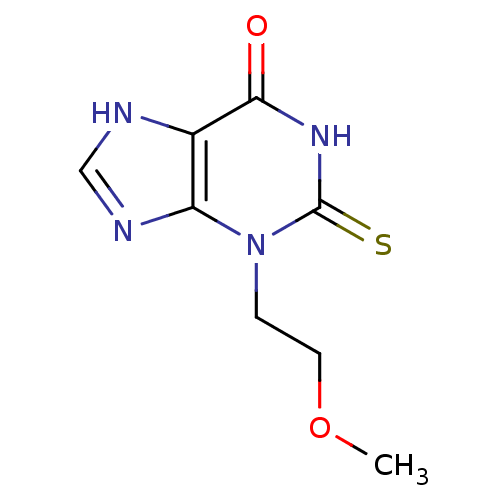 (2-Thioxanthine, TX5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | 7.4 | 21 |
AstraZeneca R&D | Assay Description The chlorination activity of MPO was determined by measuring the production of hypocholorous acid. | J Biol Chem 286: 37578-89 (2011) Article DOI: 10.1074/jbc.M111.266981 BindingDB Entry DOI: 10.7270/Q2M32TCV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM92469 (2-Thioxanthine, TX4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 7.4 | 21 |
AstraZeneca R&D | Assay Description The chlorination activity of MPO was determined by measuring the production of hypocholorous acid. | J Biol Chem 286: 37578-89 (2011) Article DOI: 10.1074/jbc.M111.266981 BindingDB Entry DOI: 10.7270/Q2M32TCV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM92468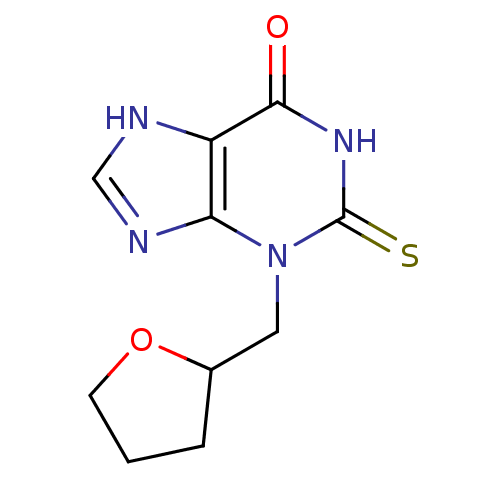 (2-Thioxanthine, TX3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | 7.4 | 21 |
AstraZeneca R&D | Assay Description The chlorination activity of MPO was determined by measuring the production of hypocholorous acid. | J Biol Chem 286: 37578-89 (2011) Article DOI: 10.1074/jbc.M111.266981 BindingDB Entry DOI: 10.7270/Q2M32TCV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM92466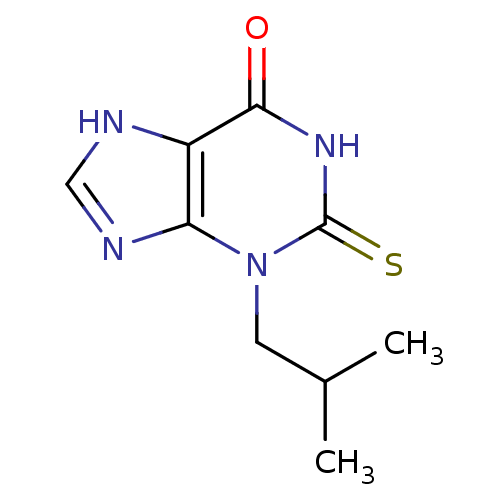 (2-Thioxanthine, TX1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | 7.4 | 21 |
AstraZeneca R&D | Assay Description The chlorination activity of MPO was determined by measuring the production of hypocholorous acid. | J Biol Chem 286: 37578-89 (2011) Article DOI: 10.1074/jbc.M111.266981 BindingDB Entry DOI: 10.7270/Q2M32TCV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM92467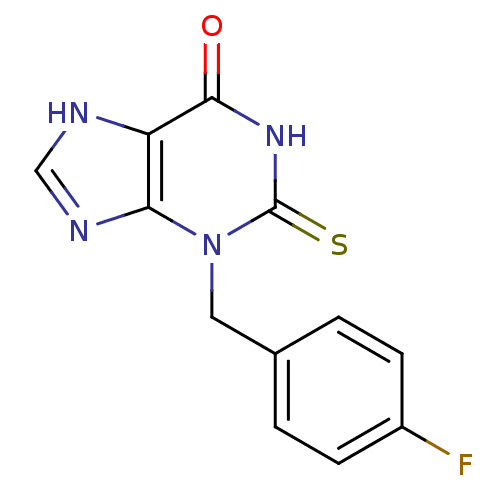 (2-Thioxanthine, TX2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 7.4 | 21 |
