

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysophosphatidic acid receptor 1 (Rattus norvegicus) | BDBM50211650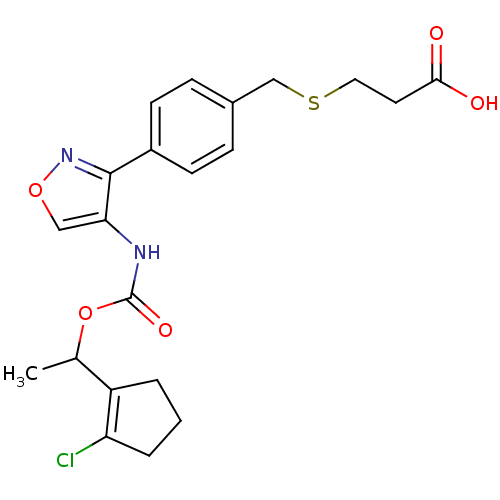 (3-(4-(4-((1-(2-chlorocyclopent-1-enyl)ethoxy)carbo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Antagonist activity at LPA1 receptor in rat hepatic stellate cells assessed as inhibition of lysophosphatidic acid-induced intracellular calcium infl... | Bioorg Med Chem Lett 17: 3736-40 (2007) Article DOI: 10.1016/j.bmcl.2007.04.024 BindingDB Entry DOI: 10.7270/Q2CZ36T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Rattus norvegicus) | BDBM50211649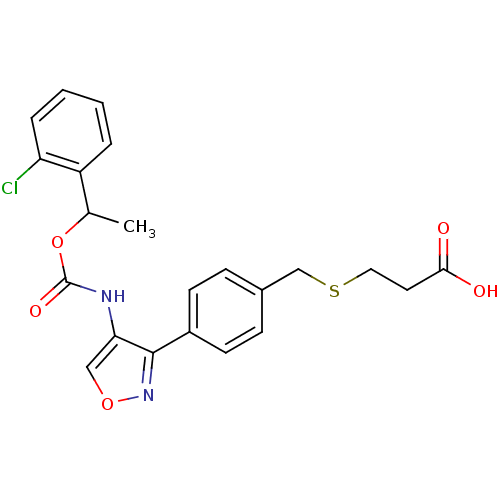 (3-(4-(4-((1-(2-chlorophenyl)ethoxy)carbonyl)isoxaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Antagonist activity at LPA1 receptor in rat hepatic stellate cells assessed as inhibition of lysophosphatidic acid-induced intracellular calcium infl... | Bioorg Med Chem Lett 17: 3736-40 (2007) Article DOI: 10.1016/j.bmcl.2007.04.024 BindingDB Entry DOI: 10.7270/Q2CZ36T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Rattus norvegicus) | BDBM50211647 (3-(4-(4-((1-(2-chlorocyclohex-1-enyl)ethoxy)carbon...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Antagonist activity at LPA1 receptor in rat hepatic stellate cells assessed as inhibition of lysophosphatidic acid-induced intracellular calcium infl... | Bioorg Med Chem Lett 17: 3736-40 (2007) Article DOI: 10.1016/j.bmcl.2007.04.024 BindingDB Entry DOI: 10.7270/Q2CZ36T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Rattus norvegicus) | BDBM50211646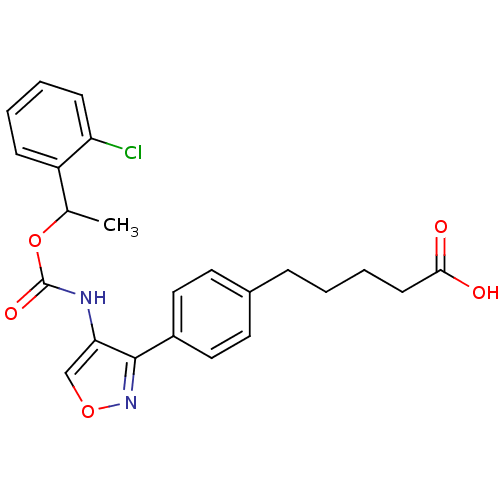 (5-(4-(4-((1-(2-chlorophenyl)ethoxy)carbonyl)isoxaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Antagonist activity at LPA1 receptor in rat hepatic stellate cells assessed as inhibition of lysophosphatidic acid-induced intracellular calcium infl... | Bioorg Med Chem Lett 17: 3736-40 (2007) Article DOI: 10.1016/j.bmcl.2007.04.024 BindingDB Entry DOI: 10.7270/Q2CZ36T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Rattus norvegicus) | BDBM50211655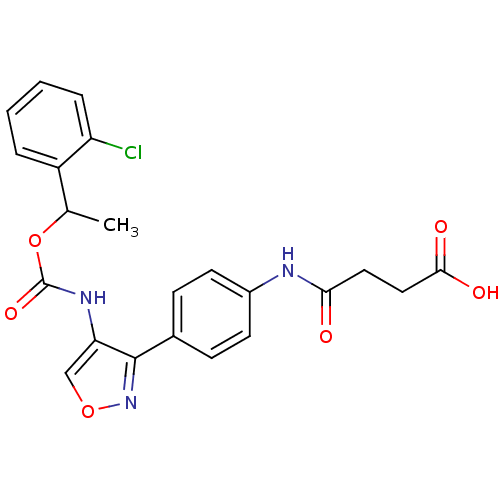 (4-(4-(4-((1-(2-chlorophenyl)ethoxy)carbonyl)isoxaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Antagonist activity