 Found 72 hits of Enzyme Inhibition Constant Data
Found 72 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159110

(1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...)Show InChI InChI=1S/C20H32N2O/c1-3-12-21(13-4-1)16-7-17-23-20-10-8-19(9-11-20)18-22-14-5-2-6-15-22/h8-11H,1-7,12-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317720

((4-cyclobutyl-1,4-diazepan-1-yl)((2R,4S)-4-(4-fluo...)Show SMILES CS(=O)(=O)N1C[C@H](C[C@@H]1C(=O)N1CCCN(CC1)C1CCC1)Oc1ccc(F)cc1 |r| Show InChI InChI=1S/C21H30FN3O4S/c1-30(27,28)25-15-19(29-18-8-6-16(22)7-9-18)14-20(25)21(26)24-11-3-10-23(12-13-24)17-4-2-5-17/h6-9,17,19-20H,2-5,10-15H2,1H3/t19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317711

(((2R,4S)-4-(4-chloro-3-methylphenoxy)pyrrolidin-2-...)Show SMILES Cc1cc(O[C@@H]2CN[C@H](C2)C(=O)N2CCCN(CC2)C2CCC2)ccc1Cl |r| Show InChI InChI=1S/C21H30ClN3O2/c1-15-12-17(6-7-19(15)22)27-18-13-20(23-14-18)21(26)25-9-3-8-24(10-11-25)16-4-2-5-16/h6-7,12,16,18,20,23H,2-5,8-11,13-14H2,1H3/t18-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317706

(1-((2R,4S)-2-(4-cyclobutyl-1,4-diazepane-1-carbony...)Show SMILES CC(=O)N1C[C@H](C[C@@H]1C(=O)N1CCCN(CC1)C1CCC1)Oc1ccc(F)cc1 |r| Show InChI InChI=1S/C22H30FN3O3/c1-16(27)26-15-20(29-19-8-6-17(23)7-9-19)14-21(26)22(28)25-11-3-10-24(12-13-25)18-4-2-5-18/h6-9,18,20-21H,2-5,10-15H2,1H3/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317698
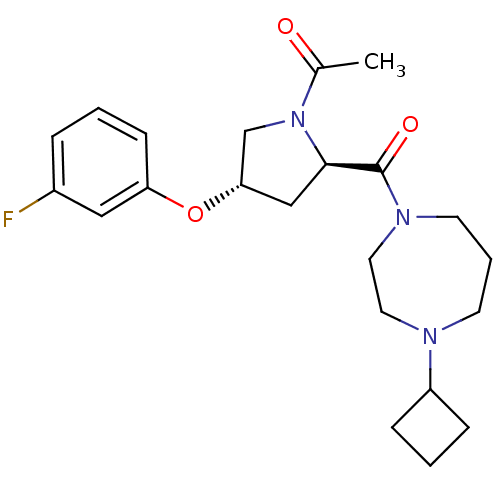
(1-((2R,4S)-2-(4-cyclobutyl-1,4-diazepane-1-carbony...)Show SMILES CC(=O)N1C[C@H](C[C@@H]1C(=O)N1CCCN(CC1)C1CCC1)Oc1cccc(F)c1 |r| Show InChI InChI=1S/C22H30FN3O3/c1-16(27)26-15-20(29-19-8-2-5-17(23)13-19)14-21(26)22(28)25-10-4-9-24(11-12-25)18-6-3-7-18/h2,5,8,13,18,20-21H,3-4,6-7,9-12,14-15H2,1H3/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317700
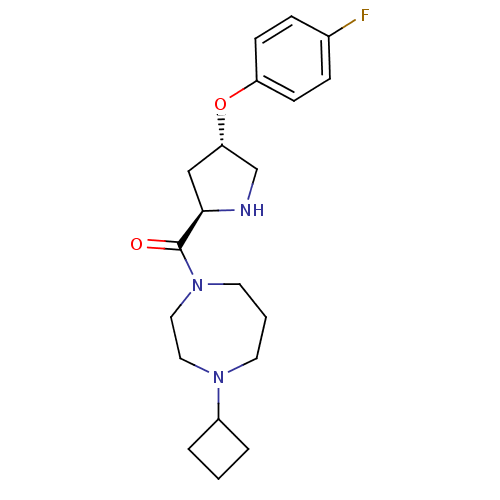
((4-cyclobutyl-1,4-diazepan-1-yl)((2R,4S)-4-(4-fluo...)Show SMILES Fc1ccc(O[C@@H]2CN[C@H](C2)C(=O)N2CCCN(CC2)C2CCC2)cc1 |r| Show InChI InChI=1S/C20H28FN3O2/c21-15-5-7-17(8-6-15)26-18-13-19(22-14-18)20(25)24-10-2-9-23(11-12-24)16-3-1-4-16/h5-8,16,18-19,22H,1-4,9-14H2/t18-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317716

((4-cyclobutyl-1,4-diazepan-1-yl)((2R,4S)-4-(4-fluo...)Show SMILES CN1C[C@H](C[C@@H]1C(=O)N1CCCN(CC1)C1CCC1)Oc1ccc(F)cc1 |r| Show InChI InChI=1S/C21H30FN3O2/c1-23-15-19(27-18-8-6-16(22)7-9-18)14-20(23)21(26)25-11-3-10-24(12-13-25)17-4-2-5-17/h6-9,17,19-20H,2-5,10-15H2,1H3/t19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317701
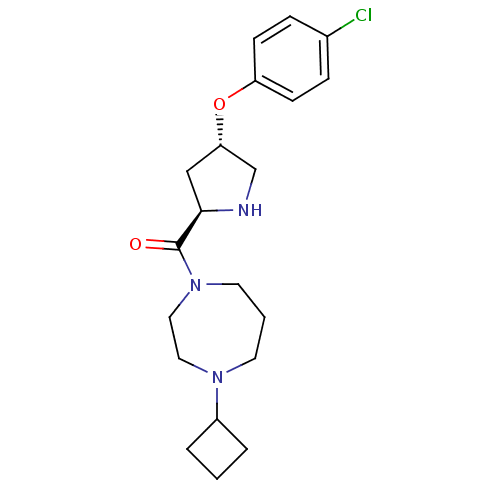
(((2R,4S)-4-(4-chlorophenoxy)pyrrolidin-2-yl)(4-cyc...)Show SMILES Clc1ccc(O[C@@H]2CN[C@H](C2)C(=O)N2CCCN(CC2)C2CCC2)cc1 |r| Show InChI InChI=1S/C20H28ClN3O2/c21-15-5-7-17(8-6-15)26-18-13-19(22-14-18)20(25)24-10-2-9-23(11-12-24)16-3-1-4-16/h5-8,16,18-19,22H,1-4,9-14H2/t18-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317728
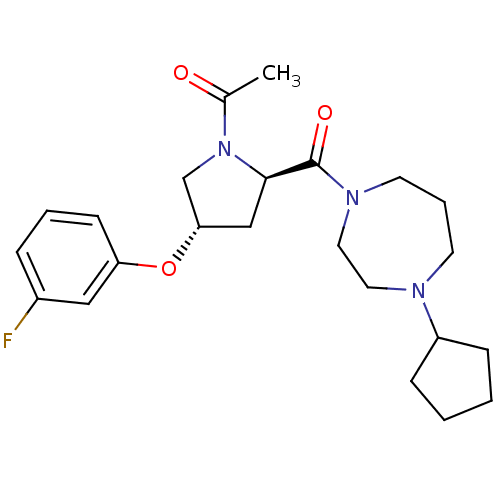
(1-((2R,4S)-2-(4-cyclopentyl-1,4-diazepane-1-carbon...)Show SMILES CC(=O)N1C[C@H](C[C@@H]1C(=O)N1CCCN(CC1)C1CCCC1)Oc1cccc(F)c1 |r| Show InChI InChI=1S/C23H32FN3O3/c1-17(28)27-16-21(30-20-9-4-6-18(24)14-20)15-22(27)23(29)26-11-5-10-25(12-13-26)19-7-2-3-8-19/h4,6,9,14,19,21-22H,2-3,5,7-8,10-13,15-16H2,1H3/t21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317703
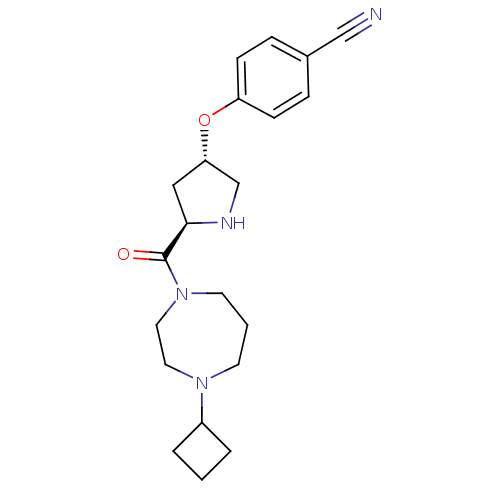
(4-((3S,5R)-5-(4-cyclobutyl-1,4-diazepane-1-carbony...)Show SMILES O=C([C@H]1C[C@@H](CN1)Oc1ccc(cc1)C#N)N1CCCN(CC1)C1CCC1 |r| Show InChI InChI=1S/C21H28N4O2/c22-14-16-5-7-18(8-6-16)27-19-13-20(23-15-19)21(26)25-10-2-9-24(11-12-25)17-3-1-4-17/h5-8,17,19-20,23H,1-4,9-13,15H2/t19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317702
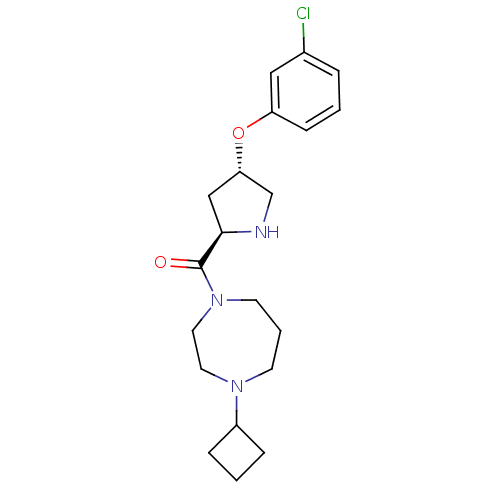
(((2R,4S)-4-(3-chlorophenoxy)pyrrolidin-2-yl)(4-cyc...)Show SMILES Clc1cccc(O[C@@H]2CN[C@H](C2)C(=O)N2CCCN(CC2)C2CCC2)c1 |r| Show InChI InChI=1S/C20H28ClN3O2/c21-15-4-1-7-17(12-15)26-18-13-19(22-14-18)20(25)24-9-3-8-23(10-11-24)16-5-2-6-16/h1,4,7,12,16,18-19,22H,2-3,5-6,8-11,13-14H2/t18-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317686