AstraZeneca R&D | Assay Description The chlorination activity of MPO was determined by measuring the production of hypocholorous acid. | J Biol Chem 286: 37578-89 (2011) Article DOI: 10.1074/jbc.M111.266981 BindingDB Entry DOI: 10.7270/Q2M32TCV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50133601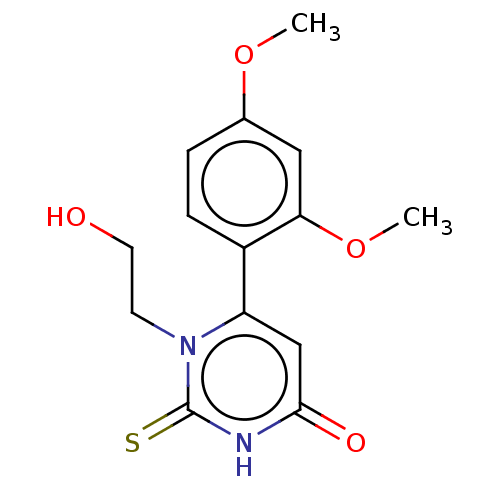 (CHEMBL3633251) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ... | J Med Chem 58: 8513-28 (2015) Article DOI: 10.1021/acs.jmedchem.5b00963 BindingDB Entry DOI: 10.7270/Q2SQ926X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50133602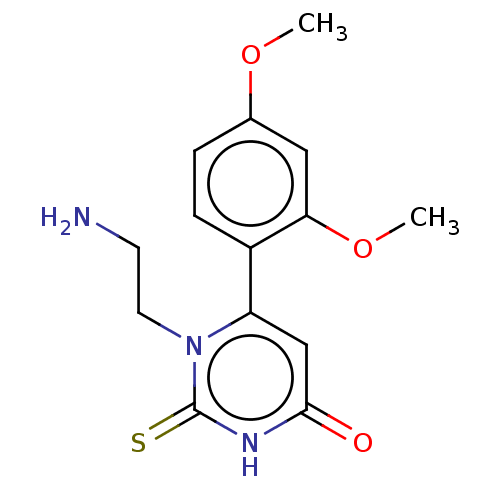 (CHEMBL3633250) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ... | J Med Chem 58: 8513-28 (2015) Article DOI: 10.1021/acs.jmedchem.5b00963 BindingDB Entry DOI: 10.7270/Q2SQ926X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50133595 (CHEMBL3633460) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ... | J Med Chem 58: 8513-28 (2015) Article DOI: 10.1021/acs.jmedchem.5b00963 BindingDB Entry DOI: 10.7270/Q2SQ926X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50133596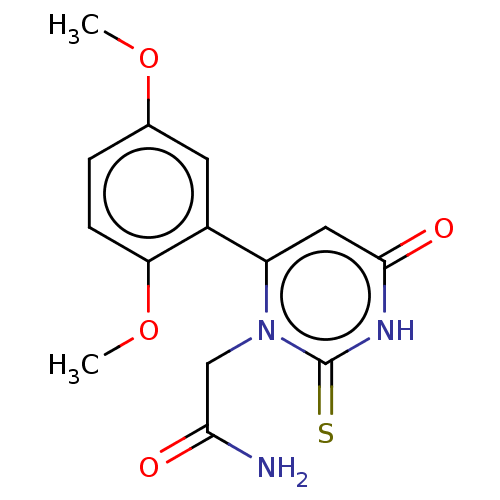 (CHEMBL3633459) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 347 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ... | J Med Chem 58: 8513-28 (2015) Article DOI: 10.1021/acs.jmedchem.5b00963 BindingDB Entry DOI: 10.7270/Q2SQ926X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50133603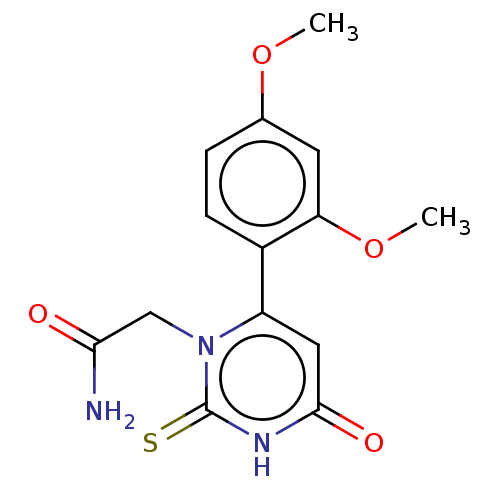 (CHEMBL3633248) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ... | J Med Chem 58: 8513-28 (2015) Article DOI: 10.1021/acs.jmedchem.5b00963 BindingDB Entry DOI: 10.7270/Q2SQ926X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50133600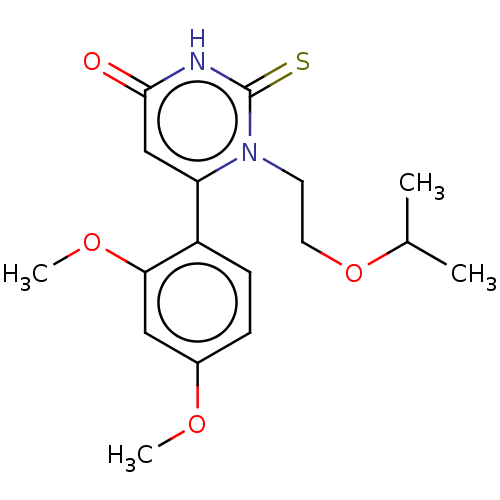 (CHEMBL3633457) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of peroxidase activity of MPO isolated from human polynuclear leukocytes using Amplex Red as substrate assessed as formation of resorufin ... | J Med Chem 58: 8513-28 (2015) Article DOI: 10.1021/acs.jmedchem.5b00963 BindingDB Entry DOI: 10.7270/Q2SQ926X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50002435 (3-Isopropoxy-5-methoxy-benzo[b]thiophene-2-carboxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of serum-opsonized zymosan stimulated myeloperoxidase release from human neutrophil | J Med Chem 35: 958-65 (1992) BindingDB Entry DOI: 10.7270/Q25T3JF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50008371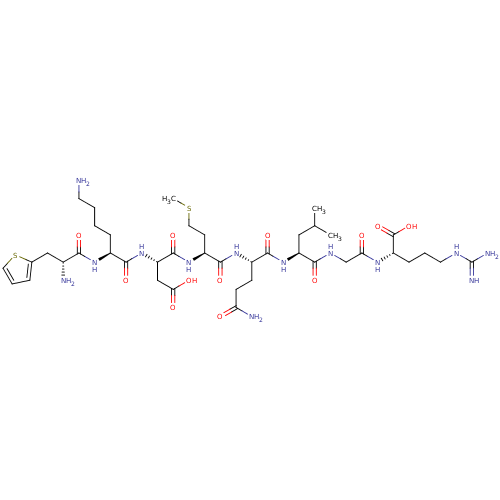 (CHEMBL127385 | H-Thi-Lys-AspMet-GIn-Leu-Gly-Arg-OH) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vivo potency of the compound was determined as the maximal lysosomal myeloperoxidase (MPO) release in PMNL assay | J Med Chem 35: 402-6 (1992) BindingDB Entry DOI: 10.7270/Q2HH6J1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50008373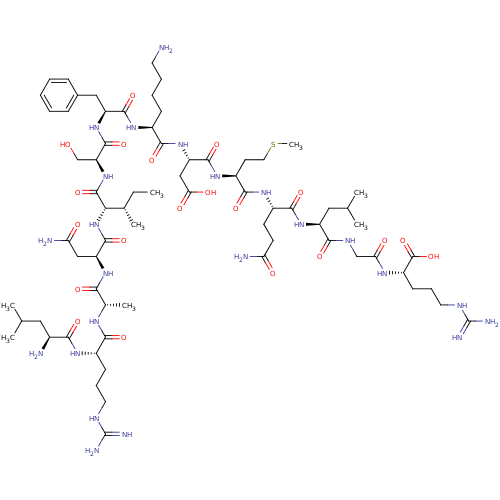 (CHEMBL439468 | H-Leu-Arg-Ala-Asn-Ile-Ser-Phe-Lys-A...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vivo potency of the compound was determined as the maximal lysosomal myeloperoxidase (MPO) release in PMNL assay | J Med Chem 35: 402-6 (1992) BindingDB Entry DOI: 10.7270/Q2HH6J1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50008382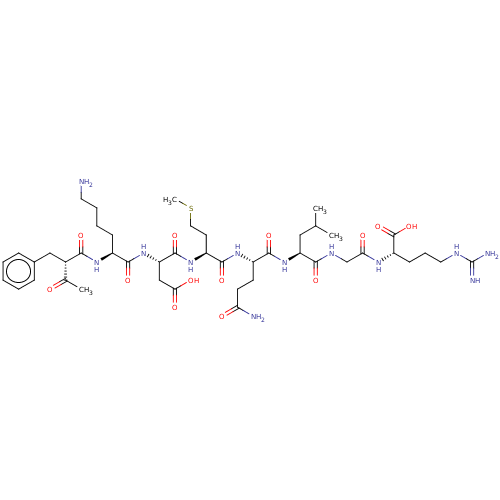 (Ac-Phe-Lys-AspMet-GIn-Leu-Gly-Arg-OH | CHEMBL23706...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vivo potency of the compound was determined as the maximal lysosomal myeloperoxidase (MPO) release in PMNL assay | J Med Chem 35: 402-6 (1992) BindingDB Entry DOI: 10.7270/Q2HH6J1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50008381 (CHEMBL441393 | H-Ile-Ser-Phe-Lys-AspMet-GIn-Leu-Gl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vivo potency of the compound was determined as the maximal lysosomal myeloperoxidase (MPO) release in PMNL assay | J Med Chem 35: 402-6 (1992) BindingDB Entry DOI: 10.7270/Q2HH6J1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50008383 (CHEMBL408929 | H-Ala-Asn-Ile-Ser-Phe-Lys-AspMet-GI...