at LPA1 receptor in rat hepatic stellate cells assessed as inhibition of lysophosphatidic acid-induced intracellular calcium infl... | Bioorg Med Chem Lett 17: 3736-40 (2007) Article DOI: 10.1016/j.bmcl.2007.04.024 BindingDB Entry DOI: 10.7270/Q2CZ36T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Rattus norvegicus) | BDBM50211645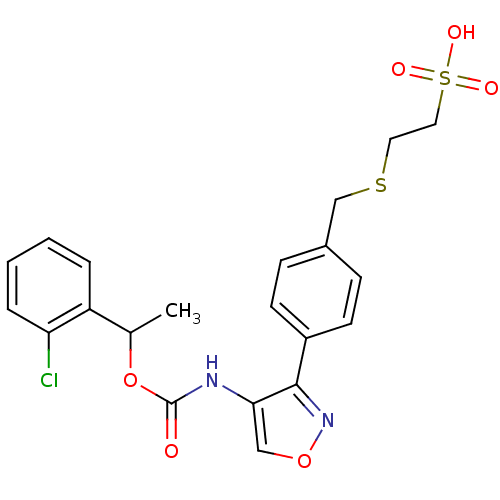 (2-(4-(4-((1-(2-chlorophenyl)ethoxy)carbonyl)isoxaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Antagonist activity at LPA1 receptor in rat hepatic stellate cells assessed as inhibition of lysophosphatidic acid-induced intracellular calcium infl... | Bioorg Med Chem Lett 17: 3736-40 (2007) Article DOI: 10.1016/j.bmcl.2007.04.024 BindingDB Entry DOI: 10.7270/Q2CZ36T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Rattus norvegicus) | BDBM50211651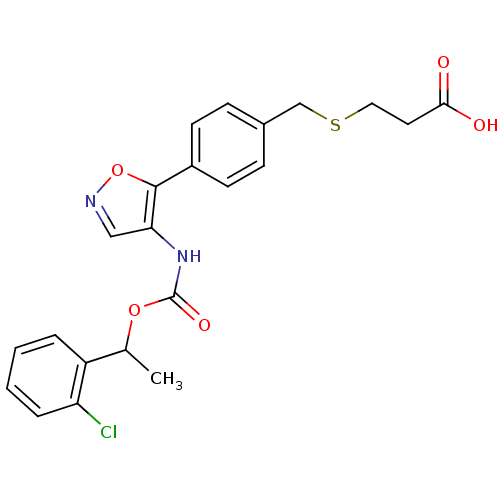 (3-(4-(4-((1-(2-chlorophenyl)ethoxy)carbonyl)isoxaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Antagonist activity at LPA1 receptor in rat hepatic stellate cells assessed as inhibition of lysophosphatidic acid-induced intracellular calcium infl... | Bioorg Med Chem Lett 17: 3736-40 (2007) Article DOI: 10.1016/j.bmcl.2007.04.024 BindingDB Entry DOI: 10.7270/Q2CZ36T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Rattus norvegicus) | BDBM50211648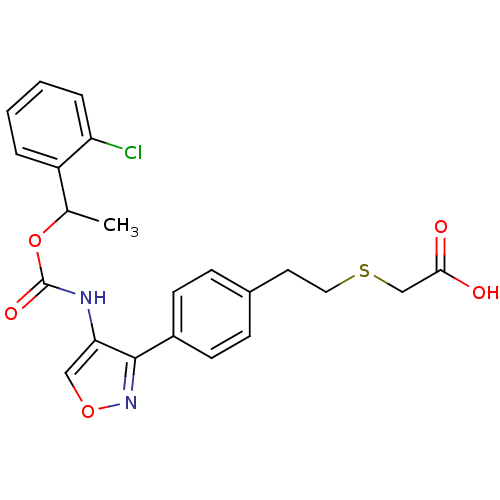 (2-(4-(4-((1-(2-chlorophenyl)ethoxy)carbonyl)isoxaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Antagonist activity at LPA1 receptor in rat hepatic stellate cells assessed as inhibition of lysophosphatidic acid-induced intracellular calcium infl... | Bioorg Med Chem Lett 17: 3736-40 (2007) Article DOI: 10.1016/j.bmcl.2007.04.024 BindingDB Entry DOI: 10.7270/Q2CZ36T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Rattus norvegicus) | BDBM50211653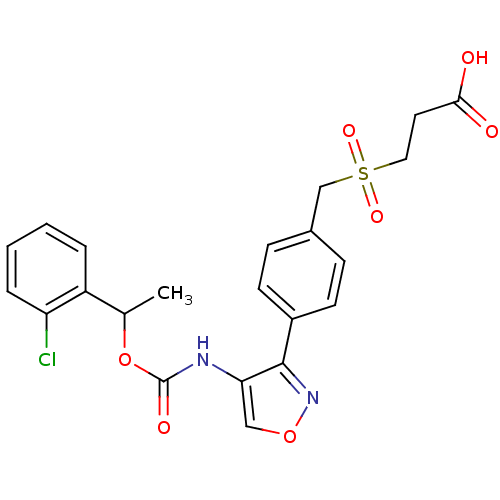 (3-(4-(4-((1-(2-chlorophenyl)ethoxy)carbonyl)isoxaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Antagonist activity at LPA1 receptor in rat hepatic stellate cells assessed as inhibition of lysophosphatidic acid-induced intracellular calcium infl... | Bioorg Med Chem Lett 17: 3736-40 (2007) Article DOI: 10.1016/j.bmcl.2007.04.024 BindingDB Entry DOI: 