 Found 42 hits of Enzyme Inhibition Constant Data
Found 42 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50154078
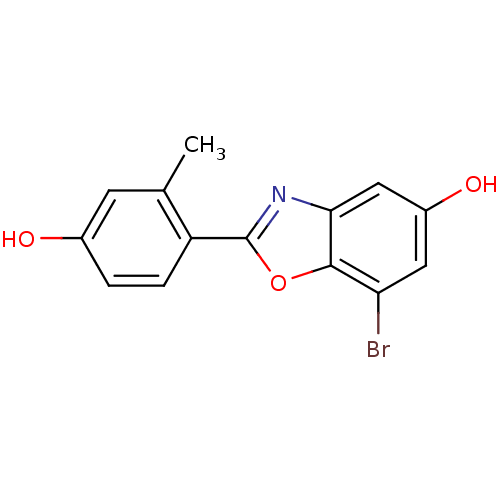
(7-Bromo-2-(4-hydroxy-2-methyl-phenyl)-benzooxazol-...)Show InChI InChI=1S/C14H10BrNO3/c1-7-4-8(17)2-3-10(7)14-16-12-6-9(18)5-11(15)13(12)19-14/h2-6,17-18H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of estrogen receptor beta |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50154137
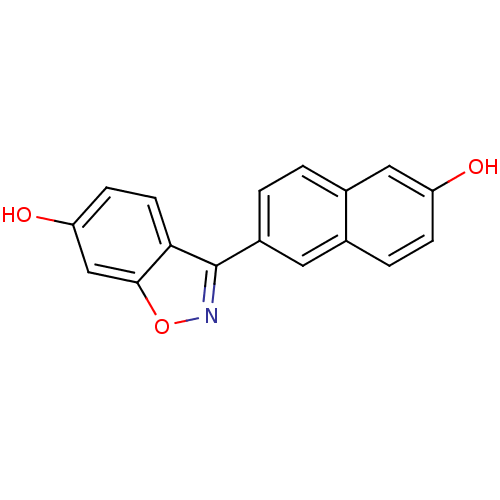
(3-(6-HYDROXY-NAPHTHALEN-2-YL)-BENZO[D]ISOOXAZOL-6-...)Show InChI InChI=1S/C17H11NO3/c19-13-4-3-10-7-12(2-1-11(10)8-13)17-15-6-5-14(20)9-16(15)21-18-17/h1-9,19-20H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of estrogen receptor beta |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50154062

(2-(5-HYDROXY-NAPHTHALEN-1-YL)-1,3-BENZOOXAZOL-6-OL...)Show InChI InChI=1S/C17H11NO3/c19-10-7-8-14-16(9-10)21-17(18-14)13-5-1-4-12-11(13)3-2-6-15(12)20/h1-9,19-20H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of estrogen receptor beta |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50148169
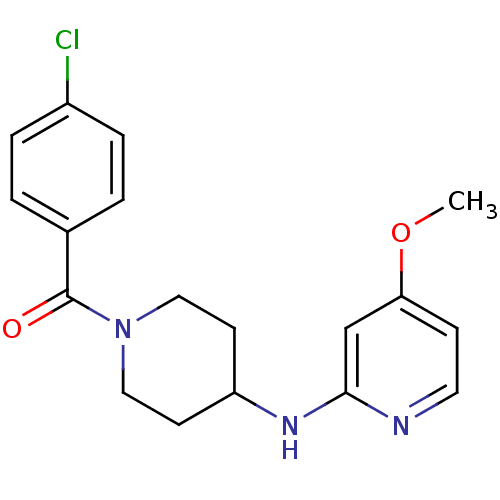
((4-Chloro-phenyl)-[4-(4-methoxy-pyridin-2-ylamino)...)Show InChI InChI=1S/C18H20ClN3O2/c1-24-16-6-9-20-17(12-16)21-15-7-10-22(11-8-15)18(23)13-2-4-14(19)5-3-13/h2-6,9,12,15H,7-8,10-11H2,1H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of iNOS |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50124525
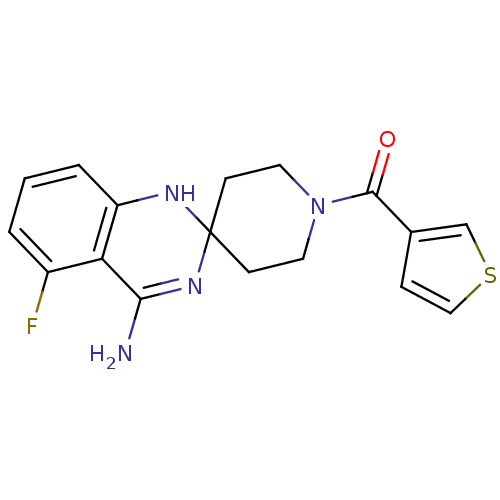
