

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM50415462 (CHEMBL589384) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of BChE from equine serum by Ellman's method | J Med Chem 53: 2094-103 (2010) Article DOI: 10.1021/jm901616h BindingDB Entry DOI: 10.7270/Q2KW5H8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.13 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of BChE from equine serum by Ellman's method | J Med Chem 53: 2094-103 (2010) Article DOI: 10.1021/jm901616h BindingDB Entry DOI: 10.7270/Q2KW5H8J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50415464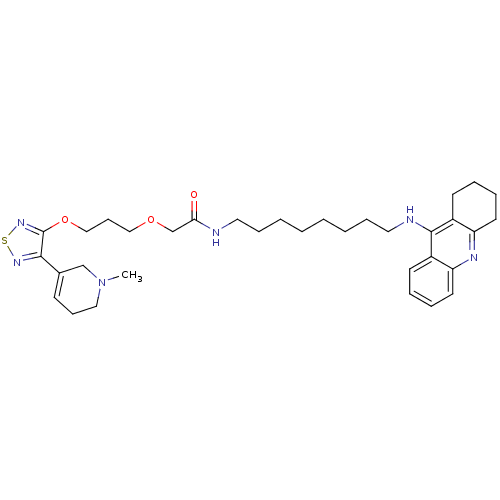 (CHEMBL589405) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.13 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of BChE from equine serum by Ellman's method | J Med Chem 53: 2094-103 (2010) Article DOI: 10.1021/jm901616h BindingDB Entry DOI: 10.7270/Q2KW5H8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50415465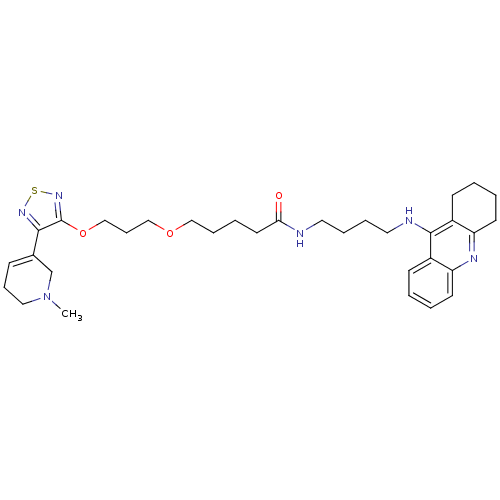 (CHEMBL589409) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.89 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of BChE from equine serum by Ellman's method | J Med Chem 53: 2094-103 (2010) Article DOI: 10.1021/jm901616h BindingDB Entry DOI: 10.7270/Q2KW5H8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50415461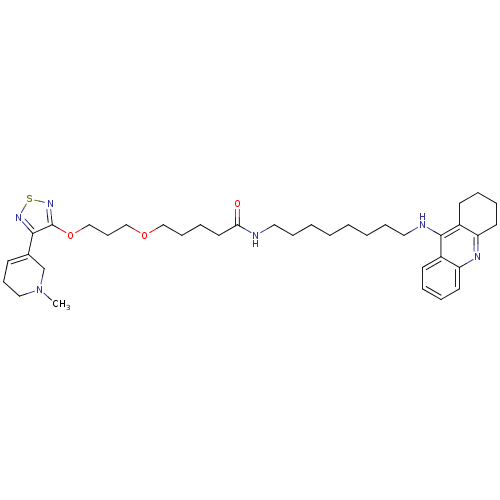 (CHEMBL589352) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.89 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of BChE from equine serum by Ellman's method | J Med Chem 53: 2094-103 (2010) Article DOI: 10.1021/jm901616h BindingDB Entry DOI: 10.7270/Q2KW5H8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50415461 (CHEMBL589352) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.17 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Med Chem 53: 2094-103 (2010) Article DOI: 10.1021/jm901616h BindingDB Entry DOI: 10.7270/Q2KW5H8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50415462 (CHEMBL589384) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.46 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Med Chem 53: 2094-103 (2010) Article DOI: 10.1021/jm901616h BindingDB Entry DOI: 10.7270/Q2KW5H8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50415464 (CHEMBL589405) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.61 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Med Chem 53: 2094-103 (2010) Article DOI: 10.1021/jm901616h BindingDB Entry DOI: 10.7270/Q2KW5H8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50415476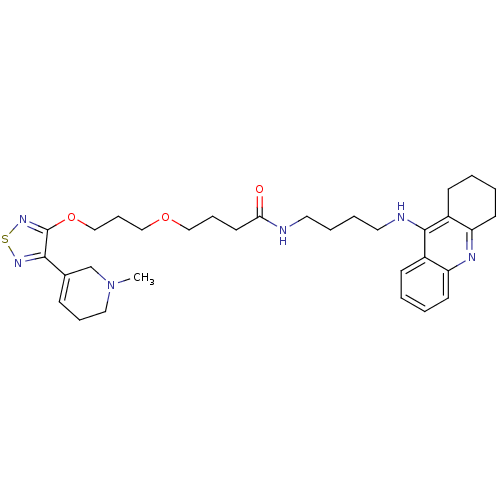 (CHEMBL589496) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.76 