((4-cyclobutyl-1,4-diazepan-1-yl)((2R,4S)-4-(pyridi...)Show SMILES O=C([C@H]1C[C@@H](CN1)Oc1cccnc1)N1CCCN(CC1)C1CCC1 |r| Show InChI InChI=1S/C19H28N4O2/c24-19(23-9-3-8-22(10-11-23)15-4-1-5-15)18-12-17(14-21-18)25-16-6-2-7-20-13-16/h2,6-7,13,15,17-18,21H,1,3-5,8-12,14H2/t17-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317688

(1-((2R,4S)-2-(4-cyclobutyl-1,4-diazepane-1-carbony...)Show SMILES CC(=O)N1C[C@H](C[C@@H]1C(=O)N1CCCN(CC1)C1CCC1)Oc1ccsc1 |r| Show InChI InChI=1S/C20H29N3O3S/c1-15(24)23-13-18(26-17-6-11-27-14-17)12-19(23)20(25)22-8-3-7-21(9-10-22)16-4-2-5-16/h6,11,14,16,18-19H,2-5,7-10,12-13H2,1H3/t18-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317713
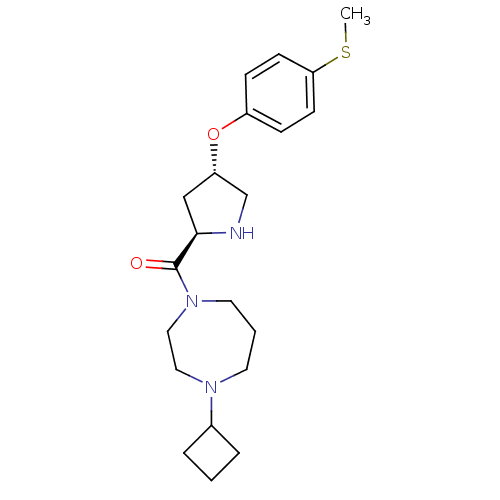
((4-cyclobutyl-1,4-diazepan-1-yl)((2R,4S)-4-(4-(met...)Show SMILES CSc1ccc(O[C@@H]2CN[C@H](C2)C(=O)N2CCCN(CC2)C2CCC2)cc1 |r| Show InChI InChI=1S/C21H31N3O2S/c1-27-19-8-6-17(7-9-19)26-18-14-20(22-15-18)21(25)24-11-3-10-23(12-13-24)16-4-2-5-16/h6-9,16,18,20,22H,2-5,10-15H2,1H3/t18-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317708
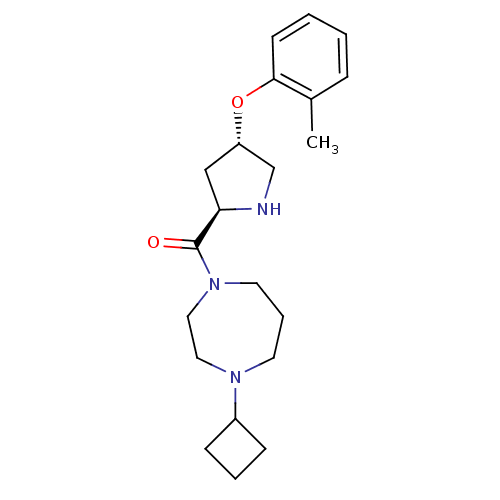
((4-cyclobutyl-1,4-diazepan-1-yl)((2R,4S)-4-(o-toly...)Show SMILES Cc1ccccc1O[C@@H]1CN[C@H](C1)C(=O)N1CCCN(CC1)C1CCC1 |r| Show InChI InChI=1S/C21H31N3O2/c1-16-6-2-3-9-20(16)26-18-14-19(22-15-18)21(25)24-11-5-10-23(12-13-24)17-7-4-8-17/h2-3,6,9,17-19,22H,4-5,7-8,10-15H2,1H3/t18-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317705

