

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin K (Homo sapiens (Human)) | BDBM50095493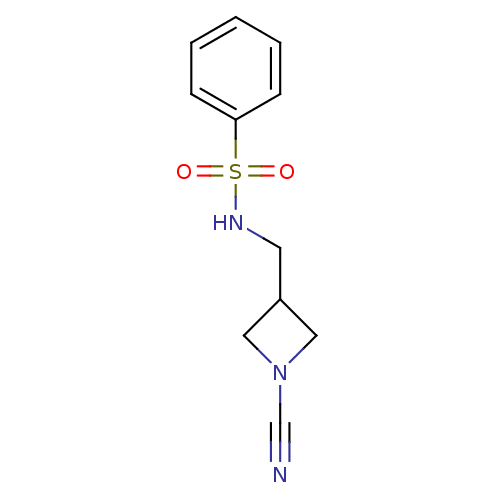 (CHEMBL276169 | N-(1-Cyano-azetidin-3-ylmethyl)-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory constant of the compound against human cathepsin K | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095489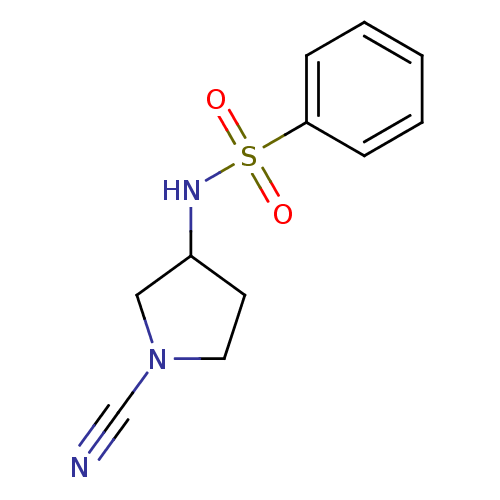 (CHEMBL275080 | N-(1-Cyano-pyrrolidin-3-yl)-benzene...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory constant of the compound against human cathepsin K | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095491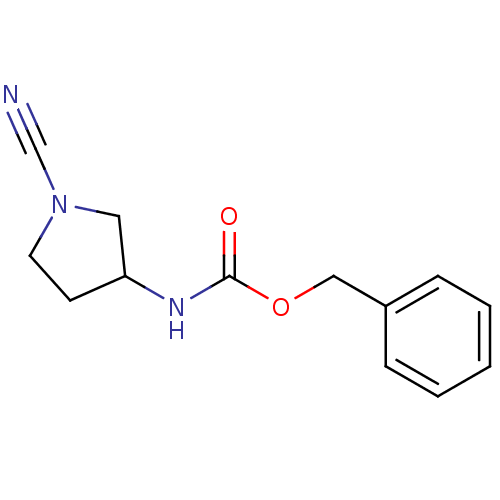 ((1-Cyano-pyrrolidin-3-yl)-carbamic acid benzyl est...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory constant of the compound against human cathepsin K | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095488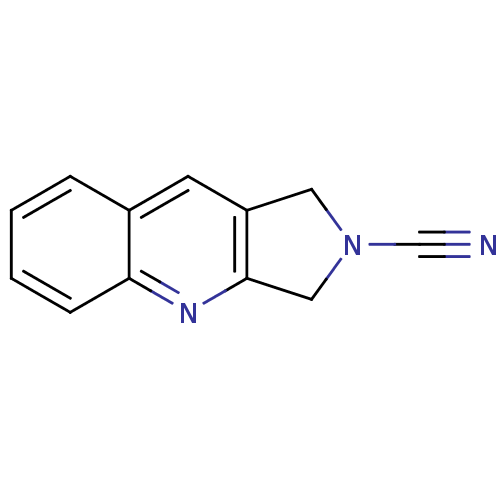 (1,3-Dihydro-pyrrolo[3,4-b]quinoline-2-carbonitrile...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory constant of the compound against human cathepsin K | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095487 (CHEMBL8796 | Cyclohexanecarboxylic acid (1-cyano-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin K using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50095487 (CHEMBL8796 | Cyclohexanecarboxylic acid (1-cyano-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin L using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095487 (CHEMBL8796 | Cyclohexanecarboxylic acid (1-cyano-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin K receptor using gelatinase assay | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095487 (CHEMBL8796 | Cyclohexanecarboxylic acid (1-cyano-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 5.5 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of Cathepsin K at pH 5.5 | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095493 (CHEMBL276169 | N-(1-Cyano-azetidin-3-ylmethyl)-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory constant of the compound against human cathepsin K | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095489 (CHEMBL275080 | N-(1-Cyano-pyrrolidin-3-yl)-benzene...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin K receptor using gelatinase assay | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095491 ((1-Cyano-pyrrolidin-3-yl)-carbamic acid benzyl est...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin K receptor using gelatinase assay | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095493 (CHEMBL276169 | N-(1-Cyano-azetidin-3-ylmethyl)-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin K using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095483 (3-Cyclohexylmethoxy-azetidine-1-carbonitrile | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin K using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095487 (CHEMBL8796 | Cyclohexanecarboxylic acid (1-cyano-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | 5.5 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Effect of 10 mM of GSH on the inhibitory activity of compound against Cathepsin K, at pH 5.5 | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095487 (CHEMBL8796 | Cyclohexanecarboxylic acid (1-cyano-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of Cathepsin K at pH 7 | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095491 ((1-Cyano-pyrrolidin-3-yl)-carbamic acid benzyl est...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory constant of the compound against human cathepsin K | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095491 ((1-Cyano-pyrrolidin-3-yl)-carbamic acid benzyl est...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin K using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095486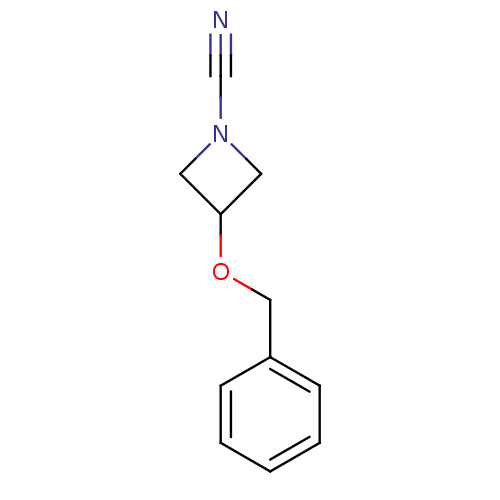 (3-Benzyloxy-azetidine-1-carbonitrile | CHEMBL8547) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin K using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50095493 (CHEMBL276169 | N-(1-Cyano-azetidin-3-ylmethyl)-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin L using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095491 ((1-Cyano-pyrrolidin-3-yl)-carbamic acid benzyl est...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of Cathepsin K at pH 7 | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095489 (CHEMBL275080 | N-(1-Cyano-pyrrolidin-3-yl)-benzene...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin K using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50095491 ((1-Cyano-pyrrolidin-3-yl)-carbamic acid benzyl est...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin L using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095491 ((1-Cyano-pyrrolidin-3-yl)-carbamic acid benzyl est...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | 5.5 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of Cathepsin K at pH 5.5 | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095498 (CHEMBL8577 | N-(1-Cyano-azetidin-3-ylmethyl)-benza...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin K using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50095489 (CHEMBL275080 | N-(1-Cyano-pyrrolidin-3-yl)-benzene...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin L using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095489 (CHEMBL275080 | N-(1-Cyano-pyrrolidin-3-yl)-benzene...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory constant of the compound against human cathepsin K | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095491 ((1-Cyano-pyrrolidin-3-yl)-carbamic acid benzyl est...