

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM21337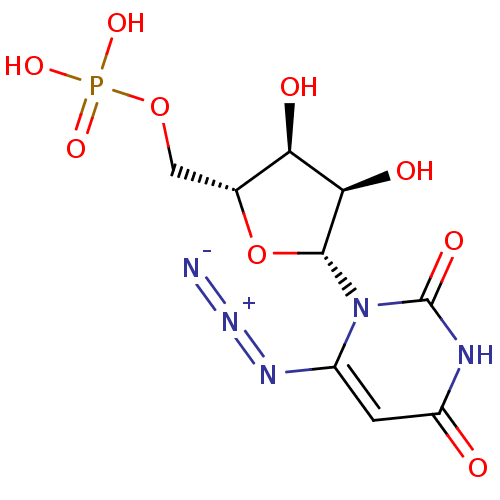 (6-Azido-uridine 5-O-Monophosphate | C6-Uridine Der...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 200 | -42.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 55 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 52: 1648-58 (2009) Article DOI: 10.1021/jm801224t BindingDB Entry DOI: 10.7270/Q2RR1WJB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM27946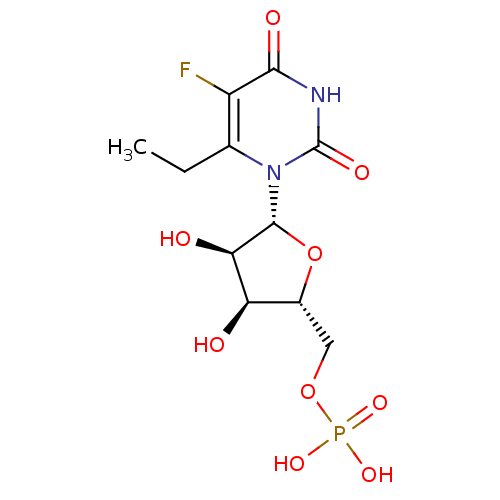 (uridine derivative, 43 | {[(2R,3S,4R,5R)-5-(6-ethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 350 | -38.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 52: 1648-58 (2009) Article DOI: 10.1021/jm801224t BindingDB Entry DOI: 10.7270/Q2RR1WJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM27944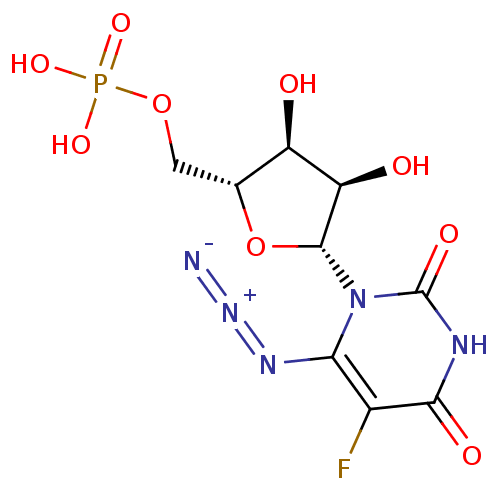 (uridine derivative, 41 | {[(2R,3S,4R,5R)-5-(6-azid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 360 | -40.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 55 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 52: 1648-58 (2009) Article DOI: 10.1021/jm801224t BindingDB Entry DOI: 10.7270/Q2RR1WJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM21338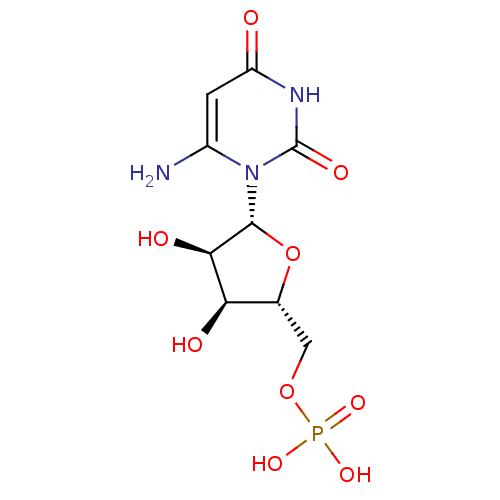 (6-Amino-uridine 5-O-Monophosphate | C6-Uridine Der...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 840 | -38.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 55 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 52: 1648-58 (2009) Article DOI: 10.1021/jm801224t BindingDB Entry DOI: 10.7270/Q2RR1WJB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM27945 (uridine derivative, 42 | {[(2R,3S,4R,5R)-5-(6-amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.14E+4 | -31.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 55 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 52: 1648-58 (2009) Article DOI: 10.1021/jm801224t BindingDB Entry DOI: 10.7270/Q2RR1WJB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM27945 (uridine derivative, 42 | {[(2R,3S,4R,5R)-5-(6-amin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.66E+4 | -28.