(4-((3S,5R)-5-(4-cyclopentyl-1,4-diazepane-1-carbon...)Show SMILES O=C([C@H]1C[C@@H](CN1)Oc1ccc(cc1)C#N)N1CCCN(CC1)C1CCCC1 |r| Show InChI InChI=1S/C22H30N4O2/c23-15-17-6-8-19(9-7-17)28-20-14-21(24-16-20)22(27)26-11-3-10-25(12-13-26)18-4-1-2-5-18/h6-9,18,20-21,24H,1-5,10-14,16H2/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317699
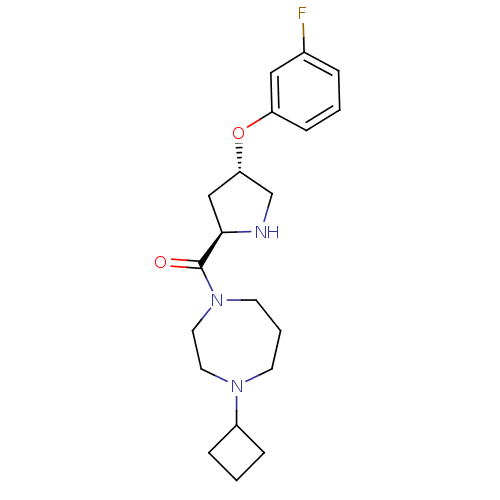
((4-cyclobutyl-1,4-diazepan-1-yl)((2R,4S)-4-(3-fluo...)Show SMILES Fc1cccc(O[C@@H]2CN[C@H](C2)C(=O)N2CCCN(CC2)C2CCC2)c1 |r| Show InChI InChI=1S/C20H28FN3O2/c21-15-4-1-7-17(12-15)26-18-13-19(22-14-18)20(25)24-9-3-8-23(10-11-24)16-5-2-6-16/h1,4,7,12,16,18-19,22H,2-3,5-6,8-11,13-14H2/t18-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317687
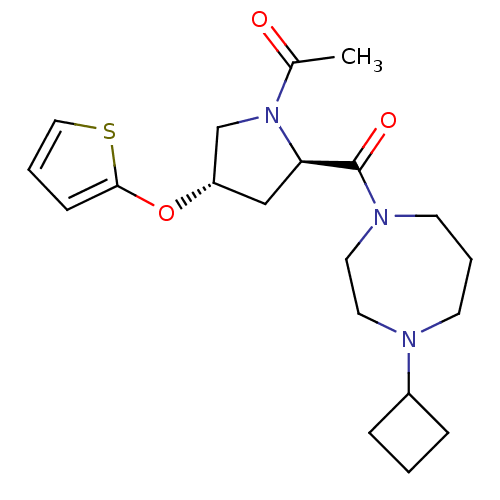
(1-((2R,4S)-2-(4-cyclobutyl-1,4-diazepane-1-carbony...)Show SMILES CC(=O)N1C[C@H](C[C@@H]1C(=O)N1CCCN(CC1)C1CCC1)Oc1cccs1 |r| Show InChI InChI=1S/C20H29N3O3S/c1-15(24)23-14-17(26-19-7-3-12-27-19)13-18(23)20(25)22-9-4-8-21(10-11-22)16-5-2-6-16/h3,7,12,16-18H,2,4-6,8-11,13-14H2,1H3/t17-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317704
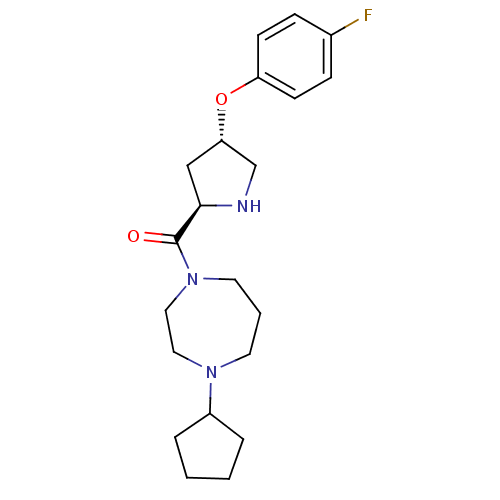
((4-cyclopentyl-1,4-diazepan-1-yl)((2R,4S)-4-(4-flu...)Show SMILES Fc1ccc(O[C@@H]2CN[C@H](C2)C(=O)N2CCCN(CC2)C2CCCC2)cc1 |r| Show InChI InChI=1S/C21H30FN3O2/c22-16-6-8-18(9-7-16)27-19-14-20(23-15-19)21(26)25-11-3-10-24(12-13-25)17-4-1-2-5-17/h6-9,17,19-20,23H,1-5,10-15H2/t19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317714
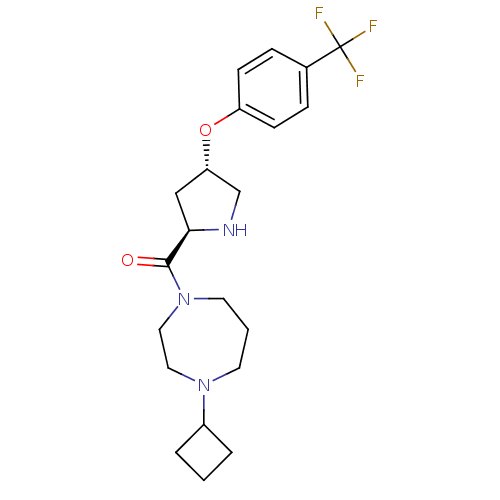
((4-cyclobutyl-1,4-diazepan-1-yl)((2R,4S)-4-(4-(tri...)Show SMILES FC(F)(F)c1ccc(O[C@@H]2CN[C@H](C2)C(=O)N2CCCN(CC2)C2CCC2)cc1 |r| Show InChI InChI=1S/C21H28F3N3O2/c22-21(23,24)15-5-7-17(8-6-15)29-18-13-19(25-14-18)20(28)27-10-2-9-26(11-12-27)16-3-1-4-16/h5-8,16,18-19,25H,1-4,9-14H2/t18-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317685
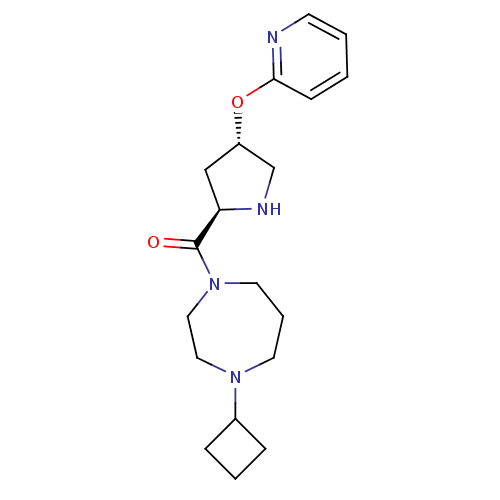
((4-cyclobutyl-1,4-diazepan-1-yl)((2R,4S)-4-(pyridi...)Show SMILES O=C([C@H]1C[C@@H](CN1)Oc1ccccn1)N1CCCN(CC1)C1CCC1 |r| Show InChI InChI=1S/C19H28N4O2/c24-19(23-10-4-9-22(11-12-23)15-5-3-6-15)17-13-16(14-21-17)25-18-7-1-2-8-20-18/h1-2,7-8,15-17,21H,3-6,9-14H2/t16-,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317710
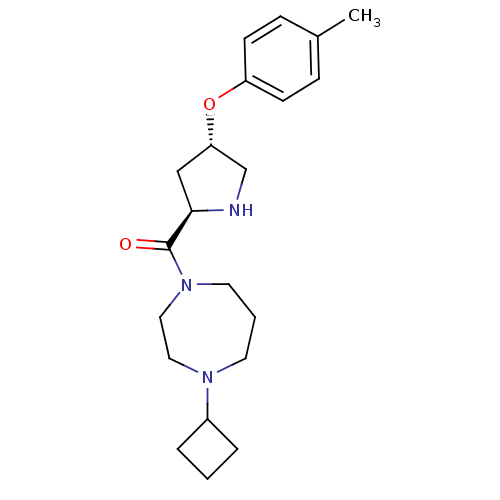
((4-cyclobutyl-1,4-diazepan-1-yl)((2R,4S)-4-(p-toly...)Show SMILES Cc1ccc(O[C@@H]2CN[C@H](C2)C(=O)N2CCCN(CC2)C2CCC2)cc1 |r| Show InChI InChI=1S/C21H31N3O2/c1-16-6-8-18(9-7-16)26-19-14-20(22-15-19)21(25)24-11-3-10-23(12-13-24)17-4-2-5-17/h6-9,17,19-20,22H,2-5,10-15H2,1H3/t19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317712
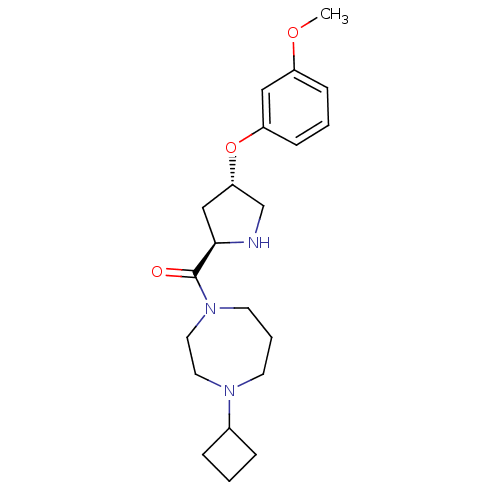
((4-cyclobutyl-1,4-diazepan-1-yl)((2R,4S)-4-(3-meth...)Show SMILES COc1cccc(O[C@@H]2CN[C@H](C2)C(=O)N2CCCN(CC2)C2CCC2)c1 |r| Show InChI InChI=1S/C21H31N3O3/c1-26-17-7-3-8-18(13-17)27-19-14-20(22-15-19)21(25)24-10-4-9-23(11-12-24)16-5-2-6-16/h3,7-8,13,16,19-20,22H,2,4-6,9-12,14-15H2,1H3/t19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317709

((4-cyclobutyl-1,4-diazepan-1-yl)((2R,4S)-4-(m-toly...)Show SMILES Cc1cccc(O[C@@H]2CN[C@H](C2)C(=O)N2CCCN(CC2)C2CCC2)c1 |r| Show InChI InChI=1S/C21H31N3O2/c1-16-5-2-8-18(13-16)26-19-14-20(22-15-19)21(25)24-10-4-9-23(11-12-24)17-6-3-7-17/h2,5,8,13,17,19-20,22H,3-4,6-7,9-12,14-15H2,1H3/t19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317707
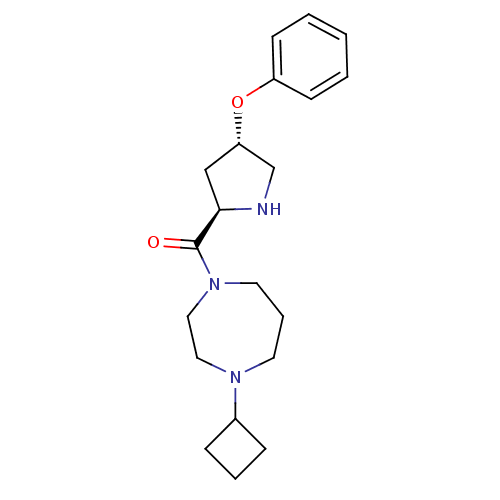
((4-cyclobutyl-1,4-diazepan-1-yl)((2R,4S)-4-phenoxy...)Show SMILES O=C([C@H]1C[C@@H](CN1)Oc1ccccc1)N1CCCN(CC1)C1CCC1 |r| Show InChI InChI=1S/C20H29N3O2/c24-20(23-11-5-10-22(12-13-23)16-6-4-7-16)19-14-18(15-21-19)25-17-8-2-1-3-9-17/h1-3,8-9,16,18-19,21H,4-7,10-15H2/t18-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317684