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.30E+5 | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vivo potency of the compound was determined as the maximal lysosomal myeloperoxidase (MPO) release in PMNL assay | J Med Chem 35: 402-6 (1992) BindingDB Entry DOI: 10.7270/Q2HH6J1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50035200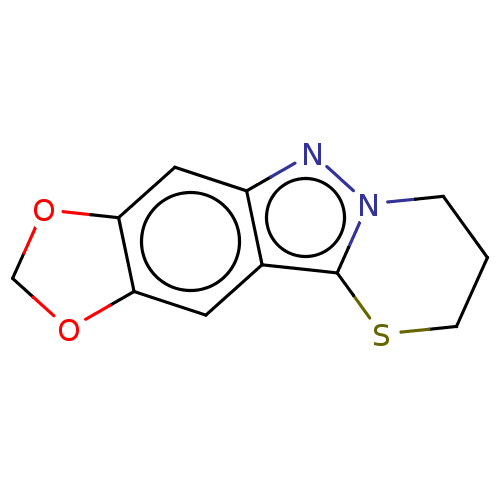 (CHEMBL3343355) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of myeloperoxidase (unknown origin) assessed as reduction in enzymatic hypochlorous acid production by spectrophotometry based taurine chl... | Bioorg Med Chem 22: 6422-9 (2014) Article DOI: 10.1016/j.bmc.2014.09.044 BindingDB Entry DOI: 10.7270/Q2M0472H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50035201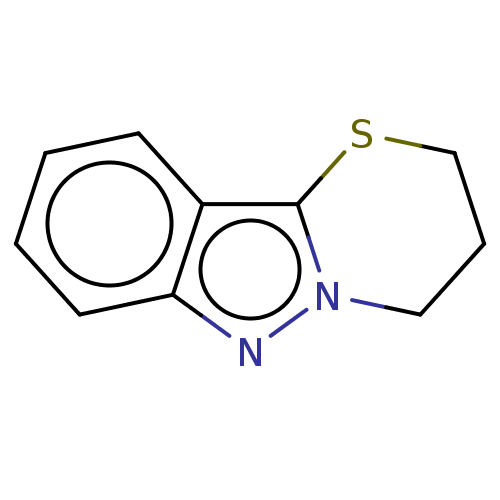 (CHEMBL3343354) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 213 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of myeloperoxidase (unknown origin) assessed as reduction in enzymatic hypochlorous acid production by spectrophotometry based taurine chl... | Bioorg Med Chem 22: 6422-9 (2014) Article DOI: 10.1016/j.bmc.2014.09.044 BindingDB Entry DOI: 10.7270/Q2M0472H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50035203 (CHEMBL3343353) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of myeloperoxidase (unknown origin) assessed as reduction in enzymatic hypochlorous acid production by spectrophotometry based taurine chl... | Bioorg Med Chem 22: 6422-9 (2014) Article DOI: 10.1016/j.bmc.2014.09.044 BindingDB Entry DOI: 10.7270/Q2M0472H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50035214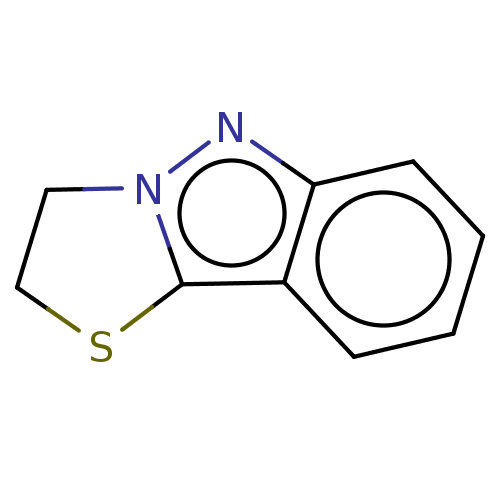 (CHEMBL3343352) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of myeloperoxidase (unknown origin) assessed as reduction in enzymatic hypochlorous acid production by spectrophotometry based taurine chl... | Bioorg Med Chem 22: 6422-9 (2014) Article DOI: 10.1016/j.bmc.2014.09.044 BindingDB Entry DOI: 10.7270/Q2M0472H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50035215 (CHEMBL3343351) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 