(4'-amino-5'-fluorospiro[hexahydropyridine-4,2'-(1'...)Show SMILES NC1=NC2(CCN(CC2)C(=O)c2ccsc2)Nc2cccc(F)c12 |t:1| Show InChI InChI=1S/C17H17FN4OS/c18-12-2-1-3-13-14(12)15(19)21-17(20-13)5-7-22(8-6-17)16(23)11-4-9-24-10-11/h1-4,9-10,20H,5-8H2,(H2,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of iNOS |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50035206

(4-(5-Methoxy-1a,2,3,7b-tetrahydro-1H-cyclopropa[a]...)Show InChI InChI=1S/C17H17NO/c1-19-13-3-5-14-12(10-13)2-4-15-16(17(14)15)11-6-8-18-9-7-11/h3,5-10,15-17H,2,4H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of aromatase |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM19956
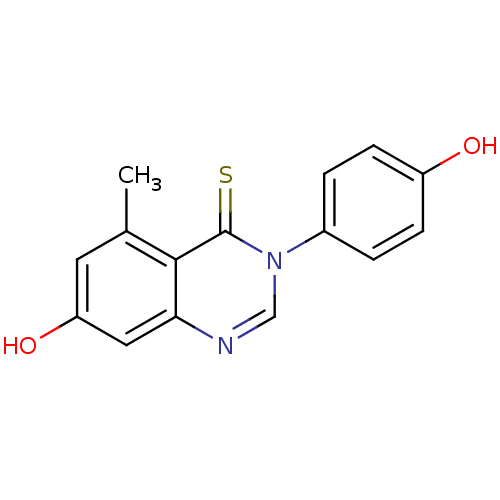
(3-arylquinazolinethione, 1bd | 7-hydroxy-3-(4-hydr...)Show InChI InChI=1S/C15H12N2O2S/c1-9-6-12(19)7-13-14(9)15(20)17(8-16-13)10-2-4-11(18)5-3-10/h2-8,18-19H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of estrogen receptor beta |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50148164
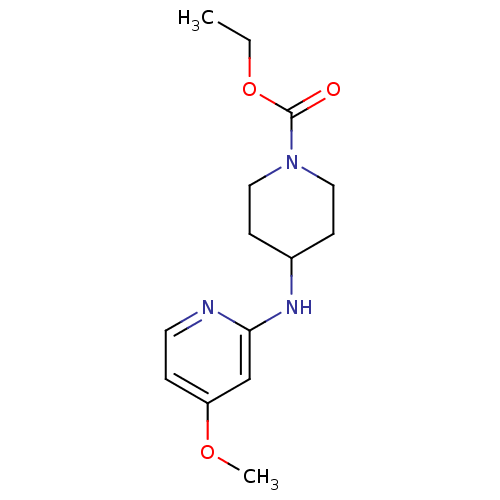
(4-(4-Methoxy-pyridin-2-ylamino)-piperidine-1-carbo...)Show InChI InChI=1S/C14H21N3O3/c1-3-20-14(18)17-8-5-11(6-9-17)16-13-10-12(19-2)4-7-15-13/h4,7,10-11H,3,5-6,8-9H2,1-2H3,(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of iNOS |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM10009
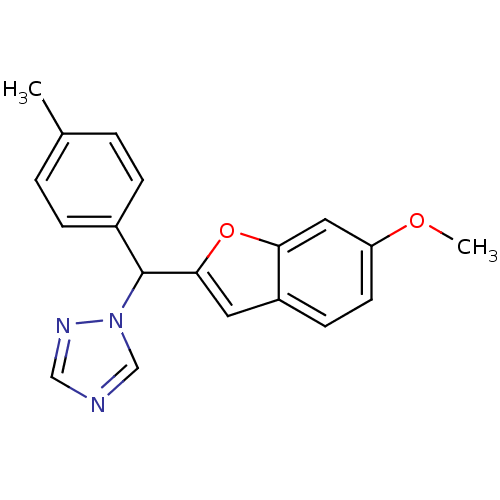
(1-[(6-Methoxybenzofuran-2-yl)-p-tolylmethyl]-1H-1,...)Show InChI InChI=1S/C19H17N3O2/c1-13-3-5-14(6-4-13)19(22-12-20-11-21-22)18-9-15-7-8-16(23-2)10-17(15)24-18/h3-12,19H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of aromatase |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM10002
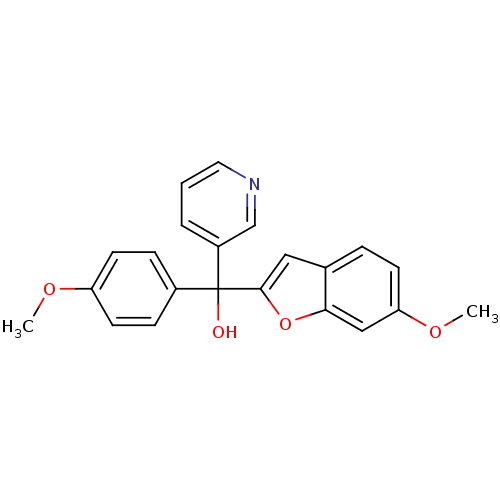