10.7270/Q2CZ36T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Rattus norvegicus) | BDBM50211654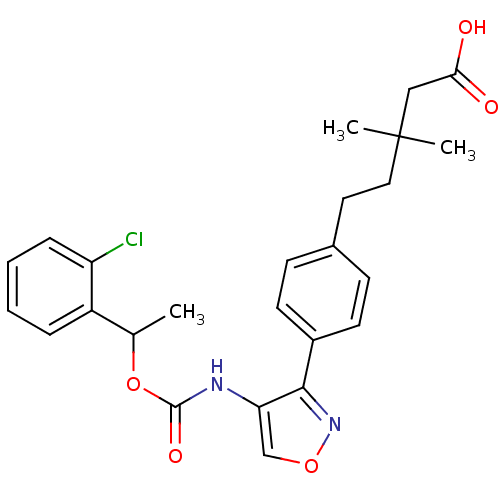 (5-(4-(4-((1-(2-chlorophenyl)ethoxy)carbonyl)isoxaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Antagonist activity at LPA1 receptor in rat hepatic stellate cells assessed as inhibition of lysophosphatidic acid-induced intracellular calcium infl... | Bioorg Med Chem Lett 17: 3736-40 (2007) Article DOI: 10.1016/j.bmcl.2007.04.024 BindingDB Entry DOI: 10.7270/Q2CZ36T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50211648 (2-(4-(4-((1-(2-chlorophenyl)ethoxy)carbonyl)isoxaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Antagonist activity at human recombinant LPA1 receptor expressed in CHOK1 cells assessed as inhibition of lysophosphatidic acid-induced intracellular... | Bioorg Med Chem Lett 17: 3736-40 (2007) Article DOI: 10.1016/j.bmcl.2007.04.024 BindingDB Entry DOI: 10.7270/Q2CZ36T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50211650 (3-(4-(4-((1-(2-chlorocyclopent-1-enyl)ethoxy)carbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Antagonist activity at human recombinant LPA1 receptor expressed in CHOK1 cells assessed as inhibition of lysophosphatidic acid-induced intracellular... | Bioorg Med Chem Lett 17: 3736-40 (2007) Article DOI: 10.1016/j.bmcl.2007.04.024 BindingDB Entry DOI: 10.7270/Q2CZ36T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50211655 (4-(4-(4-((1-(2-chlorophenyl)ethoxy)carbonyl)isoxaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Antagonist activity at human recombinant LPA1 receptor expressed in CHOK1 cells assessed as inhibition of lysophosphatidic acid-induced intracellular... | Bioorg Med Chem Lett 17: 3736-40 (2007) Article DOI: 10.1016/j.bmcl.2007.04.024 BindingDB Entry DOI: 10.7270/Q2CZ36T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Rattus norvegicus) | BDBM50170859 (3-(4-(4-((1-(2-chlorophenyl)ethoxy)carbonyl)-3-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Antagonist activity at LPA1 receptor in rat hepatic stellate cells assessed as inhibition of lysophosphatidic acid-induced intracellular calcium infl... | Bioorg Med Chem Lett 17: 3736-40 (2007) Article DOI: 10.1016/j.bmcl.2007.04.024 BindingDB