403 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of myeloperoxidase (unknown origin) assessed as reduction in enzymatic hypochlorous acid production by spectrophotometry based taurine chl... | Bioorg Med Chem 22: 6422-9 (2014) Article DOI: 10.1016/j.bmc.2014.09.044 BindingDB Entry DOI: 10.7270/Q2M0472H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50035216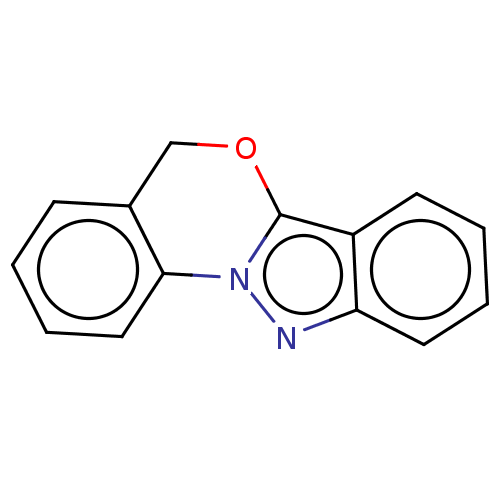 (CHEMBL3343350) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of myeloperoxidase (unknown origin) assessed as reduction in enzymatic hypochlorous acid production by spectrophotometry based taurine chl... | Bioorg Med Chem 22: 6422-9 (2014) Article DOI: 10.1016/j.bmc.2014.09.044 BindingDB Entry DOI: 10.7270/Q2M0472H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM94407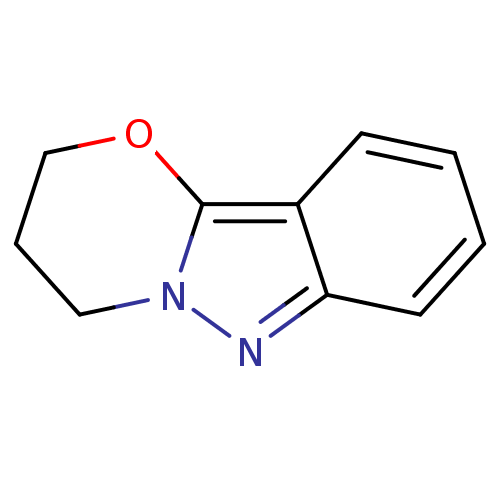 (3,4-dihydro-2H-[1,3]oxazin[3,2-b]indazole | 3,4-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 359 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of myeloperoxidase (unknown origin) assessed as reduction in enzymatic hypochlorous acid production by spectrophotometry based taurine chl... | Bioorg Med Chem 22: 6422-9 (2014) Article DOI: 10.1016/j.bmc.2014.09.044 BindingDB Entry DOI: 10.7270/Q2M0472H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50035217 (CHEMBL3343349) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of myeloperoxidase (unknown origin) assessed as reduction in enzymatic hypochlorous acid production by spectrophotometry based taurine chl... | Bioorg Med Chem 22: 6422-9 (2014) Article DOI: 10.1016/j.bmc.2014.09.044 BindingDB Entry DOI: 10.7270/Q2M0472H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50035219 (CHEMBL2141595) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of myeloperoxidase (unknown origin) assessed as reduction in enzymatic hypochlorous acid production by spectrophotometry based taurine chl... | Bioorg Med Chem 22: 6422-9 (2014) Article DOI: 