((6-Methoxybenzofuran-2-yl)-(4-methoxyphenyl)-3-pyr...)Show SMILES COc1ccc(cc1)C(O)(c1cc2ccc(OC)cc2o1)c1cccnc1 Show InChI InChI=1S/C22H19NO4/c1-25-18-9-6-16(7-10-18)22(24,17-4-3-11-23-14-17)21-12-15-5-8-19(26-2)13-20(15)27-21/h3-14,24H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of aromatase |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9471
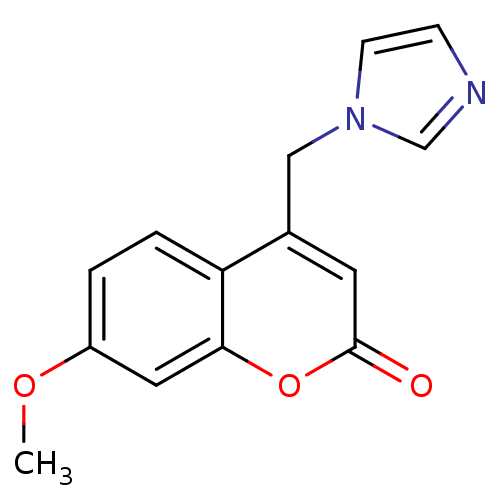
(4-(1H-Imidazol-1-ylmethyl)-7-methoxy-2H-chromen-2-...)Show InChI InChI=1S/C14H12N2O3/c1-18-11-2-3-12-10(8-16-5-4-15-9-16)6-14(17)19-13(12)7-11/h2-7,9H,8H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of aromatase |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50124528
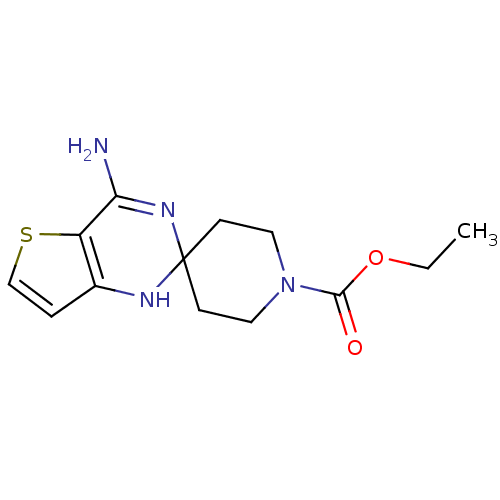
(CHEMBL170176 | ethyl 4'-amino-1H,1'H-spiro[piperid...)Show InChI InChI=1S/C13H18N4O2S/c1-2-19-12(18)17-6-4-13(5-7-17)15-9-3-8-20-10(9)11(14)16-13/h3,8,15H,2,4-7H2,1H3,(H2,14,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of iNOS |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50376217
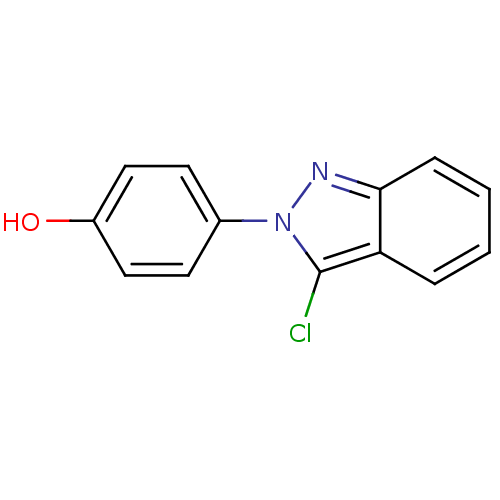
(CHEMBL362476)Show InChI InChI=1S/C13H9ClN2O/c14-13-11-3-1-2-4-12(11)15-16(13)9-5-7-10(17)8-6-9/h1-8,17H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of estrogen receptor beta |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50091813
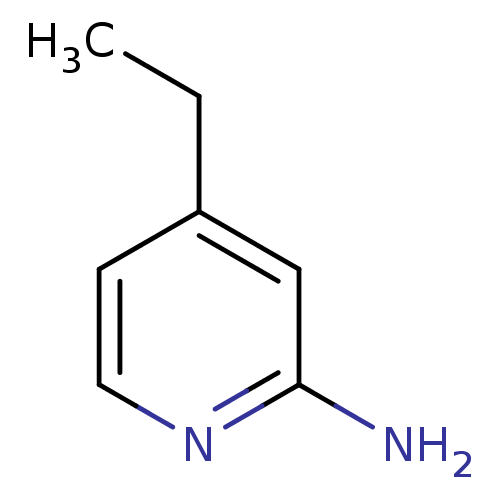
(4-Ethyl-pyridin-2-ylamine | 4-ethylpyridin-2-amine...)Show InChI InChI=1S/C7H10N2/c1-2-6-3-4-9-7(8)5-6/h3-5H,2H2,1H3,(H2,8,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of iNOS |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50376221
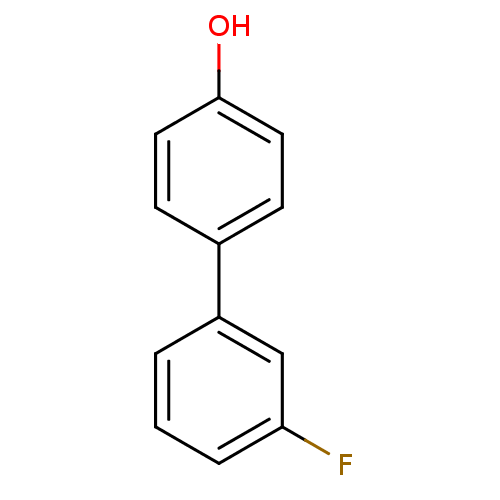