((4-cyclobutyl-1,4-diazepan-1-yl)((2R,4S)-4-(thioph...)Show SMILES O=C([C@H]1C[C@@H](CN1)Oc1ccsc1)N1CCCN(CC1)C1CCC1 |r| Show InChI InChI=1S/C18H27N3O2S/c22-18(17-11-16(12-19-17)23-15-5-10-24-13-15)21-7-2-6-20(8-9-21)14-3-1-4-14/h5,10,13-14,16-17,19H,1-4,6-9,11-12H2/t16-,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317689
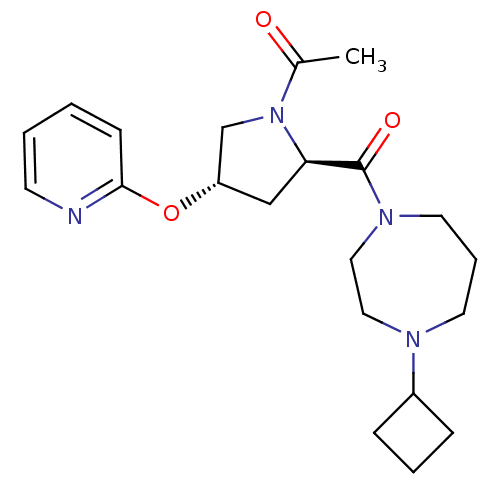
(1-((2R,4S)-2-(4-cyclobutyl-1,4-diazepane-1-carbony...)Show SMILES CC(=O)N1C[C@H](C[C@@H]1C(=O)N1CCCN(CC1)C1CCC1)Oc1ccccn1 |r| Show InChI InChI=1S/C21H30N4O3/c1-16(26)25-15-18(28-20-8-2-3-9-22-20)14-19(25)21(27)24-11-5-10-23(12-13-24)17-6-4-7-17/h2-3,8-9,17-19H,4-7,10-15H2,1H3/t18-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317690

(1-((2R,4S)-2-(4-cyclobutyl-1,4-diazepane-1-carbony...)Show SMILES CC(=O)N1C[C@H](C[C@@H]1C(=O)N1CCCN(CC1)C1CCC1)Oc1cccnc1 |r| Show InChI InChI=1S/C21H30N4O3/c1-16(26)25-15-19(28-18-7-3-8-22-14-18)13-20(25)21(27)24-10-4-9-23(11-12-24)17-5-2-6-17/h3,7-8,14,17,19-20H,2,4-6,9-13,15H2,1H3/t19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317727
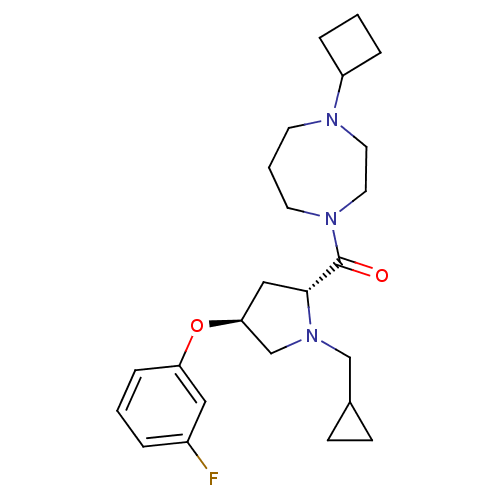
((4-cyclobutyl-1,4-diazepan-1-yl)((2R,4S)-1-(cyclop...)Show SMILES Fc1cccc(O[C@H]2C[C@@H](N(CC3CC3)C2)C(=O)N2CCCN(CC2)C2CCC2)c1 |r| Show InChI InChI=1S/C24H34FN3O2/c25-19-4-1-7-21(14-19)30-22-15-23(28(17-22)16-18-8-9-18)24(29)27-11-3-10-26(12-13-27)20-5-2-6-20/h1,4,7,14,18,20,22-23H,2-3,5-6,8-13,15-17H2/t22-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317726

((4-cyclobutyl-1,4-diazepan-1-yl)((2R,4S)-1-(cyclop...)Show SMILES Fc1cccc(O[C@H]2C[C@@H](N(C2)C(=O)C2CC2)C(=O)N2CCCN(CC2)C2CCC2)c1 |r| Show InChI InChI=1S/C24H32FN3O3/c25-18-4-1-7-20(14-18)31-21-15-22(28(16-21)23(29)17-8-9-17)24(30)27-11-3-10-26(12-13-27)19-5-2-6-19/h1,4,7,14,17,19,21-22H,2-3,5-6,8-13,15-16H2/t21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317694

((S)-(2-benzylpyrrolidin-2-yl)(4-cyclobutyl-1,4-dia...)Show SMILES O=C(N1CCCN(CC1)C1CCC1)[C@@]1(Cc2ccccc2)CCCN1 |r| Show InChI InChI=1S/C21H31N3O/c25-20(24-14-6-13-23(15-16-24)19-9-4-10-19)21(11-5-12-22-21)17-18-7-2-1-3-8-18/h1-3,7-8,19,22H,4-6,9-17H2/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317717
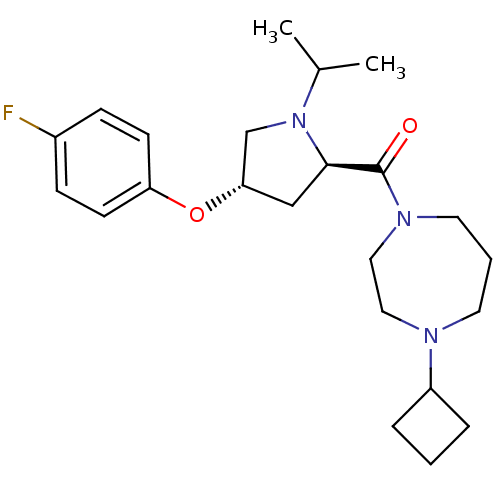
((4-cyclobutyl-1,4-diazepan-1-yl)((2R,4S)-4-(4-fluo...)Show SMILES CC(C)N1C[C@H](C[C@@H]1C(=O)N1CCCN(CC1)C1CCC1)Oc1ccc(F)cc1 |r| Show InChI InChI=1S/C23H34FN3O2/c1-17(2)27-16-21(29-20-9-7-18(24)8-10-20)15-22(27)23(28)26-12-4-11-25(13-14-26)19-5-3-6-19/h7-10,17,19,21-22H,3-6,11-16H2,1-2H3/t21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317724
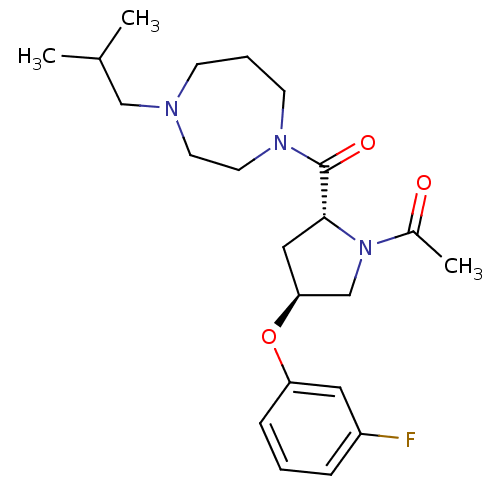
(1-((2R,4S)-4-(3-fluorophenoxy)-2-(4-isobutyl-1,4-d...)Show SMILES CC(C)CN1CCCN(CC1)C(=O)[C@H]1C[C@@H](CN1C(C)=O)Oc1cccc(F)c1 |r| Show InChI InChI=1S/C22H32FN3O3/c1-16(2)14-24-8-5-9-25(11-10-24)22(28)21-13-20(15-26(21)17(3)27)29-19-7-4-6-18(23)12-19/h4,6-7,12,16,20-21H,5,8-11,13-15H2,1-3H3/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317718
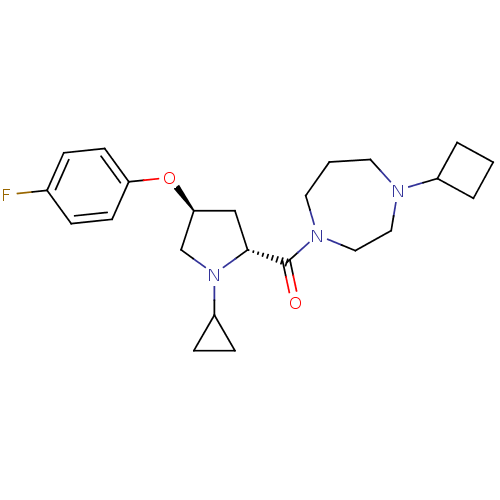
((4-cyclobutyl-1,4-diazepan-1-yl)((2R,4S)-1-cyclopr...)Show SMILES Fc1ccc(O[C@H]2C[C@@H](N(C2)C2CC2)C(=O)N2CCCN(CC2)C2CCC2)cc1 |r| Show InChI InChI=1S/C23H32FN3O2/c24-17-5-9-20(10-6-17)29-21-15-22(27(16-21)19-7-8-19)23(28)26-12-2-11-25(13-14-26)18-3-1-4-18/h5-6,9-10,18-19,21-22H,1-4,7-8,11-16H2/t21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317691
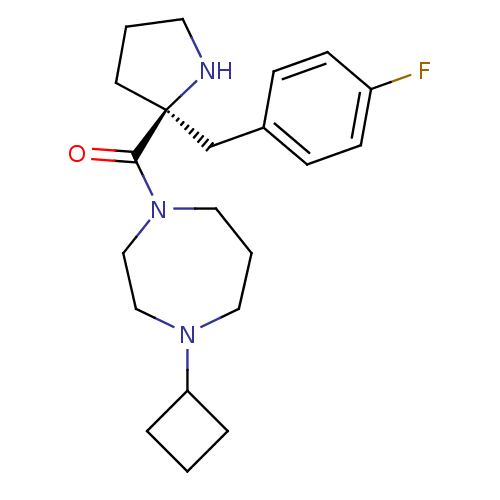
((S)-(4-cyclobutyl-1,4-diazepan-1-yl)(2-(4-fluorobe...)Show SMILES Fc1ccc(C[C@@]2(CCCN2)C(=O)N2CCCN(CC2)C2CCC2)cc1 |r| Show InChI InChI=1S/C21H30FN3O/c22-18-8-6-17(7-9-18)16-21(10-2-11-23-21)20(26)25-13-3-12-24(14-15-25)19-4-1-5-19/h6-9,19,23H,1-5,10-16H2/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317725