10.1016/j.bmc.2014.09.044 BindingDB Entry DOI: 10.7270/Q2M0472H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50035220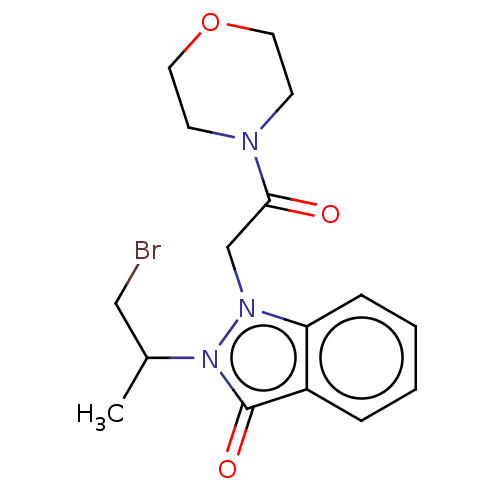 (CHEMBL3343348) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of myeloperoxidase (unknown origin) assessed as reduction in enzymatic hypochlorous acid production by spectrophotometry based taurine chl... | Bioorg Med Chem 22: 6422-9 (2014) Article DOI: 10.1016/j.bmc.2014.09.044 BindingDB Entry DOI: 10.7270/Q2M0472H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50035221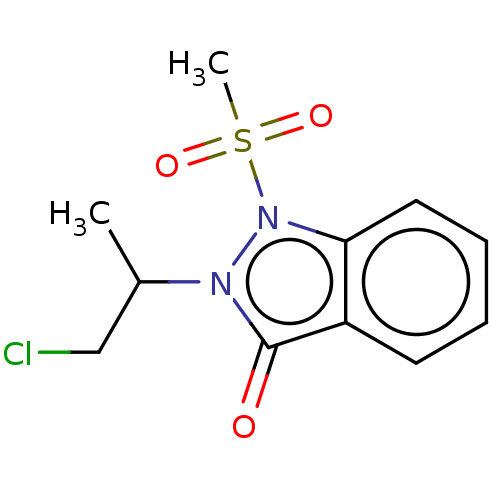 (CHEMBL2360147) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of myeloperoxidase (unknown origin) assessed as reduction in enzymatic hypochlorous acid production by spectrophotometry based taurine chl... | Bioorg Med Chem 22: 6422-9 (2014) Article DOI: 10.1016/j.bmc.2014.09.044 BindingDB Entry DOI: 10.7270/Q2M0472H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50035222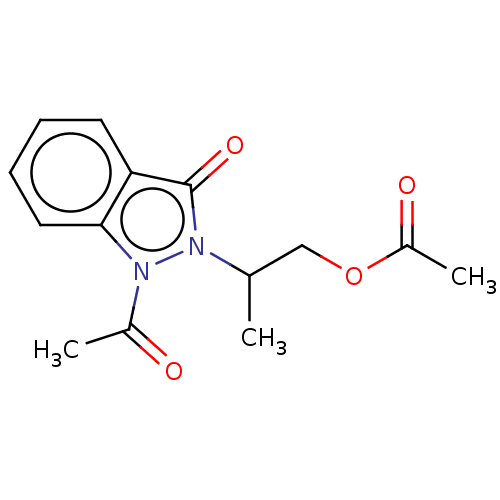 (CHEMBL3341774) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of myeloperoxidase (unknown origin) assessed as reduction in enzymatic hypochlorous acid production by spectrophotometry based taurine chl... | Bioorg Med Chem 22: 6422-9 (2014) Article DOI: 10.1016/j.bmc.2014.09.044 BindingDB Entry DOI: 10.7270/Q2M0472H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50035223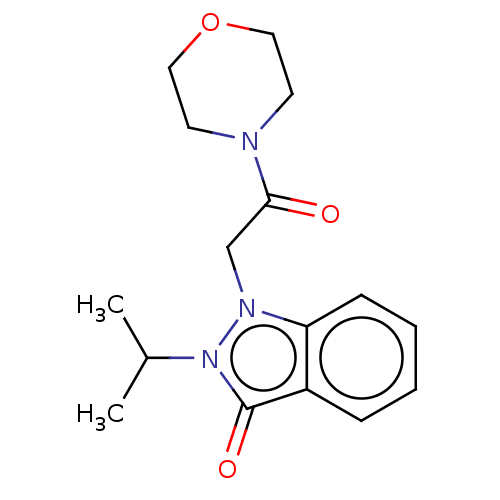 (CHEMBL2137882) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of myeloperoxidase (unknown origin) assessed as reduction in enzymatic hypochlorous acid production by spectrophotometry based taurine chl... | Bioorg Med Chem 22: 6422-9 (2014) Article DOI: 10.1016/j.bmc.2014.09.044 BindingDB Entry DOI: 10.7270/Q2M0472H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50035224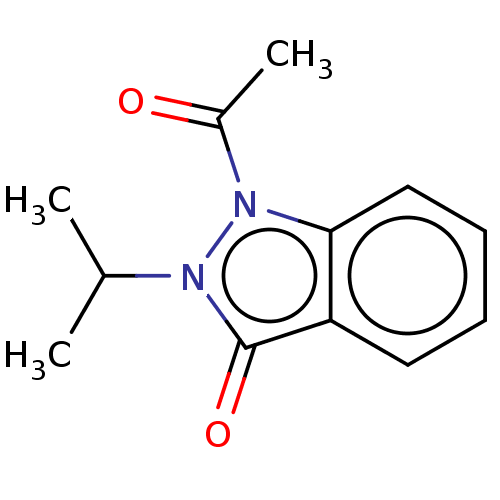 (CHEMBL3343347) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of myeloperoxidase (unknown origin) assessed as reduction in enzymatic