(CHEMBL261781)Show InChI InChI=1S/C12H9FO/c13-11-3-1-2-10(8-11)9-4-6-12(14)7-5-9/h1-8,14H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of estrogen receptor beta |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50237936
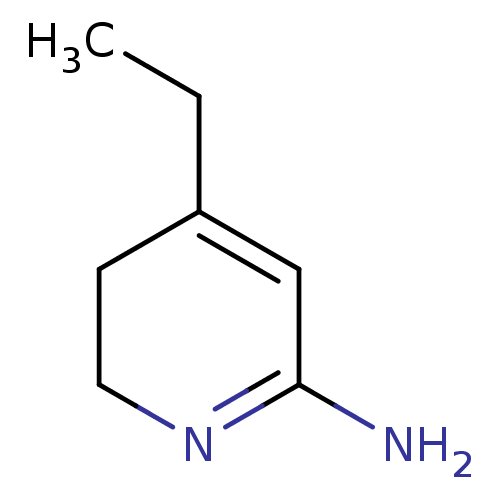
(4-Ethyl-5,6-dihydro-1H-pyridin-(2Z)-ylideneamine |...)Show InChI InChI=1S/C7H12N2/c1-2-6-3-4-9-7(8)5-6/h5H,2-4H2,1H3,(H2,8,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of iNOS |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50376215

(CHEMBL409050)Show InChI InChI=1S/C13H17N3/c1-8-4-2-7-10-11(8)12(14)16-13(15-10)9-5-3-6-9/h2,4,7,9,13,15H,3,5-6H2,1H3,(H2,14,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of iNOS |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50117550
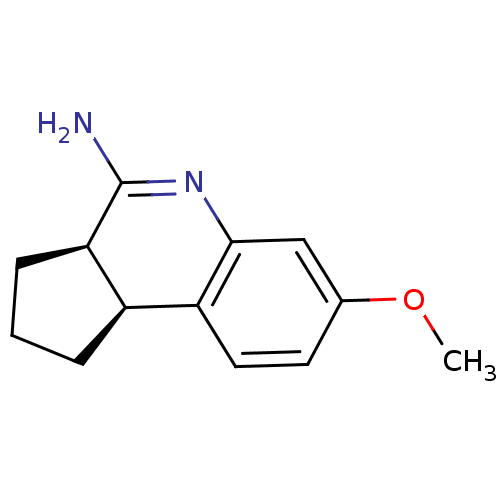
((3aR,9bS)-7-methoxy-2,3,3a,9b-tetrahydro-1H-cyclop...)Show InChI InChI=1S/C13H16N2O/c1-16-8-5-6-10-9-3-2-4-11(9)13(14)15-12(10)7-8/h5-7,9,11H,2-4H2,1H3,(H2,14,15)/t9-,11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of iNOS |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM10025
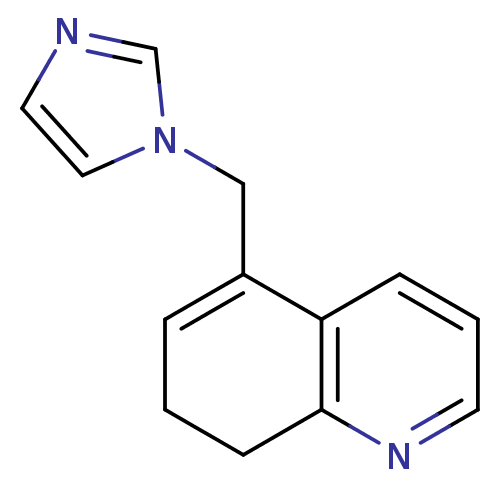
(5-(1H-imidazol-1-ylmethyl)-7,8-dihydroquinoline | ...)Show InChI InChI=1S/C13H13N3/c1-3-11(9-16-8-7-14-10-16)12-4-2-6-15-13(12)5-1/h2-4,6-8,10H,1,5,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of aromatase |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50376223
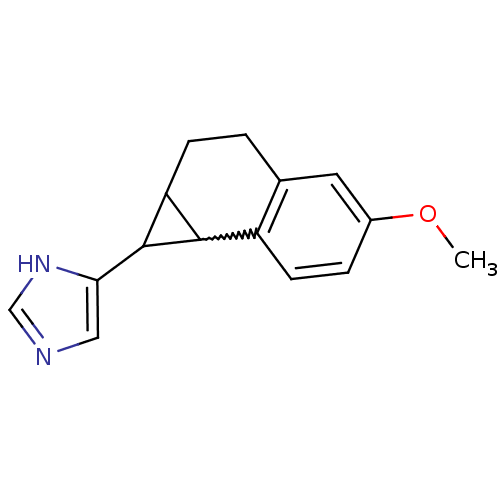
(CHEMBL438549)Show InChI InChI=1S/C15H16N2O/c1-18-10-3-5-11-9(6-10)2-4-12-14(11)15(12)13-7-16-8-17-13/h3,5-8,12,14-15H,2,4H2,1H3,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of aromatase |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50376225