((2R,4S)-ethyl 2-(4-cyclobutyl-1,4-diazepane-1-carb...)Show SMILES CCOC(=O)N1C[C@H](C[C@@H]1C(=O)N1CCCN(CC1)C1CCC1)Oc1cccc(F)c1 |r| Show InChI InChI=1S/C23H32FN3O4/c1-2-30-23(29)27-16-20(31-19-9-3-6-17(24)14-19)15-21(27)22(28)26-11-5-10-25(12-13-26)18-7-4-8-18/h3,6,9,14,18,20-21H,2,4-5,7-8,10-13,15-16H2,1H3/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317719
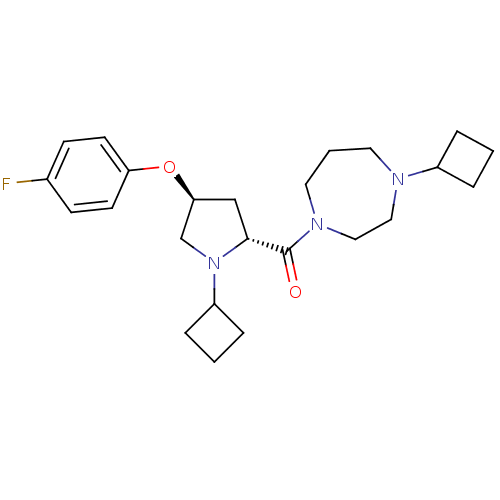
((4-cyclobutyl-1,4-diazepan-1-yl)((2R,4S)-1-cyclobu...)Show SMILES Fc1ccc(O[C@H]2C[C@@H](N(C2)C2CCC2)C(=O)N2CCCN(CC2)C2CCC2)cc1 |r| Show InChI InChI=1S/C24H34FN3O2/c25-18-8-10-21(11-9-18)30-22-16-23(28(17-22)20-6-2-7-20)24(29)27-13-3-12-26(14-15-27)19-4-1-5-19/h8-11,19-20,22-23H,1-7,12-17H2/t22-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317715
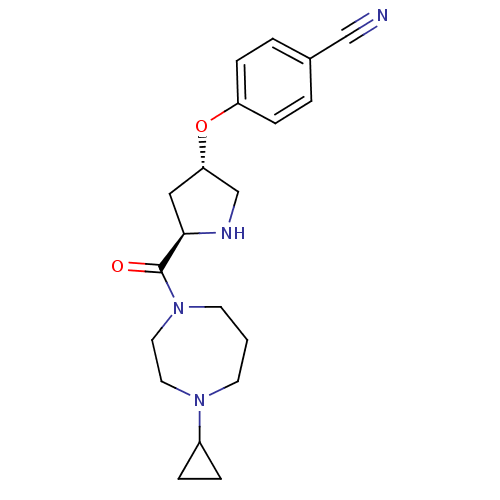
(4-((3S,5R)-5-(4-cyclopropyl-1,4-diazepane-1-carbon...)Show SMILES O=C([C@H]1C[C@@H](CN1)Oc1ccc(cc1)C#N)N1CCCN(CC1)C1CC1 |r| Show InChI InChI=1S/C20H26N4O2/c21-13-15-2-6-17(7-3-15)26-18-12-19(22-14-18)20(25)24-9-1-8-23(10-11-24)16-4-5-16/h2-3,6-7,16,18-19,22H,1,4-5,8-12,14H2/t18-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50317698

(1-((2R,4S)-2-(4-cyclobutyl-1,4-diazepane-1-carbony...)Show SMILES CC(=O)N1C[C@H](C[C@@H]1C(=O)N1CCCN(CC1)C1CCC1)Oc1cccc(F)c1 |r| Show InChI InChI=1S/C22H30FN3O3/c1-16(27)26-15-20(29-19-8-2-5-17(23)13-19)14-21(26)22(28)25-10-4-9-24(11-12-25)18-6-3-7-18/h2,5,8,13,18,20-21H,3-4,6-7,9-12,14-15H2,1H3/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to rat histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317677