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 52: 1648-58 (2009) Article DOI: 10.1021/jm801224t BindingDB Entry DOI: 10.7270/Q2RR1WJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM27946 (uridine derivative, 43 | {[(2R,3S,4R,5R)-5-(6-ethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.90E+4 | -28.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 55 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 52: 1648-58 (2009) Article DOI: 10.1021/jm801224t BindingDB Entry DOI: 10.7270/Q2RR1WJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM21335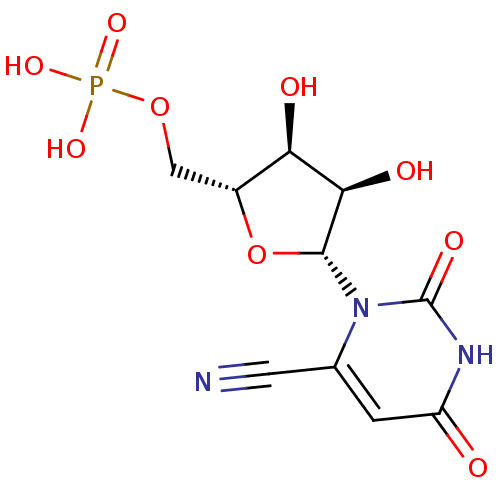 (6-cyanouridine 5-monophosphate | C6-Uridine Deriva...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 2.90E+4 | -28.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 55 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 52: 1648-58 (2009) Article DOI: 10.1021/jm801224t BindingDB Entry DOI: 10.7270/Q2RR1WJB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM27943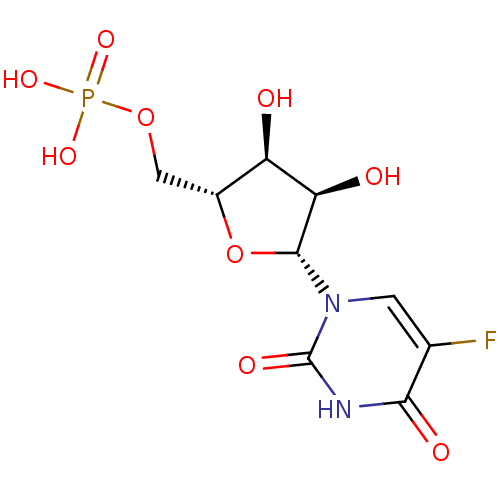 (uridine derivative, 39 | {[(2R,3S,4R,5R)-5-(5-fluo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 9.80E+4 | -23.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 52: 1648-58 (2009) Article DOI: 10.1021/jm801224t BindingDB Entry DOI: 10.7270/Q2RR1WJB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM21339 (6-Methyl-uridine 5-O-Monophosphate | C6-Uridine De...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.34E+5 | -24.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 55 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 52: 1648-58 (2009) Article DOI: 10.1021/jm801224t BindingDB Entry DOI: 10.7270/Q2RR1WJB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM27943 (uridine derivative, 39 | {[(2R,3S,4R,5R)-5-(5-fluo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 6.45E+5 | -20.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 55 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 52: 1648-58 (2009) Article DOI: 10.1021/jm801224t BindingDB Entry DOI: 10.7270/Q2RR1WJB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||