(CHEMBL258553)Show InChI InChI=1S/C13H9NO/c14-9-11-3-1-2-4-13(11)10-5-7-12(15)8-6-10/h1-8,15H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of estrogen receptor beta |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50168378

(2-methyl-6-o-tolylnaphthalene | 6-(2-Hydroxy-pheny...)Show InChI InChI=1S/C16H12O2/c17-14-8-7-11-9-13(6-5-12(11)10-14)15-3-1-2-4-16(15)18/h1-10,17-18H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of estrogen receptor beta |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50376219
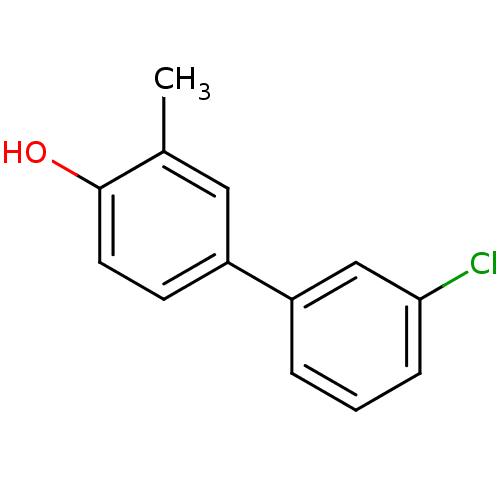
(CHEMBL258509)Show InChI InChI=1S/C13H11ClO/c1-9-7-11(5-6-13(9)15)10-3-2-4-12(14)8-10/h2-8,15H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of estrogen receptor beta |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50376220

(CHEMBL258552)Show InChI InChI=1S/C13H12O2/c1-15-13-8-4-11(5-9-13)10-2-6-12(14)7-3-10/h2-9,14H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of estrogen receptor beta |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50041326

(4-((2,3-dihydro-1H-inden-2-yl)methyl)pyridine | 4-...)Show InChI InChI=1S/C15H15N/c1-2-4-15-11-13(10-14(15)3-1)9-12-5-7-16-8-6-12/h1-8,13H,9-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of aromatase |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50124524
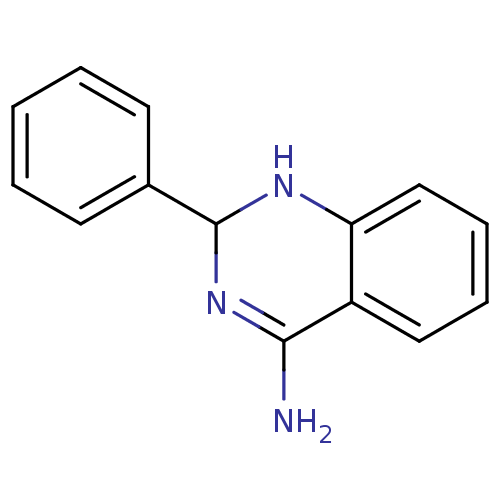
(2-Phenyl-1,2-dihydro-quinazolin-4-ylamine | 2-phen...)Show InChI InChI=1S/C14H13N3/c15-13-11-8-4-5-9-12(11)16-14(17-13)10-6-2-1-3-7-10/h1-9,14,16H,(H2,15,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of iNOS |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM8906