((4-cyclobutyl-1,4-diazepan-1-yl)((2S,4R)-4-(3-fluo...)Show SMILES Fc1cccc(O[C@H]2CN[C@@H](C2)C(=O)N2CCCN(CC2)C2CCC2)c1 |r| Show InChI InChI=1S/C20H28FN3O2/c21-15-4-1-7-17(12-15)26-18-13-19(22-14-18)20(25)24-9-3-8-23(10-11-24)16-5-2-6-16/h1,4,7,12,16,18-19,22H,2-3,5-6,8-11,13-14H2/t18-,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317692
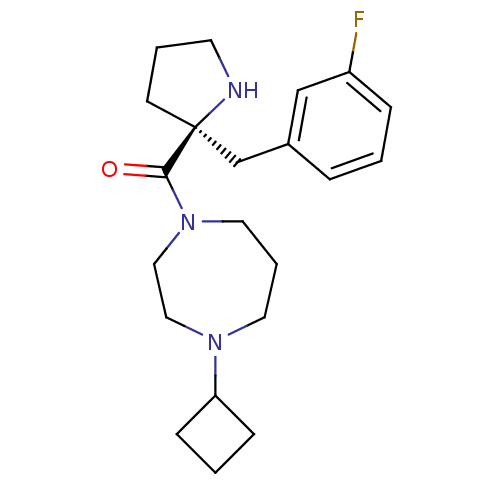
((S)-(4-cyclobutyl-1,4-diazepan-1-yl)(2-(3-fluorobe...)Show SMILES Fc1cccc(C[C@@]2(CCCN2)C(=O)N2CCCN(CC2)C2CCC2)c1 |r| Show InChI InChI=1S/C21H30FN3O/c22-18-6-1-5-17(15-18)16-21(9-3-10-23-21)20(26)25-12-4-11-24(13-14-25)19-7-2-8-19/h1,5-6,15,19,23H,2-4,7-14,16H2/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317678
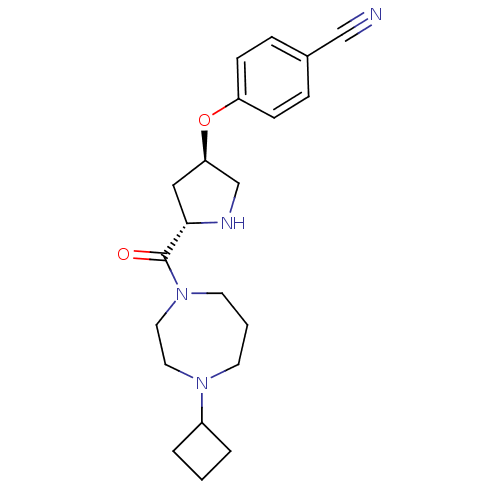
(4-((3R,5S)-5-(4-cyclobutyl-1,4-diazepane-1-carbony...)Show SMILES O=C([C@@H]1C[C@H](CN1)Oc1ccc(cc1)C#N)N1CCCN(CC1)C1CCC1 |r| Show InChI InChI=1S/C21H28N4O2/c22-14-16-5-7-18(8-6-16)27-19-13-20(23-15-19)21(26)25-10-2-9-24(11-12-25)17-3-1-4-17/h5-8,17,19-20,23H,1-4,9-13,15H2/t19-,20+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317674
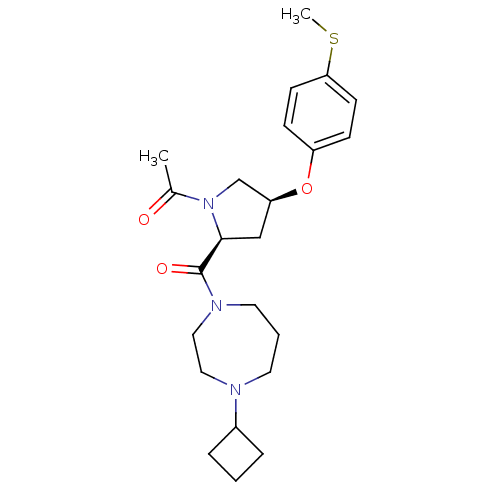
(1-((2S,4S)-2-(4-cyclobutyl-1,4-diazepane-1-carbony...)Show SMILES CSc1ccc(O[C@H]2C[C@H](N(C2)C(C)=O)C(=O)N2CCCN(CC2)C2CCC2)cc1 |r| Show InChI InChI=1S/C23H33N3O3S/c1-17(27)26-16-20(29-19-7-9-21(30-2)10-8-19)15-22(26)23(28)25-12-4-11-24(13-14-25)18-5-3-6-18/h7-10,18,20,22H,3-6,11-16H2,1-2H3/t20-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317723
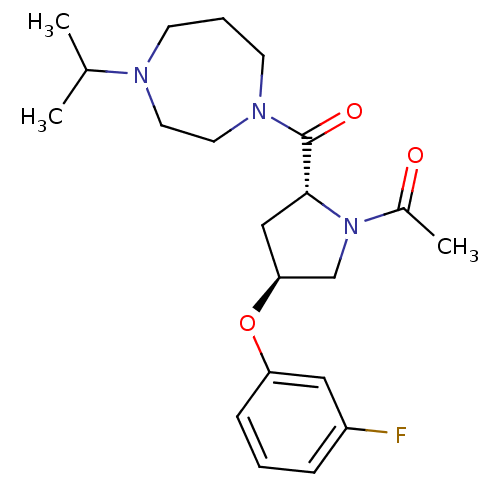
(1-((2R,4S)-4-(3-fluorophenoxy)-2-(4-isopropyl-1,4-...)Show SMILES CC(C)N1CCCN(CC1)C(=O)[C@H]1C[C@@H](CN1C(C)=O)Oc1cccc(F)c1 |r| Show InChI InChI=1S/C21H30FN3O3/c1-15(2)23-8-5-9-24(11-10-23)21(27)20-13-19(14-25(20)16(3)26)28-18-7-4-6-17(22)12-18/h4,6-7,12,15,19-20H,5,8-11,13-14H2,1-3H3/t19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317681
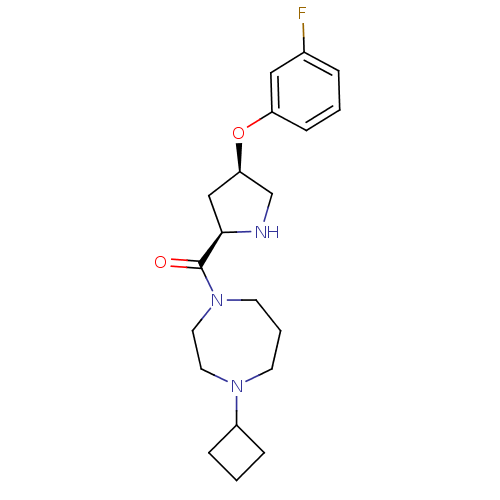
((4-cyclobutyl-1,4-diazepan-1-yl)((2R,4R)-4-(3-fluo...)Show SMILES Fc1cccc(O[C@H]2CN[C@H](C2)C(=O)N2CCCN(CC2)C2CCC2)c1 |r| Show InChI InChI=1S/C20H28FN3O2/c21-15-4-1-7-17(12-15)26-18-13-19(22-14-18)20(25)24-9-3-8-23(10-11-24)16-5-2-6-16/h1,4,7,12,16,18-19,22H,2-3,5-6,8-11,13-14H2/t18-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317676
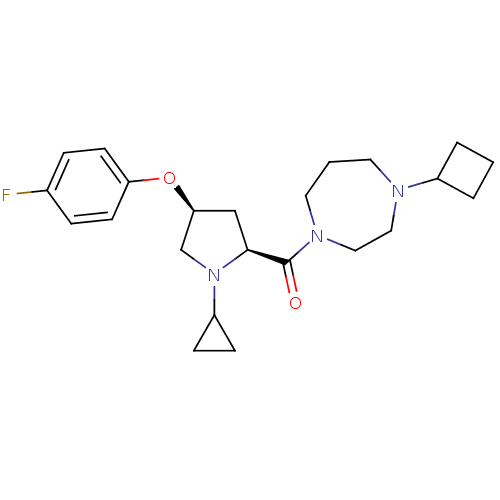
((4-cyclobutyl-1,4-diazepan-1-yl)((2S,4S)-1-cyclopr...)Show SMILES Fc1ccc(O[C@H]2C[C@H](N(C2)C2CC2)C(=O)N2CCCN(CC2)C2CCC2)cc1 |r| Show InChI InChI=1S/C23H32FN3O2/c24-17-5-9-20(10-6-17)29-21-15-22(27(16-21)19-7-8-19)23(28)26-12-2-11-25(13-14-26)18-3-1-4-18/h5-6,9-10,18-19,21-22H,1-4,7-8,11-16H2/t21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317729
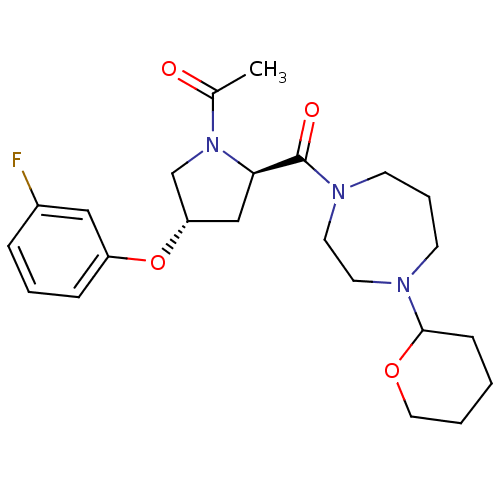
(1-((2R,4S)-4-(3-fluorophenoxy)-2-(4-(tetrahydro-2H...)Show SMILES CC(=O)N1C[C@H](C[C@@H]1C(=O)N1CCCN(CC1)C1CCCCO1)Oc1cccc(F)c1 |r| Show InChI InChI=1S/C23H32FN3O4/c1-17(28)27-16-20(31-19-7-4-6-18(24)14-19)15-21(27)23(29)26-10-5-9-25(11-12-26)22-8-2-3-13-30-22/h4,6-7,14,20-22H,2-3,5,8-13,15-16H2,1H3/t20-,21+,22?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317682
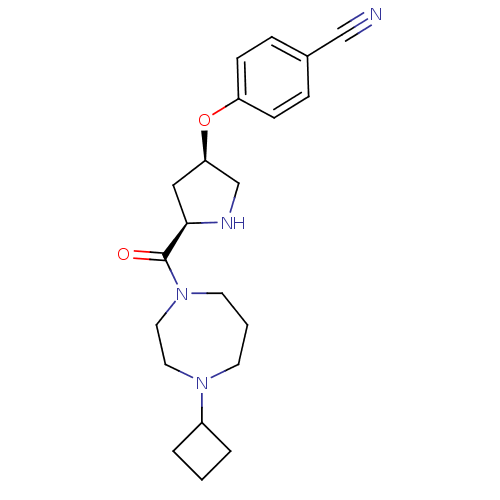
(4-((3R,5R)-5-(4-cyclobutyl-1,4-diazepane-1-carbony...)Show SMILES O=C([C@H]1C[C@H](CN1)Oc1ccc(cc1)C#N)N1CCCN(CC1)C1CCC1 |r| Show InChI InChI=1S/C21H28N4O2/c22-14-16-5-7-18(8-6-16)27-19-13-20(23-15-19)21(26)25-10-2-9-24(11-12-25)17-3-1-4-17/h5-8,17,19-20,23H,1-4,9-13,15H2/t19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317679