Entry DOI: 10.7270/Q2CZ36T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50211647 (3-(4-(4-((1-(2-chlorocyclohex-1-enyl)ethoxy)carbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Antagonist activity at human recombinant LPA1 receptor expressed in CHOK1 cells assessed as inhibition of lysophosphatidic acid-induced intracellular... | Bioorg Med Chem Lett 17: 3736-40 (2007) Article DOI: 10.1016/j.bmcl.2007.04.024 BindingDB Entry DOI: 10.7270/Q2CZ36T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50211646 (5-(4-(4-((1-(2-chlorophenyl)ethoxy)carbonyl)isoxaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Antagonist activity at human recombinant LPA1 receptor expressed in CHOK1 cells assessed as inhibition of lysophosphatidic acid-induced intracellular... | Bioorg Med Chem Lett 17: 3736-40 (2007) Article DOI: 10.1016/j.bmcl.2007.04.024 BindingDB Entry DOI: 10.7270/Q2CZ36T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50211649 (3-(4-(4-((1-(2-chlorophenyl)ethoxy)carbonyl)isoxaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Antagonist activity at human recombinant LPA1 receptor expressed in CHOK1 cells assessed as inhibition of lysophosphatidic acid-induced intracellular... | Bioorg Med Chem Lett 17: 3736-40 (2007) Article DOI: 10.1016/j.bmcl.2007.04.024 BindingDB Entry DOI: 10.7270/Q2CZ36T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50211651 (3-(4-(4-((1-(2-chlorophenyl)ethoxy)carbonyl)isoxaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Antagonist activity at human recombinant LPA1 receptor expressed in CHOK1 cells assessed as inhibition of lysophosphatidic acid-induced intracellular... | Bioorg Med Chem Lett 17: 3736-40 (2007) Article DOI: 10.1016/j.bmcl.2007.04.024 BindingDB Entry DOI: 10.7270/Q2CZ36T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50211653 (3-(4-(4-((1-(2-chlorophenyl)ethoxy)carbonyl)isoxaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Antagonist activity at human recombinant LPA1 receptor expressed in CHOK1 cells assessed as inhibition of lysophosphatidic acid-induced intracellular... | Bioorg Med Chem Lett 17: 3736-40 (2007) Article DOI: 10.1016/j.bmcl.2007.04.024 BindingDB Entry DOI: 10.7270/Q2CZ36T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50211654 (5-(4-(4-((1-(2-chlorophenyl)ethoxy)carbonyl)isoxaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Antagonist activity at human recombinant LPA1 receptor expressed in CHOK1 cells assessed as inhibition of lysophosphatidic acid-induced intracellular... | Bioorg Med Chem Lett 17: 3736-40 (2007) Article DOI: 10.1016/j.bmcl.2007.04.024 BindingDB Entry DOI: 10.7270/Q2CZ36T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50211645 (2-(4-(4-((1-(2-chlorophenyl)ethoxy)carbonyl)isoxaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Antagonist activity at human recombinant LPA1 receptor expressed in CHOK1 cells assessed as inhibition of lysophosphatidic acid-induced intracellular... | Bioorg Med Chem Lett 17: 3736-40 (2007) Article DOI: 10.1016/j.bmcl.2007.04.024 BindingDB Entry DOI: 10.7270/Q2CZ36T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50170859 (3-(4-(4-((1-(2-chlorophenyl)ethoxy)carbonyl)-3-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Antagonist activity at human recombinant LPA1 receptor expressed in CHOK1 cells assessed as inhibition of lysophosphatidic acid-induced intracellular... | Bioorg Med Chem Lett 17: 3736-40 (2007) Article DOI: 10.1016/j.bmcl.2007.04.024 BindingDB Entry DOI: 10.7270/Q2CZ36T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Rattus norvegicus) | BDBM50211652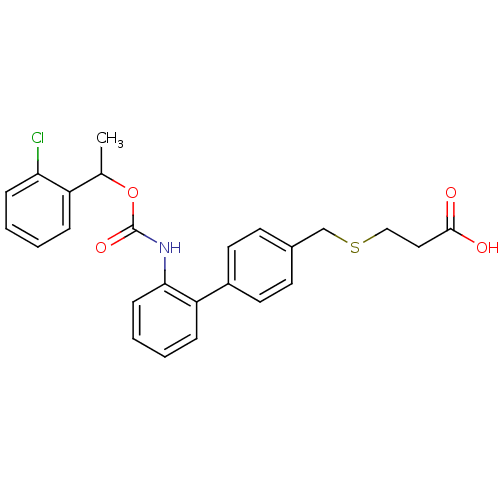 (3-{2'-[1-(2-chloro-phenyl)-ethoxycarbonylamino]-bi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Antagonist activity at LPA1 receptor in rat hepatic stellate cells assessed as inhibition of lysophosphatidic acid-induced intracellular calcium infl... | Bioorg Med Chem Lett 17: 3736-40 (2007) Article DOI: 10.1016/j.bmcl.2007.04.024 BindingDB Entry DOI: 10.7270/Q2CZ36T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50211652 (3-{2'-[1-(2-chloro-phenyl)-ethoxycarbonylamino]-bi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Antagonist activity at human recombinant LPA1 receptor expressed in CHOK1 cells assessed as inhibition of lysophosphatidic acid-induced intracellular... | Bioorg Med Chem Lett 17: 3736-40 (2007) Article DOI: 10.1016/j.bmcl.2007.04.024 BindingDB Entry DOI: 10.7270/Q2CZ36T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||