

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate receptor 2 (Homo sapiens (Human)) | BDBM50207594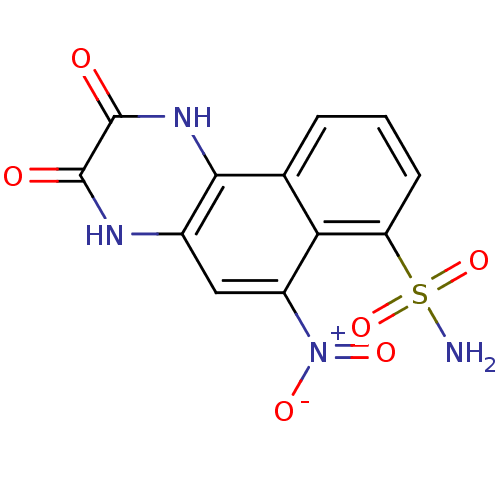 (2,3-Dihydroxy-6-nitro-benzo[f]quinoxaline-7-sulfon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Ability to displace binding of [3H]AMPA to recombinant human Ionotropic glutamate receptor AMPA 2 | J Med Chem 45: 4383-6 (2002) BindingDB Entry DOI: 10.7270/Q2XP75N4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate receptor 4 (Homo sapiens (Human)) | BDBM50207594 (2,3-Dihydroxy-6-nitro-benzo[f]quinoxaline-7-sulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Ability to displace binding of [3H]AMPA to recombinant human Ionotropic glutamate receptor AMPA 4 | J Med Chem 45: 4383-6 (2002) BindingDB Entry DOI: 10.7270/Q2XP75N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 3 (Homo sapiens (Human)) | BDBM50207594 (2,3-Dihydroxy-6-nitro-benzo[f]quinoxaline-7-sulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Ability to displace binding of [3H]AMPA to recombinant human Ionotropic glutamate receptor AMPA 3 | J Med Chem 45: 4383-6 (2002) BindingDB Entry DOI: 10.7270/Q2XP75N4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate receptor 1 (Homo sapiens (Human)) | BDBM50207594 (2,3-Dihydroxy-6-nitro-benzo[f]quinoxaline-7-sulfon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Ability to displace binding of [3H]AMPA to recombinant human Ionotropic glutamate receptor AMPA 1 | J Med Chem 45: 4383-6 (2002) BindingDB Entry DOI: 10.7270/Q2XP75N4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate receptor 2 (Homo sapiens (Human)) | BDBM86751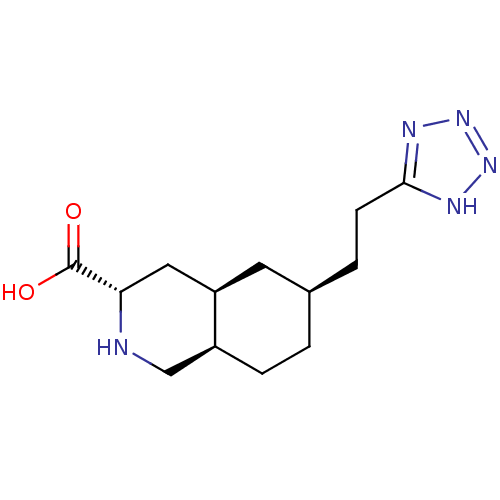 (CHEMBL14935 | LY 293558 | LY-293558) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Ability to displace binding of [3H]AMPA to recombinant human Ionotropic glutamate receptor AMPA 2 | J Med Chem 45: 4383-6 (2002) BindingDB Entry DOI: 10.7270/Q2XP75N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 1 (Homo sapiens (Human)) | BDBM86751 (CHEMBL14935 | LY 293558 | LY-293558) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Ability to displace binding of [3H]AMPA to recombinant human Ionotropic glutamate receptor AMPA 1 | J Med Chem 45: 4383-6 (2002) BindingDB Entry DOI: 10.7270/Q2XP75N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 2 (Homo sapiens (Human)) | BDBM50207594 (2,3-Dihydroxy-6-nitro-benzo[f]quinoxaline-7-sulfon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Ability to displace binding of [3H]AMPA to recombinant human AMPA receptor Ionotropic glutamate receptor ionotropic kainate 2 | J Med Chem 45: 4383-6 (2002) BindingDB Entry DOI: 10.7270/Q2XP75N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 3 (Homo sapiens (Human)) | BDBM86751 (CHEMBL14935 | LY 293558 | LY-293558) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Ability to displace binding of [3H]AMPA to recombinant human Ionotropic glutamate receptor AMPA 3 | J Med Chem 45: 4383-6 (2002) BindingDB Entry DOI: 10.7270/Q2XP75N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 4 (Homo sapiens (Human)) | BDBM86751 (CHEMBL14935 | LY 293558 | LY-293558) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Ability to displace binding of [3H]AMPA to recombinant human Ionotropic glutamate receptor AMPA 4 | J Med Chem 45: 4383-6 (2002) BindingDB Entry DOI: 10.7270/Q2XP75N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 2 (Homo sapiens (Human)) | BDBM86751 (CHEMBL14935 | LY 293558 | LY-293558) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Ability to displace binding of [3H]AMPA