((4-cyclobutyl-1,4-diazepan-1-yl)((2S,4R)-4-(4-(met...)Show SMILES CSc1ccc(O[C@H]2CN[C@@H](C2)C(=O)N2CCCN(CC2)C2CCC2)cc1 |r| Show InChI InChI=1S/C21H31N3O2S/c1-27-19-8-6-17(7-9-19)26-18-14-20(22-15-18)21(25)24-11-3-10-23(12-13-24)16-4-2-5-16/h6-9,16,18,20,22H,2-5,10-15H2,1H3/t18-,20+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317730
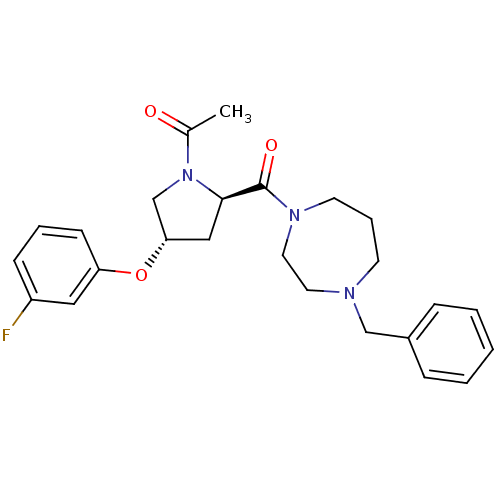
(1-((2R,4S)-2-(4-benzyl-1,4-diazepane-1-carbonyl)-4...)Show SMILES CC(=O)N1C[C@H](C[C@@H]1C(=O)N1CCCN(Cc2ccccc2)CC1)Oc1cccc(F)c1 |r| Show InChI InChI=1S/C25H30FN3O3/c1-19(30)29-18-23(32-22-10-5-9-21(26)15-22)16-24(29)25(31)28-12-6-11-27(13-14-28)17-20-7-3-2-4-8-20/h2-5,7-10,15,23-24H,6,11-14,16-18H2,1H3/t23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317673
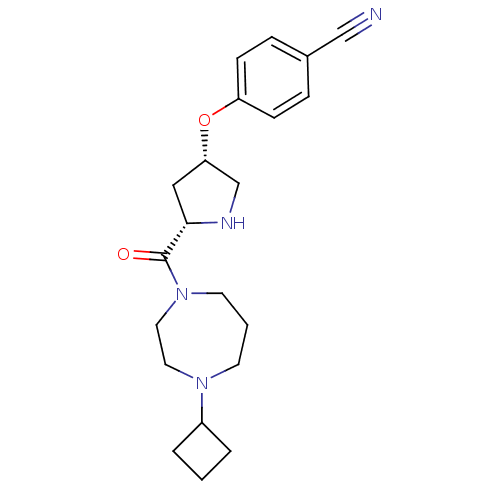
(4-((3S,5S)-5-(4-cyclobutyl-1,4-diazepane-1-carbony...)Show SMILES O=C([C@@H]1C[C@@H](CN1)Oc1ccc(cc1)C#N)N1CCCN(CC1)C1CCC1 |r| Show InChI InChI=1S/C21H28N4O2/c22-14-16-5-7-18(8-6-16)27-19-13-20(23-15-19)21(26)25-10-2-9-24(11-12-25)17-3-1-4-17/h5-8,17,19-20,23H,1-4,9-13,15H2/t19-,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 184 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317693

((S)-(4-cyclobutyl-1,4-diazepan-1-yl)(2-(4-(trifluo...)Show SMILES FC(F)(F)c1ccc(C[C@@]2(CCCN2)C(=O)N2CCCN(CC2)C2CCC2)cc1 |r| Show InChI InChI=1S/C22H30F3N3O/c23-22(24,25)18-8-6-17(7-9-18)16-21(10-2-11-26-21)20(29)28-13-3-12-27(14-15-28)19-4-1-5-19/h6-9,19,26H,1-5,10-16H2/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317695

(((2R,4S)-4-benzylpyrrolidin-2-yl)(4-cyclobutyl-1,4...)Show SMILES O=C([C@H]1C[C@H](Cc2ccccc2)CN1)N1CCCN(CC1)C1CCC1 |r| Show InChI InChI=1S/C21H31N3O/c25-21(24-11-5-10-23(12-13-24)19-8-4-9-19)20-15-18(16-22-20)14-17-6-2-1-3-7-17/h1-3,6-7,18-20,22H,4-5,8-16H2/t18-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317697
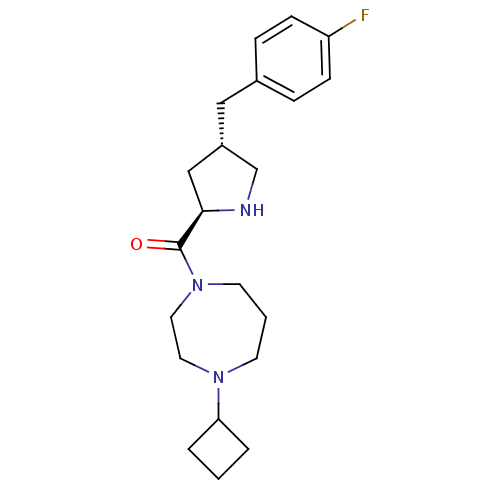
((4-cyclobutyl-1,4-diazepan-1-yl)((2R,4S)-4-(4-fluo...)Show SMILES Fc1ccc(C[C@@H]2CN[C@H](C2)C(=O)N2CCCN(CC2)C2CCC2)cc1 |r| Show InChI InChI=1S/C21H30FN3O/c22-18-7-5-16(6-8-18)13-17-14-20(23-15-17)21(26)25-10-2-9-24(11-12-25)19-3-1-4-19/h5-8,17,19-20,23H,1-4,9-15H2/t17-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317696