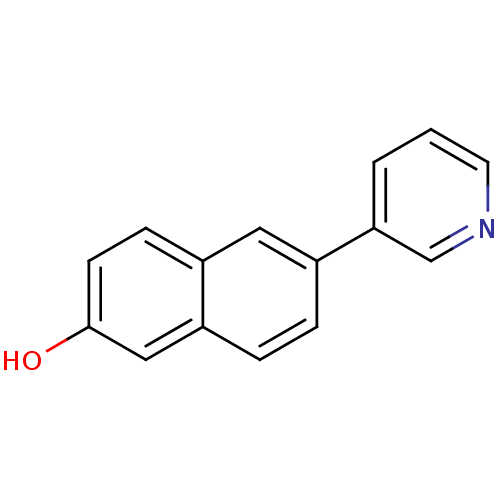
(6-(pyridin-3-yl)naphthalen-2-ol | 6-Pyridin-3-ylna...)Show InChI InChI=1S/C15H11NO/c17-15-6-5-11-8-12(3-4-13(11)9-15)14-2-1-7-16-10-14/h1-10,17H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of aromatase |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9464
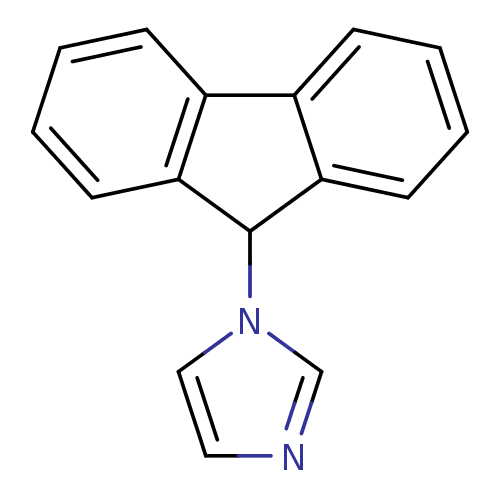
(1-(9H-Fluoren-9-yl)-1H-imidazole | CHEMBL225447 | ...)Show InChI InChI=1S/C16H12N2/c1-3-7-14-12(5-1)13-6-2-4-8-15(13)16(14)18-10-9-17-11-18/h1-11,16H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of aromatase |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM8916

(1-(2-Naphthyl)-1H-imidazole | 1-(naphthalen-2-yl)-...)Show InChI InChI=1S/C13H10N2/c1-2-4-12-9-13(6-5-11(12)3-1)15-8-7-14-10-15/h1-10H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of aromatase |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50376218
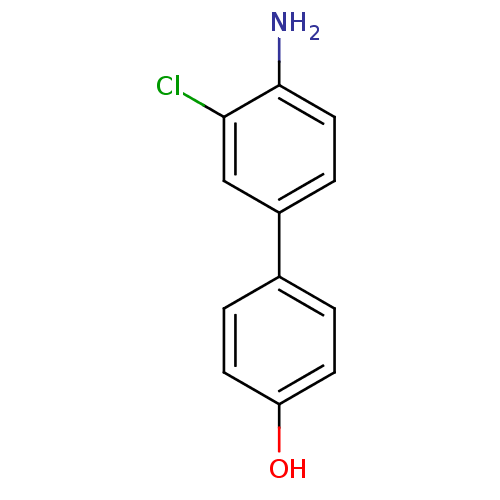
(CHEMBL261680)Show InChI InChI=1S/C12H10ClNO/c13-11-7-9(3-6-12(11)14)8-1-4-10(15)5-2-8/h1-7,15H,14H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of estrogen receptor beta |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50376216
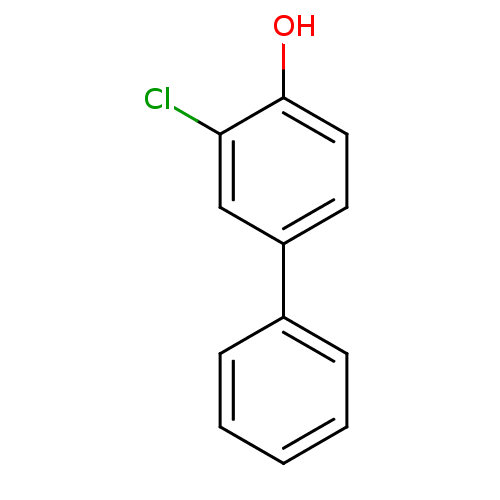
(CHEMBL406630)Show InChI InChI=1S/C12H9ClO/c13-11-8-10(6-7-12(11)14)9-4-2-1-3-5-9/h1-8,14H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of estrogen receptor beta |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50066783
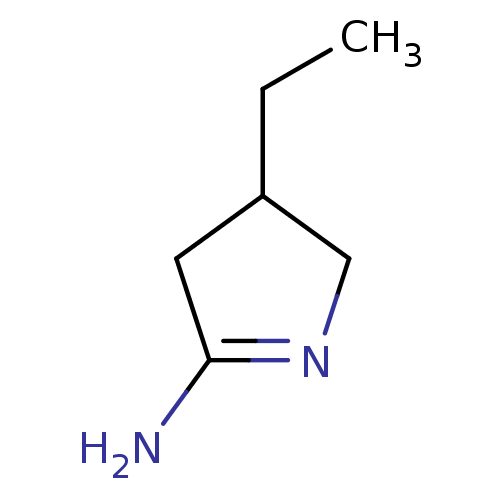
(4-Ethyl-pyrrolidin-(2E)-ylideneamine; hydrochlorid...)Show InChI InChI=1S/C6H12N2/c1-2-5-3-6(7)8-4-5/h5H,2-4H2,1H3,(H2,7,8) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of iNOS |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50097361