to recombinant human AMPA receptor Ionotropic glutamate receptor ionotropic kainate 2 | J Med Chem 45: 4383-6 (2002) BindingDB Entry DOI: 10.7270/Q2XP75N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 2 (Homo sapiens (Human)) | BDBM50207594 (2,3-Dihydroxy-6-nitro-benzo[f]quinoxaline-7-sulfon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Ability to displace binding of [3H]KA to recombinant human KA receptor Ionotropic glutamate receptor ionotropic kainate 2 expressed in EK 293 cell me... | J Med Chem 45: 4383-6 (2002) BindingDB Entry DOI: 10.7270/Q2XP75N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 2 (Homo sapiens (Human)) | BDBM86751 (CHEMBL14935 | LY 293558 | LY-293558) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Ability to displace binding of [3H]KA to recombinant human KA receptor Ionotropic glutamate receptor ionotropic kainate 2 expressed in EK 293 cell me... | J Med Chem 45: 4383-6 (2002) BindingDB Entry DOI: 10.7270/Q2XP75N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 2 (Homo sapiens (Human)) | BDBM50118701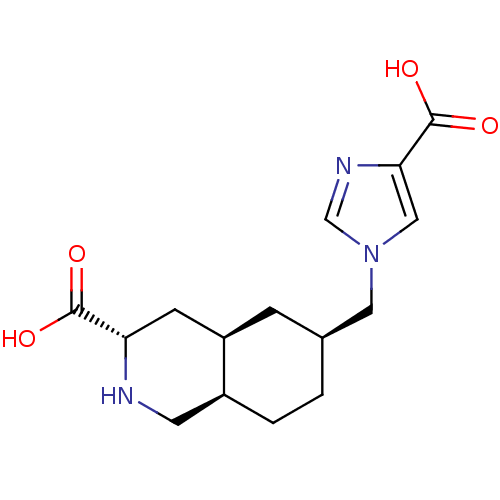 (6-(4-Carboxy-imidazol-1-ylmethyl)-decahydro-isoqui...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.17E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Ability to displace binding of [3H]AMPA to recombinant human Ionotropic glutamate receptor AMPA 2 | J Med Chem 45: 4383-6 (2002) BindingDB Entry DOI: 10.7270/Q2XP75N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 1 (Homo sapiens (Human)) | BDBM50118701 (6-(4-Carboxy-imidazol-1-ylmethyl)-decahydro-isoqui...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.34E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Ability to displace binding of [3H]AMPA to recombinant human Ionotropic glutamate receptor AMPA 1 | J Med Chem 45: 4383-6 (2002) BindingDB Entry DOI: 10.7270/Q2XP75N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 2 (Homo sapiens (Human)) | BDBM50118701 (6-(4-Carboxy-imidazol-1-ylmethyl)-decahydro-isoqui...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Ability to displace binding of [3H]KA to recombinant human KA receptor Ionotropic glutamate receptor ionotropic kainate 2 expressed in EK 293 cell me... | J Med Chem 45: 4383-6 (2002) BindingDB Entry DOI: 10.7270/Q2XP75N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 3 (Homo sapiens (Human)) | BDBM50118701 (6-(4-Carboxy-imidazol-1-ylmethyl)-decahydro-isoqui...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.47E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Ability to displace binding of [3H]AMPA to recombinant human Ionotropic glutamate receptor AMPA 3 | J Med Chem 45: 4383-6 (2002) BindingDB Entry DOI: 10.7270/Q2XP75N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 4 (Homo sapiens (Human)) | BDBM50118701 (6-(4-Carboxy-imidazol-1-ylmethyl)-decahydro-isoqui...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.62E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Ability to displace binding of [3H]AMPA to recombinant human Ionotropic glutamate receptor AMPA 4 | J Med Chem 45: 4383-6 (2002) BindingDB Entry DOI: 10.7270/Q2XP75N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 2 (Homo sapiens (Human)) | BDBM50118701 (6-(4-Carboxy-imidazol-1-ylmethyl)-decahydro-isoqui...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Ability to displace binding of [3H]AMPA to recombinant human AMPA receptor Ionotropic glutamate receptor ionotropic kainate 2 | J Med Chem 45: 4383-6 (2002) BindingDB Entry DOI: 10.7270/Q2XP75N4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||