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | 5.5 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Effect of 10 mM of GSH on the inhibitory activity of compound against Cathepsin K, at pH 5.5 | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095490 (3-Benzyloxymethyl-pyrrolidine-1-carbonitrile | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin K using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50095483 (3-Cyclohexylmethoxy-azetidine-1-carbonitrile | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin L using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50095486 (3-Benzyloxy-azetidine-1-carbonitrile | CHEMBL8547) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin L using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095485 (Azetidine-1-carbonitrile | CHEMBL8123) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin K using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095491 ((1-Cyano-pyrrolidin-3-yl)-carbamic acid benzyl est...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Effect of 10 mM of GSH on the inhibitory activity against Cathepsin K, at pH 7 | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50095490 (3-Benzyloxymethyl-pyrrolidine-1-carbonitrile | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin L using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50095487 (CHEMBL8796 | Cyclohexanecarboxylic acid (1-cyano-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin B using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50095491 ((1-Cyano-pyrrolidin-3-yl)-carbamic acid benzyl est...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin B using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095494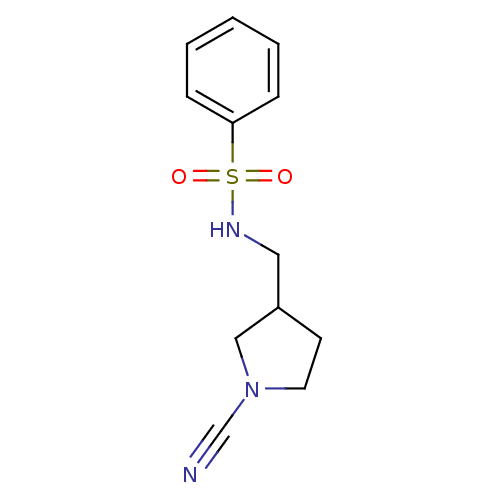 (CHEMBL8563 | N-(1-Cyano-pyrrolidin-3-ylmethyl)-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin K using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50095489 (CHEMBL275080 | N-(1-Cyano-pyrrolidin-3-yl)-benzene...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | 5.5 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against papain at a pH 5.5 | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50095498 (CHEMBL8577 | N-(1-Cyano-azetidin-3-ylmethyl)-benza...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin B using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095488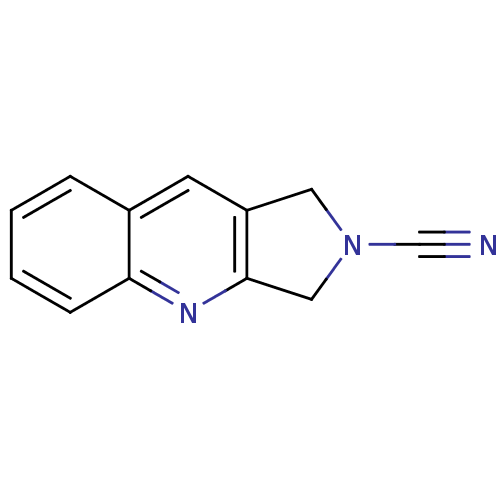 (1,3-Dihydro-pyrrolo[3,4-b]quinoline-2-carbonitrile...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of Cathepsin K at pH 7 | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095499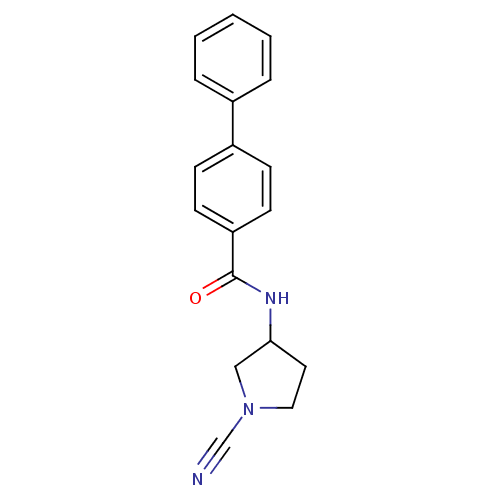 (Biphenyl-4-carboxylic acid (1-cyano-pyrrolidin-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin K using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50095499 (Biphenyl-4-carboxylic acid (1-cyano-pyrrolidin-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin B using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50095493 (CHEMBL276169 | N-(1-Cyano-azetidin-3-ylmethyl)-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin B using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095488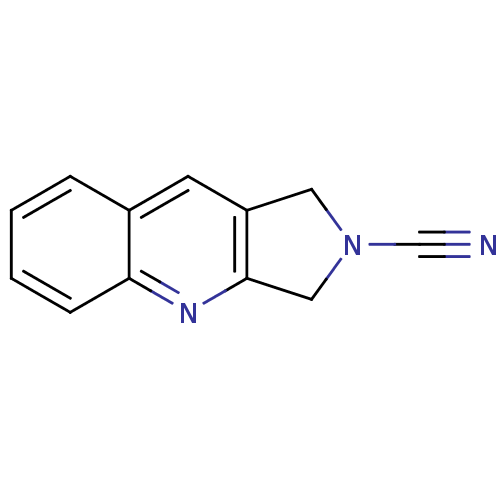 (1,3-Dihydro-pyrrolo[3,4-b]quinoline-2-carbonitrile...