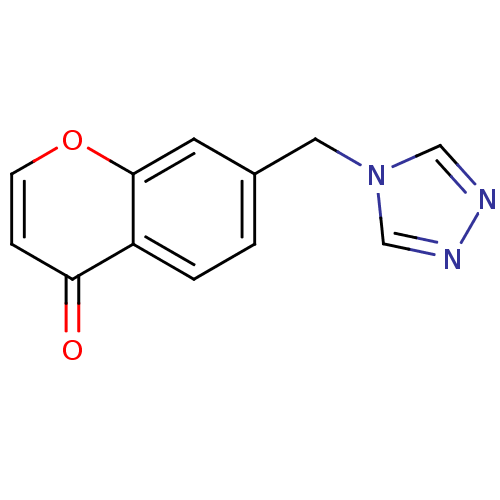
(7-((4H-1,2,4-triazol-4-yl)methyl)-4H-chromen-4-one...)Show InChI InChI=1S/C12H9N3O2/c16-11-3-4-17-12-5-9(1-2-10(11)12)6-15-7-13-14-8-15/h1-5,7-8H,6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of aromatase |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50376222
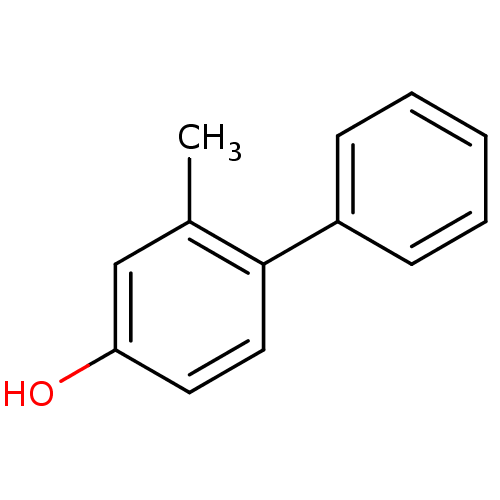
(CHEMBL261780)Show InChI InChI=1S/C13H12O/c1-10-9-12(14)7-8-13(10)11-5-3-2-4-6-11/h2-9,14H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of estrogen receptor beta |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50035207

(1-(pyridin-4-yl)-1a,2,3,7b-tetrahydro-1H-cycloprop...)Show InChI InChI=1S/C16H15NO/c18-14-3-1-2-12-11(14)4-5-13-15(16(12)13)10-6-8-17-9-7-10/h1-3,6-9,13,15-16,18H,4-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of aromatase |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM10041
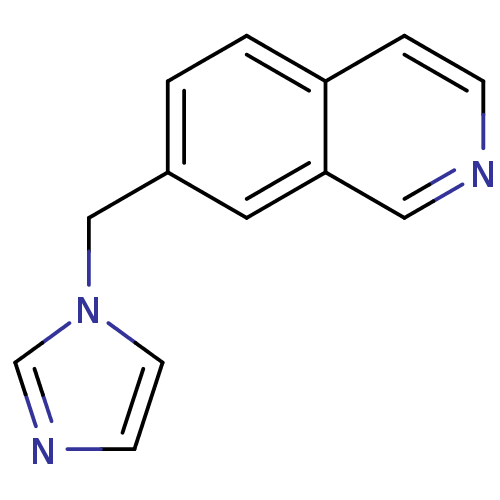
(7-(1H-imidazol-1-ylmethyl)isoquinoline | 7-[(Imida...)Show InChI InChI=1S/C13H11N3/c1-2-12-3-4-14-8-13(12)7-11(1)9-16-6-5-15-10-16/h1-8,10H,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of aromatase |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50117561
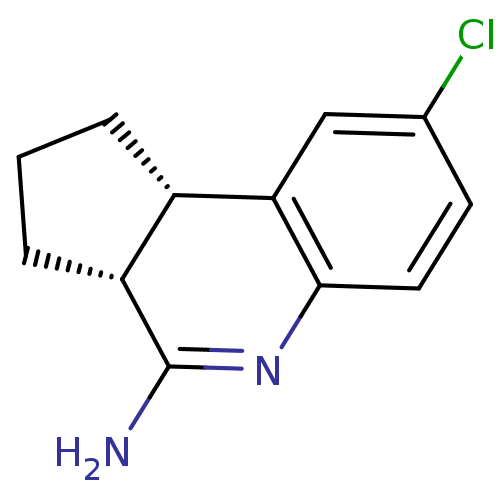
((3aR,9bS)-8-Chloro-2,3,3a,9b-tetrahydro-1H-cyclope...)Show InChI InChI=1S/C12H13ClN2/c13-7-4-5-11-10(6-7)8-2-1-3-9(8)12(14)15-11/h4-6,8-9H,1-3H2,(H2,14,15)/t8-,9+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of iNOS |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM8890