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of BChE from equine serum by Ellman's method | J Med Chem 53: 2094-103 (2010) Article DOI: 10.1021/jm901616h BindingDB Entry DOI: 10.7270/Q2KW5H8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50415476 (CHEMBL589496) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.51 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Med Chem 53: 2094-103 (2010) Article DOI: 10.1021/jm901616h BindingDB Entry DOI: 10.7270/Q2KW5H8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50415465 (CHEMBL589409) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.71 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Med Chem 53: 2094-103 (2010) Article DOI: 10.1021/jm901616h BindingDB Entry DOI: 10.7270/Q2KW5H8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50415468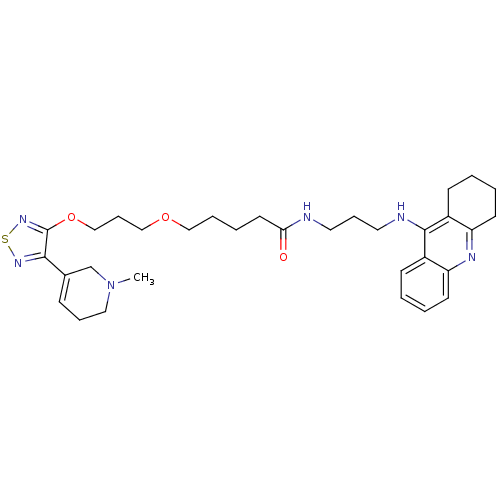 (CHEMBL590059) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.91 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of BChE from equine serum by Ellman's method | J Med Chem 53: 2094-103 (2010) Article DOI: 10.1021/jm901616h BindingDB Entry DOI: 10.7270/Q2KW5H8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50415466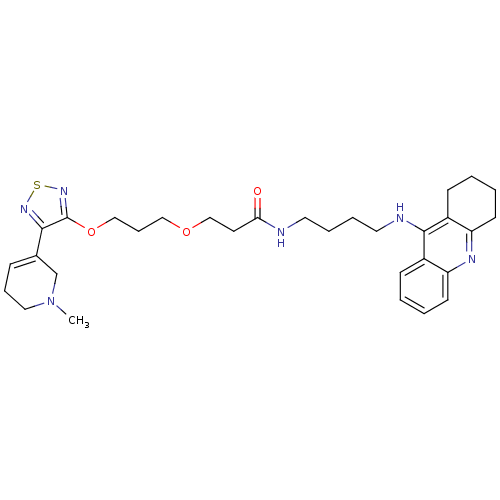 (CHEMBL589419) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.12 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of BChE from equine serum by Ellman's method | J Med Chem 53: 2094-103 (2010) Article DOI: 10.1021/jm901616h BindingDB Entry DOI: 10.7270/Q2KW5H8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50415471 (CHEMBL599652) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.77 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of BChE from equine serum by Ellman's method | J Med Chem 53: 2094-103 (2010) Article DOI: 10.1021/jm901616h BindingDB Entry DOI: 10.7270/Q2KW5H8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50415469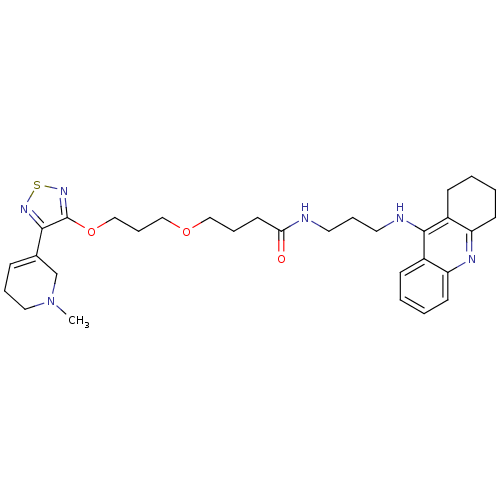 (CHEMBL590055) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of BChE from equine serum by Ellman's method | J Med Chem 53: 2094-103 (2010) Article DOI: 10.1021/jm901616h BindingDB Entry DOI: 10.7270/Q2KW5H8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50415468 (CHEMBL590059) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10.2 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Med Chem 53: 2094-103 (2010) Article DOI: 10.1021/jm901616h BindingDB Entry DOI: 10.7270/Q2KW5H8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50415470 (CHEMBL599243) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10.7 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of BChE from equine serum by