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | 5.5 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of Cathepsin K at pH 5.5 | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50095494 (CHEMBL8563 | N-(1-Cyano-pyrrolidin-3-ylmethyl)-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin L using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095488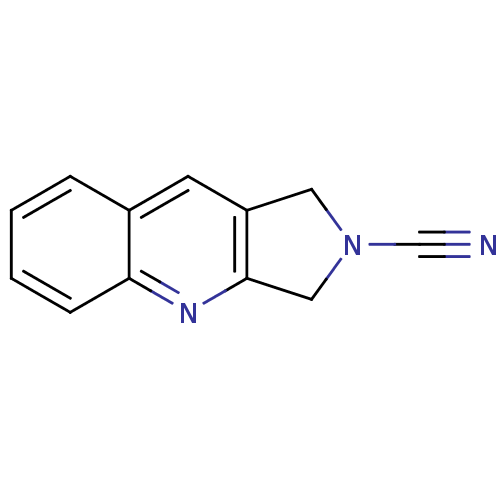 (1,3-Dihydro-pyrrolo[3,4-b]quinoline-2-carbonitrile...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | 5.5 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Effect of 10 mM of GSH on the inhibitory activity of compound against Cathepsin K, at pH 5.5 | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095488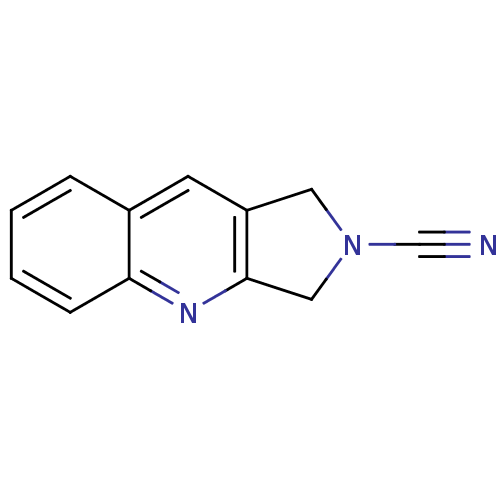 (1,3-Dihydro-pyrrolo[3,4-b]quinoline-2-carbonitrile...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin K receptor using gelatinase assay | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095492 (CHEMBL440590 | N-(1-Cyano-pyrrolidin-3-yl)-benzami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin K using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095488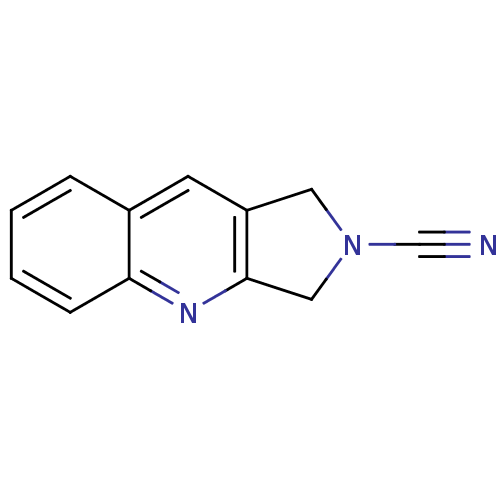 (1,3-Dihydro-pyrrolo[3,4-b]quinoline-2-carbonitrile...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin K using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095488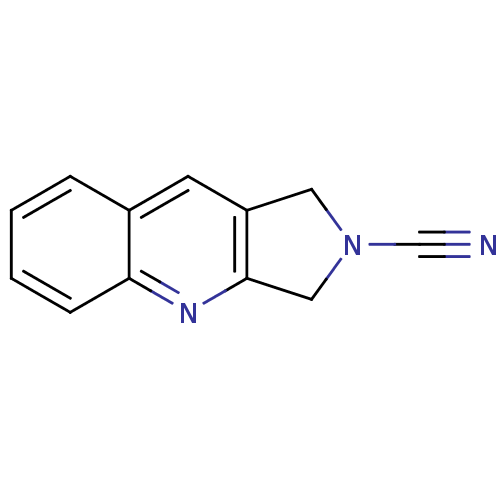 (1,3-Dihydro-pyrrolo[3,4-b]quinoline-2-carbonitrile...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory constant of the compound against human cathepsin K | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50095485 (Azetidine-1-carbonitrile | CHEMBL8123) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin L using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095485 (Azetidine-1-carbonitrile | CHEMBL8123) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | 5.5 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of Cathepsin K at pH 5.5 | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50095488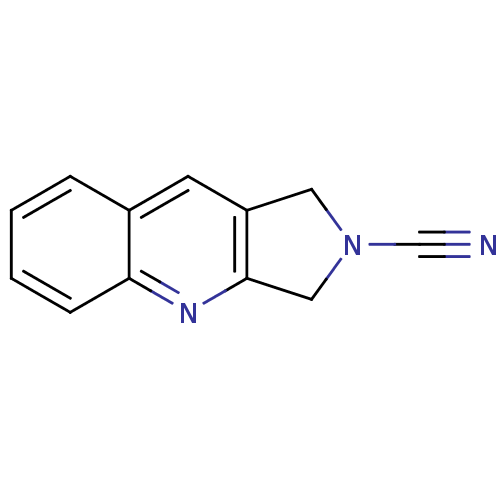 (1,3-Dihydro-pyrrolo[3,4-b]quinoline-2-carbonitrile...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin L using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095485 (Azetidine-1-carbonitrile | CHEMBL8123) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | 5.5 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Effect of 10 mM of GSH on the inhibitory activity of compound against Cathepsin K, at pH 5.5 | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50095483 (3-Cyclohexylmethoxy-azetidine-1-carbonitrile | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin B using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50095486 (3-Benzyloxy-azetidine-1-carbonitrile | CHEMBL8547) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin B using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50095498 (CHEMBL8577 | N-(1-Cyano-azetidin-3-ylmethyl)-benza...