hypochlorous acid production by spectrophotometry based taurine chl... | Bioorg Med Chem 22: 6422-9 (2014) Article DOI: 10.1016/j.bmc.2014.09.044 BindingDB Entry DOI: 10.7270/Q2M0472H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50035225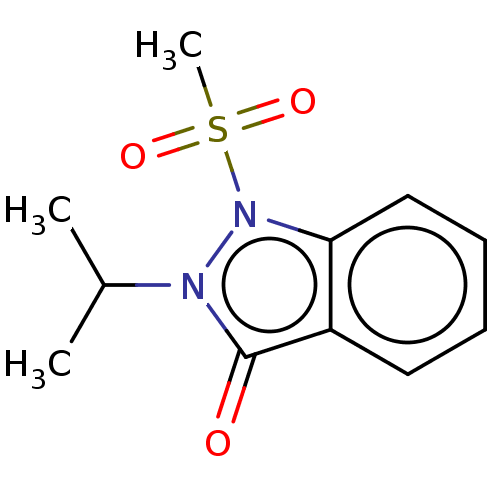 (CHEMBL3343346) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of myeloperoxidase (unknown origin) assessed as reduction in enzymatic hypochlorous acid production by spectrophotometry based taurine chl... | Bioorg Med Chem 22: 6422-9 (2014) Article DOI: 10.1016/j.bmc.2014.09.044 BindingDB Entry DOI: 10.7270/Q2M0472H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50035226 (CHEMBL3343345) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of myeloperoxidase (unknown origin) assessed as reduction in enzymatic hypochlorous acid production by spectrophotometry based taurine chl... | Bioorg Med Chem 22: 6422-9 (2014) Article DOI: 10.1016/j.bmc.2014.09.044 BindingDB Entry DOI: 10.7270/Q2M0472H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50035227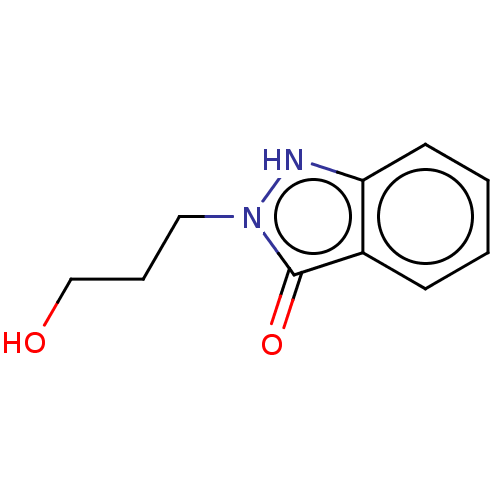 (CHEMBL3144794) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of myeloperoxidase (unknown origin) assessed as reduction in enzymatic hypochlorous acid production by spectrophotometry based taurine chl... | Bioorg Med Chem 22: 6422-9 (2014) Article DOI: 10.1016/j.bmc.2014.09.044 BindingDB Entry DOI: 10.7270/Q2M0472H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50035228 (CHEMBL3144695) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of myeloperoxidase (unknown origin) assessed as reduction in enzymatic hypochlorous acid production by spectrophotometry based taurine chl... | Bioorg Med Chem 22: 6422-9 (2014) Article DOI: 10.1016/j.bmc.2014.09.044 BindingDB Entry DOI: 10.7270/Q2M0472H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50035229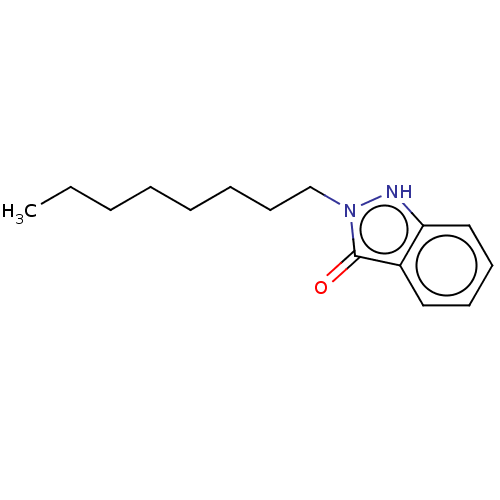 (CHEMBL3343344) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of myeloperoxidase (unknown origin) assessed as reduction in enzymatic hypochlorous acid production by spectrophotometry based taurine chl... | Bioorg Med Chem 22: 6422-9 (2014) Article DOI: 10.1016/j.bmc.2014.09.044 BindingDB Entry DOI: 10.7270/Q2M0472H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50035230 (CHEMBL3144791) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 792 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of myeloperoxidase (unknown origin) assessed as reduction in enzymatic hypochlorous acid production by spectrophotometry based taurine chl... | Bioorg Med Chem 22: 6422-9 (2014) Article DOI: 10.1016/j.bmc.2014.09.044 BindingDB Entry DOI: 10.7270/Q2M0472H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50035231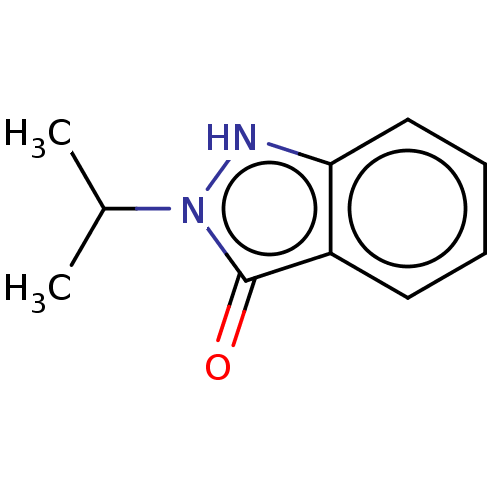 (CHEMBL3145355) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of myeloperoxidase (unknown origin) assessed as reduction in enzymatic hypochlorous acid production by spectrophotometry based taurine chl... | Bioorg Med Chem 22: 6422-9 (2014) Article DOI: 10.1016/j.bmc.2014.09.044 BindingDB Entry DOI: 10.7270/Q2M0472H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50035232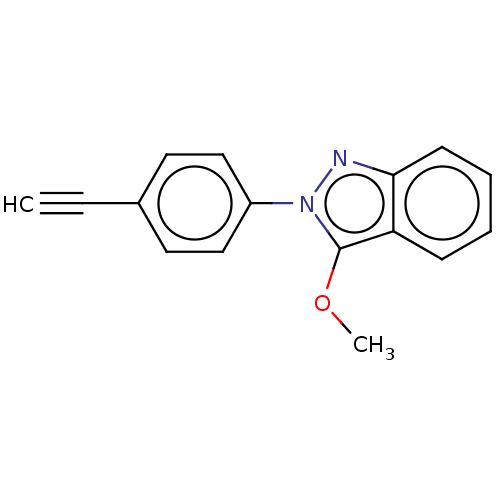 (CHEMBL3343343) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of myeloperoxidase (unknown origin) assessed as reduction in enzymatic hypochlorous acid production by spectrophotometry based taurine chl... | Bioorg Med Chem 22: 6422-9 (2014) Article DOI: 10.1016/j.bmc.2014.09.044 BindingDB Entry DOI: 10.7270/Q2M0472H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50035233 (CHEMBL3343342) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of myeloperoxidase (unknown origin) assessed as reduction in enzymatic hypochlorous acid production by spectrophotometry based taurine chl... | Bioorg Med Chem 22: 6422-9 (2014) Article DOI: 10.1016/j.bmc.2014.09.044 BindingDB Entry DOI: 10.7270/Q2M0472H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1037 total ) | Next | Last >> |