Ellman's method | J Med Chem 53: 2094-103 (2010) Article DOI: 10.1021/jm901616h BindingDB Entry DOI: 10.7270/Q2KW5H8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50415469 (CHEMBL590055) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10.7 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Med Chem 53: 2094-103 (2010) Article DOI: 10.1021/jm901616h BindingDB Entry DOI: 10.7270/Q2KW5H8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50415467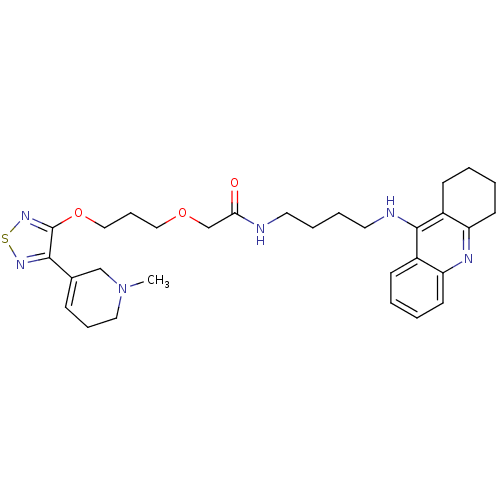 (CHEMBL597801) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10.7 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of BChE from equine serum by Ellman's method | J Med Chem 53: 2094-103 (2010) Article DOI: 10.1021/jm901616h BindingDB Entry DOI: 10.7270/Q2KW5H8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50415466 (CHEMBL589419) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10.7 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Med Chem 53: 2094-103 (2010) Article DOI: 10.1021/jm901616h BindingDB Entry DOI: 10.7270/Q2KW5H8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50415467 (CHEMBL597801) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12.0 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Med Chem 53: 2094-103 (2010) Article DOI: 10.1021/jm901616h BindingDB Entry DOI: 10.7270/Q2KW5H8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50415473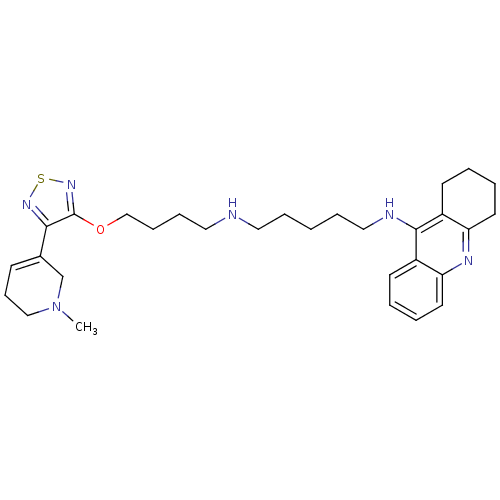 (CHEMBL599651) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12.3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of BChE from equine serum by Ellman's method | J Med Chem 53: 2094-103 (2010) Article DOI: 10.1021/jm901616h BindingDB Entry DOI: 10.7270/Q2KW5H8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50415463 (CHEMBL589495) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13.2 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of BChE from equine serum by Ellman's method | J Med Chem 53: 2094-103 (2010) Article DOI: 10.1021/jm901616h BindingDB Entry DOI: 10.7270/Q2KW5H8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50415472 (CHEMBL589978) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13.5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of BChE from equine serum by Ellman's method | J Med Chem 53: 2094-103 (2010) Article DOI: 10.1021/jm901616h BindingDB Entry DOI: 10.7270/Q2KW5H8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50415470 (CHEMBL599243) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13.5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Med Chem 53: 2094-103 (2010) Article DOI: 10.1021/jm901616h BindingDB Entry DOI: 