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin L using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095489 (CHEMBL275080 | N-(1-Cyano-pyrrolidin-3-yl)-benzene...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin K receptor using bone resorption assay | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50095499 (Biphenyl-4-carboxylic acid (1-cyano-pyrrolidin-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin L using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095485 (Azetidine-1-carbonitrile | CHEMBL8123) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of Cathepsin K at pH 7 | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095495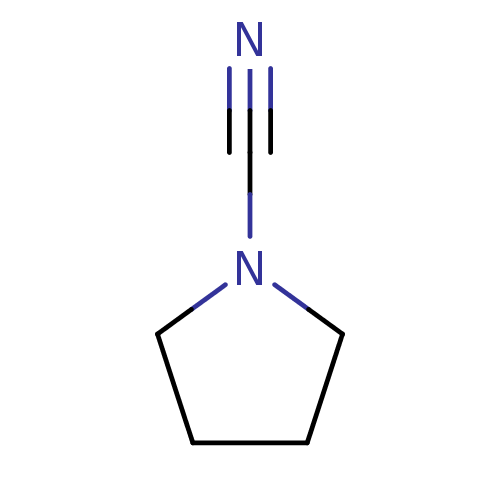 (CHEMBL262697 | Pyrrolidine-1-carbonitrile) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin K using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50095490 (3-Benzyloxymethyl-pyrrolidine-1-carbonitrile | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin B using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50095489 (CHEMBL275080 | N-(1-Cyano-pyrrolidin-3-yl)-benzene...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin B using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095491 ((1-Cyano-pyrrolidin-3-yl)-carbamic acid benzyl est...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin K receptor using bone resorption assay | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095495 (CHEMBL262697 | Pyrrolidine-1-carbonitrile) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | 5.5 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Effect of 10 mM of GSH on the inhibitory activity of compound against Cathepsin K, at pH 5.5 | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50095494 (CHEMBL8563 | N-(1-Cyano-pyrrolidin-3-ylmethyl)-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin B using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095495 (CHEMBL262697 | Pyrrolidine-1-carbonitrile) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.63E+3 | n/a | n/a | n/a | n/a | 5.5 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of Cathepsin K at pH 5.5 | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50095492 (CHEMBL440590 | N-(1-Cyano-pyrrolidin-3-yl)-benzami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin L using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50095485 (Azetidine-1-carbonitrile | CHEMBL8123) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin B using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50095488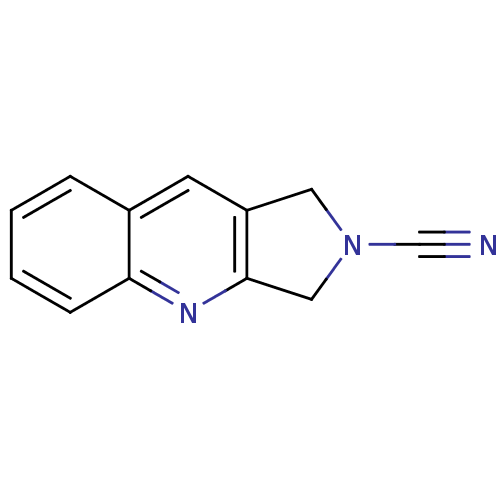 (1,3-Dihydro-pyrrolo[3,4-b]quinoline-2-carbonitrile...