

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phospholipase A2 (Apis mellifera) | BDBM50269843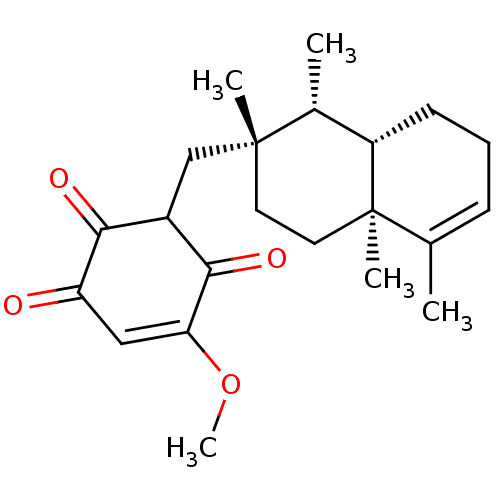 (Bolinaquinone | CHEMBL477692) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Napoli Federico II Curated by ChEMBL | Assay Description Inhibition of bee venom group 3 secretory phospholipase A2 by liquid scintillation counting | J Nat Prod 64: 612-5 (2001) BindingDB Entry DOI: 10.7270/Q28915MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50269843 (Bolinaquinone | CHEMBL477692) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Napoli Federico II Curated by ChEMBL | Assay Description Inhibition of human synovial recombinant group 2 secretory phospholipase A2 by liquid scintillation counting | J Nat Prod 64: 612-5 (2001) BindingDB Entry DOI: 10.7270/Q28915MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, major isoenzyme (Sus scrofa (pig)) | BDBM50269843 (Bolinaquinone | CHEMBL477692) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Napoli Federico II Curated by ChEMBL | Assay Description Inhibition of pig pancreatic group 1 secretory phospholipase A2 by liquid scintillation counting | J Nat Prod 64: 612-5 (2001) BindingDB Entry DOI: 10.7270/Q28915MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50269844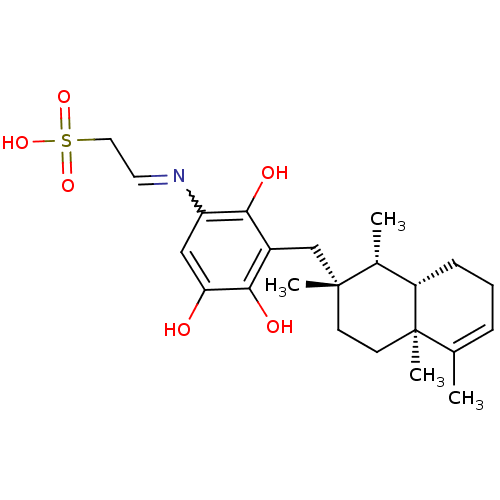 (CHEMBL517216 | Dysidine) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Napoli Federico II Curated by ChEMBL | Assay Description Inhibition of human synovial recombinant group 2 secretory phospholipase A2 by liquid scintillation counting | J Nat Prod 64: 612-5 (2001) BindingDB Entry DOI: 10.7270/Q28915MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50250399 (5-Hydroxy-4-{(R)-6-hydroxy-5-[(E)-4-methyl-6-(2,6,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Napoli Federico II Curated by ChEMBL | Assay Description Inhibition of human synovial recombinant group 2 secretory phospholipase A2 by liquid scintillation counting | J Nat Prod 64: 612-5 (2001) BindingDB Entry DOI: 10.7270/Q28915MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 (Apis mellifera) | BDBM50250399 (5-Hydroxy-4-{(R)-6-hydroxy-5-[(E)-4-methyl-6-(2,6,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Napoli Federico II Curated by ChEMBL | Assay Description Inhibition of bee venom group 3 secretory phospholipase A2 by liquid scintillation counting | J Nat Prod 64: 612-5 (2001) BindingDB Entry DOI: 10.7270/Q28915MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||