10.7270/Q2KW5H8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50415471 (CHEMBL599652) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14.8 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Med Chem 53: 2094-103 (2010) Article DOI: 10.1021/jm901616h BindingDB Entry DOI: 10.7270/Q2KW5H8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50415474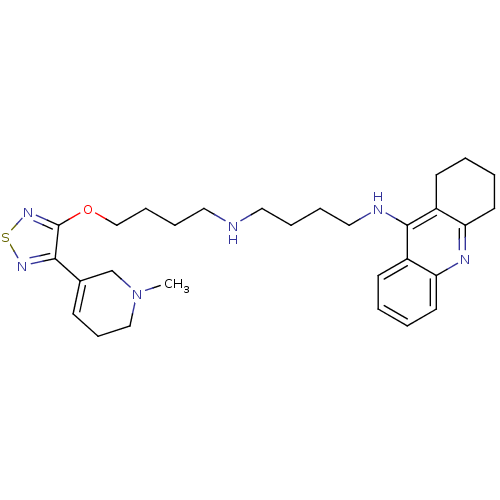 (CHEMBL599650) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16.2 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of BChE from equine serum by Ellman's method | J Med Chem 53: 2094-103 (2010) Article DOI: 10.1021/jm901616h BindingDB Entry DOI: 10.7270/Q2KW5H8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50415473 (CHEMBL599651) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21.9 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Med Chem 53: 2094-103 (2010) Article DOI: 10.1021/jm901616h BindingDB Entry DOI: 10.7270/Q2KW5H8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50415463 (CHEMBL589495) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22.9 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Med Chem 53: 2094-103 (2010) Article DOI: 10.1021/jm901616h BindingDB Entry DOI: 10.7270/Q2KW5H8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50415472 (CHEMBL589978) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26.3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Med Chem 53: 2094-103 (2010) Article DOI: 10.1021/jm901616h BindingDB Entry DOI: 10.7270/Q2KW5H8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50415475 (CHEMBL597192) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28.8 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of BChE from equine serum by Ellman's method | J Med Chem 53: 2094-103 (2010) Article DOI: 10.1021/jm901616h BindingDB Entry DOI: 10.7270/Q2KW5H8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50415474 (CHEMBL599650) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43.6 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Med Chem 53: 2094-103 (2010) Article DOI: 10.1021/jm901616h BindingDB Entry DOI: 10.7270/Q2KW5H8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 45.7 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Med Chem 53: 2094-103 (2010) Article DOI: 10.1021/jm901616h BindingDB Entry DOI: 10.7270/Q2KW5H8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50415475 (CHEMBL597192) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63.1 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Med Chem 53: 2094-103 (2010) Article DOI: 10.1021/jm901616h BindingDB Entry DOI: 10.7270/Q2KW5H8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||