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin B using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095495 (CHEMBL262697 | Pyrrolidine-1-carbonitrile) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of Cathepsin K at pH 7 | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095495 (CHEMBL262697 | Pyrrolidine-1-carbonitrile) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Effect of 10 mM of GSH on the inhibitory activity against Cathepsin K, at pH 7 | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50095495 (CHEMBL262697 | Pyrrolidine-1-carbonitrile) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin L using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50095492 (CHEMBL440590 | N-(1-Cyano-pyrrolidin-3-yl)-benzami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin B using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095488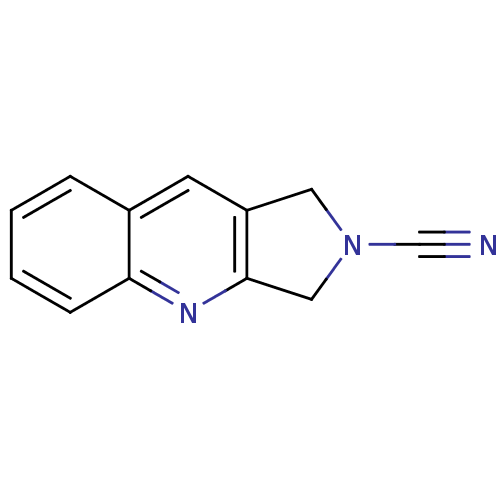 (1,3-Dihydro-pyrrolo[3,4-b]quinoline-2-carbonitrile...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Effect of 10 mM of GSH on the inhibitory activity against Cathepsin K, at pH 7 | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50095496 (CHEMBL8591 | N-(1-Cyano-pyrrolidin-2-ylmethyl)-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin L using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095496 (CHEMBL8591 | N-(1-Cyano-pyrrolidin-2-ylmethyl)-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin K using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095497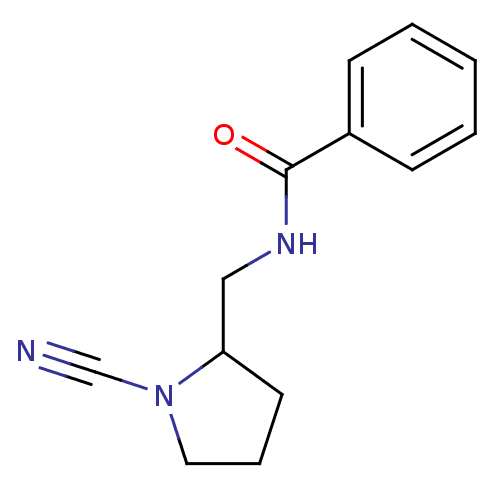 (CHEMBL8480 | N-(1-Cyano-pyrrolidin-2-ylmethyl)-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin K using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50095495 (CHEMBL262697 | Pyrrolidine-1-carbonitrile) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin B using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50095497 (CHEMBL8480 | N-(1-Cyano-pyrrolidin-2-ylmethyl)-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin L using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50095497 (CHEMBL8480 | N-(1-Cyano-pyrrolidin-2-ylmethyl)-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin B using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095484 (CHEMBL274120 | Diethyl-cyanamide) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin K using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095487 (CHEMBL8796 | Cyclohexanecarboxylic acid (1-cyano-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Effect of 10 mM of GSH on the inhibitory activity against Cathepsin K, at pH 7 | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50095484 (CHEMBL274120 | Diethyl-cyanamide) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin B using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50095484 (CHEMBL274120 | Diethyl-cyanamide) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory activity tested against Human Cathepsin L using Z-Phe-Arg-pNA as substrate | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50095485 (Azetidine-1-carbonitrile | CHEMBL8123) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Effect of 10 mM of GSH on the inhibitory activity against Cathepsin K, at pH 7 | J Med Chem 44: 94-104 (2001) BindingDB Entry DOI: 10.7270/Q2DV1J4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||