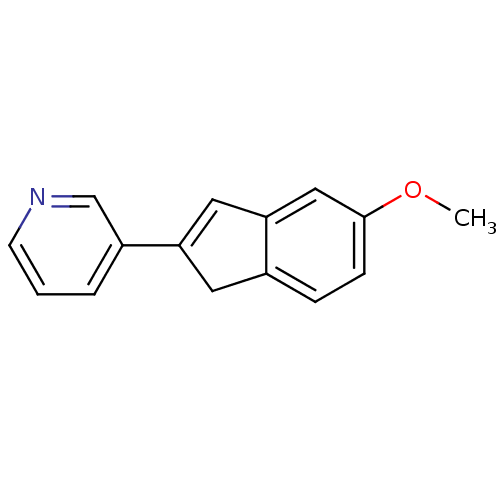
(3-(5-Methoxy-1H-inden-2-yl)pyridine | indene 3)Show InChI InChI=1S/C15H13NO/c1-17-15-5-4-11-7-13(8-14(11)9-15)12-3-2-6-16-10-12/h2-6,8-10H,7H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of aromatase |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50124534
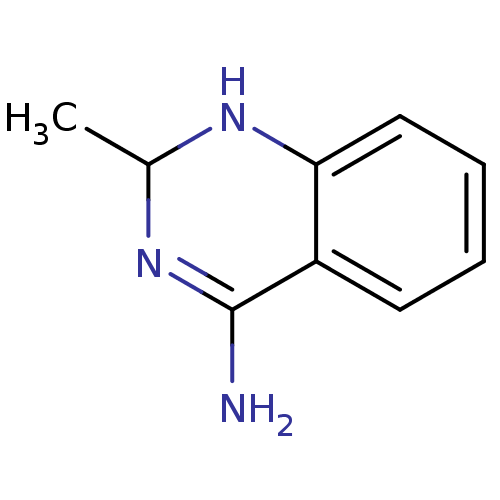
(2-Methyl-1,2-dihydro-quinazolin-4-ylamine | 2-meth...)Show InChI InChI=1S/C9H11N3/c1-6-11-8-5-3-2-4-7(8)9(10)12-6/h2-6,11H,1H3,(H2,10,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of iNOS |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50376224
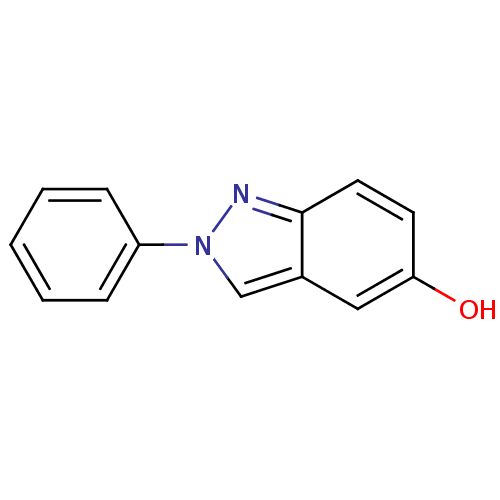
(CHEMBL179421)Show InChI InChI=1S/C13H10N2O/c16-12-6-7-13-10(8-12)9-15(14-13)11-4-2-1-3-5-11/h1-9,16H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of estrogen receptor beta |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50148171
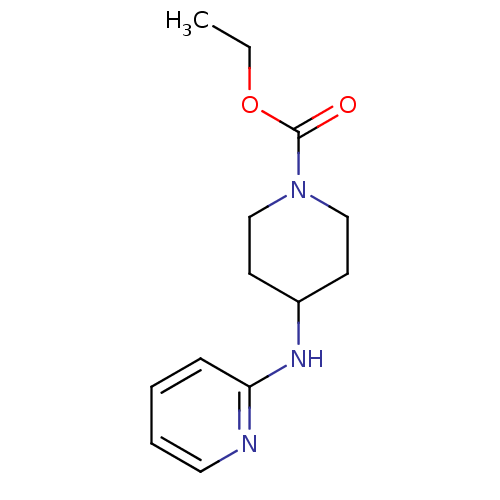
(4-(Pyridin-2-ylamino)-piperidine-1-carboxylic acid...)Show InChI InChI=1S/C13H19N3O2/c1-2-18-13(17)16-9-6-11(7-10-16)15-12-5-3-4-8-14-12/h3-5,8,11H,2,6-7,9-10H2,1H3,(H,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of iNOS |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50124541
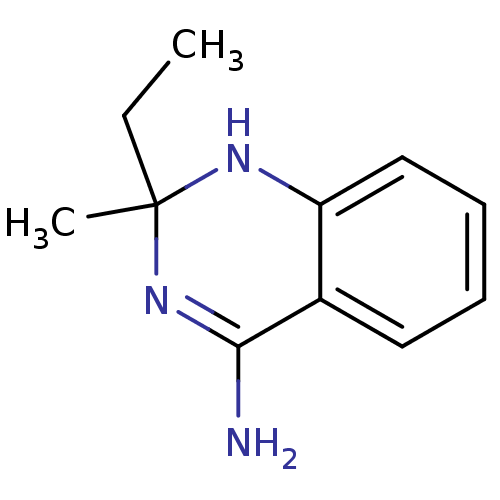
(2-Ethyl-2-methyl-1,2-dihydro-quinazolin-4-ylamine ...)Show InChI InChI=1S/C11H15N3/c1-3-11(2)13-9-7-5-4-6-8(9)10(12)14-11/h4-7,13H,3H2,1-2H3,(H2,12,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of iNOS |
J Med Chem 51: 2481-91 (2008)
Article DOI: 10.1021/jm701314u
BindingDB Entry DOI: 10.7270/Q2GX4CF5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data