((4-cyclobutyl-1,4-diazepan-1-yl)((2R,4S)-4-(3-fluo...)Show SMILES Fc1cccc(C[C@@H]2CN[C@H](C2)C(=O)N2CCCN(CC2)C2CCC2)c1 |r| Show InChI InChI=1S/C21H30FN3O/c22-18-5-1-4-16(13-18)12-17-14-20(23-15-17)21(26)25-9-3-8-24(10-11-25)19-6-2-7-19/h1,4-5,13,17,19-20,23H,2-3,6-12,14-15H2/t17-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317722
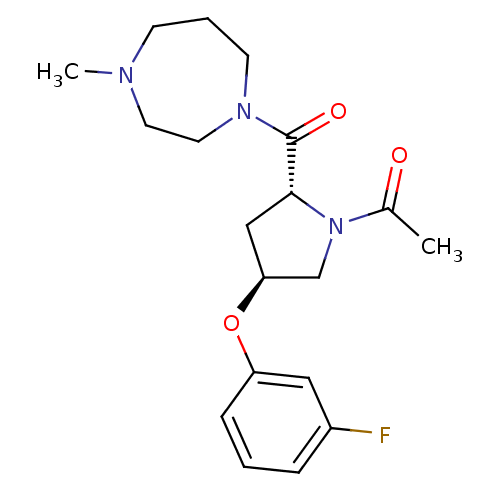
(1-((2R,4S)-4-(3-fluorophenoxy)-2-(4-methyl-1,4-dia...)Show SMILES CN1CCCN(CC1)C(=O)[C@H]1C[C@@H](CN1C(C)=O)Oc1cccc(F)c1 |r| Show InChI InChI=1S/C19H26FN3O3/c1-14(24)23-13-17(26-16-6-3-5-15(20)11-16)12-18(23)19(25)22-8-4-7-21(2)9-10-22/h3,5-6,11,17-18H,4,7-10,12-13H2,1-2H3/t17-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 455 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317680
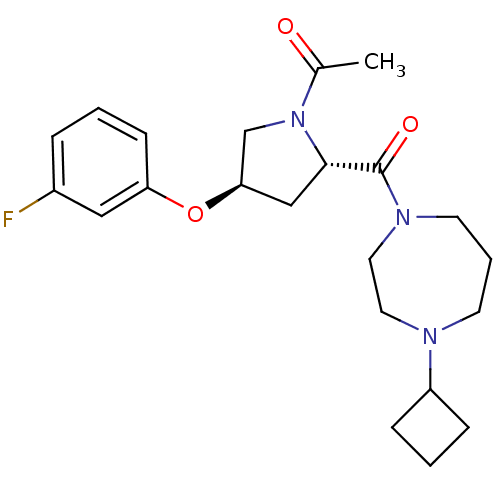
(1-((2S,4R)-2-(4-cyclobutyl-1,4-diazepane-1-carbony...)Show SMILES CC(=O)N1C[C@@H](C[C@H]1C(=O)N1CCCN(CC1)C1CCC1)Oc1cccc(F)c1 |r| Show InChI InChI=1S/C22H30FN3O3/c1-16(27)26-15-20(29-19-8-2-5-17(23)13-19)14-21(26)22(28)25-10-4-9-24(11-12-25)18-6-3-7-18/h2,5,8,13,18,20-21H,3-4,6-7,9-12,14-15H2,1H3/t20-,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317675
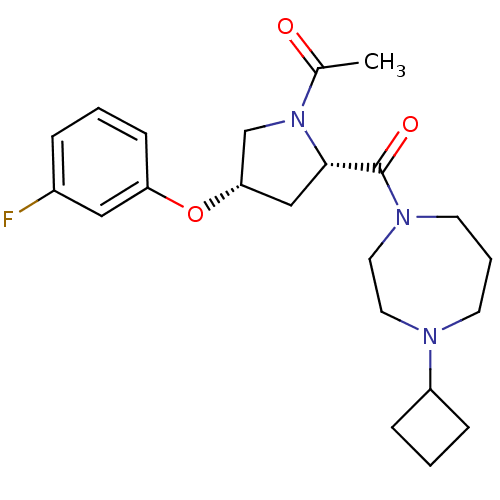
(1-((2S,4S)-2-(4-cyclobutyl-1,4-diazepane-1-carbony...)Show SMILES CC(=O)N1C[C@H](C[C@H]1C(=O)N1CCCN(CC1)C1CCC1)Oc1cccc(F)c1 |r| Show InChI InChI=1S/C22H30FN3O3/c1-16(27)26-15-20(29-19-8-2-5-17(23)13-19)14-21(26)22(28)25-10-4-9-24(11-12-25)18-6-3-7-18/h2,5,8,13,18,20-21H,3-4,6-7,9-12,14-15H2,1H3/t20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 717 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317683
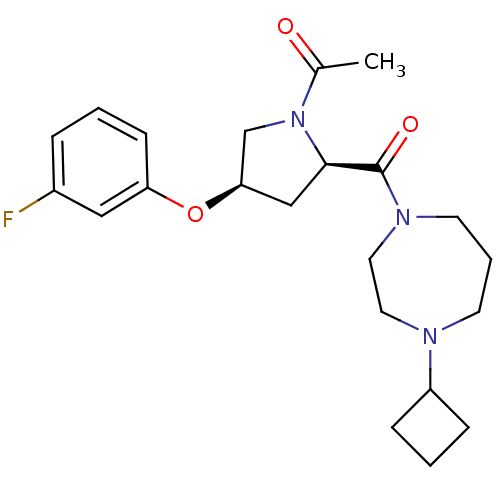
(1-((2R,4R)-2-(4-cyclobutyl-1,4-diazepane-1-carbony...)Show SMILES CC(=O)N1C[C@@H](C[C@@H]1C(=O)N1CCCN(CC1)C1CCC1)Oc1cccc(F)c1 |r| Show InChI InChI=1S/C22H30FN3O3/c1-16(27)26-15-20(29-19-8-2-5-17(23)13-19)14-21(26)22(28)25-10-4-9-24(11-12-25)18-6-3-7-18/h2,5,8,13,18,20-21H,3-4,6-7,9-12,14-15H2,1H3/t20-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50317721
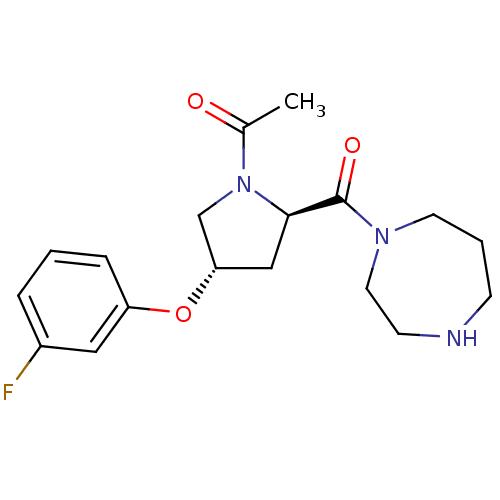
(1-((2R,4S)-2-(1,4-diazepane-1-carbonyl)-4-(3-fluor...)Show SMILES CC(=O)N1C[C@H](C[C@@H]1C(=O)N1CCCNCC1)Oc1cccc(F)c1 |r| Show InChI InChI=1S/C18H24FN3O3/c1-13(23)22-12-16(25-15-5-2-4-14(19)10-15)11-17(22)18(24)21-8-3-6-20-7-9-21/h2,4-5,10,16-17,20H,3,6-9,11-12H2,1H3/t16-,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50317701

(((2R,4S)-4-(4-chlorophenoxy)pyrrolidin-2-yl)(4-cyc...)Show SMILES Clc1ccc(O[C@@H]2CN[C@H](C2)C(=O)N2CCCN(CC2)C2CCC2)cc1 |r| Show InChI InChI=1S/C20H28ClN3O2/c21-15-5-7-17(8-6-15)26-18-13-19(22-14-18)20(25)24-10-2-9-23(11-12-24)16-3-1-4-16/h5-8,16,18-19,22H,1-4,9-14H2/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Displacement of labeled dofetilide human ERG |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50317705

(4-((3S,5R)-5-(4-cyclopentyl-1,4-diazepane-1-carbon...)Show SMILES O=C([C@H]1C[C@@H](CN1)Oc1ccc(cc1)C#N)N1CCCN(CC1)C1CCCC1 |r| Show InChI InChI=1S/C22H30N4O2/c23-15-17-6-8-19(9-7-17)28-20-14-21(24-16-20)22(27)26-11-3-10-25(12-13-26)18-4-1-2-5-18/h6-9,18,20-21,24H,1-5,10-14,16H2/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Displacement of labeled dofetilide human ERG |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50317699

((4-cyclobutyl-1,4-diazepan-1-yl)((2R,4S)-4-(3-fluo...)Show SMILES Fc1cccc(O[C@@H]2CN[C@H](C2)C(=O)N2CCCN(CC2)C2CCC2)c1 |r| Show InChI InChI=1S/C20H28FN3O2/c21-15-4-1-7-17(12-15)26-18-13-19(22-14-18)20(25)24-9-3-8-23(10-11-24)16-5-2-6-16/h1,4,7,12,16,18-19,22H,2-3,5-6,8-11,13-14H2/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp assay |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50317704

((4-cyclopentyl-1,4-diazepan-1-yl)((2R,4S)-4-(4-flu...)Show SMILES Fc1ccc(O[C@@H]2CN[C@H](C2)C(=O)N2CCCN(CC2)C2CCCC2)cc1 |r| Show InChI InChI=1S/C21H30FN3O2/c22-16-6-8-18(9-7-16)27-19-14-20(23-15-19)21(26)25-11-3-10-24(12-13-25)17-4-1-2-5-17/h6-9,17,19-20,23H,1-5,10-15H2/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Displacement of labeled dofetilide human ERG |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50317698

(1-((2R,4S)-2-(4-cyclobutyl-1,4-diazepane-1-carbony...)Show SMILES CC(=O)N1C[C@H](C[C@@H]1C(=O)N1CCCN(CC1)C1CCC1)Oc1cccc(F)c1 |r| Show InChI InChI=1S/C22H30FN3O3/c1-16(27)26-15-20(29-19-8-2-5-17(23)13-19)14-21(26)22(28)25-10-4-9-24(11-12-25)18-6-3-7-18/h2,5,8,13,18,20-21H,3-4,6-7,9-12,14-15H2,1H3/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp assay |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50317702

(((2R,4S)-4-(3-chlorophenoxy)pyrrolidin-2-yl)(4-cyc...)Show SMILES Clc1cccc(O[C@@H]2CN[C@H](C2)C(=O)N2CCCN(CC2)C2CCC2)c1 |r| Show InChI InChI=1S/C20H28ClN3O2/c21-15-4-1-7-17(12-15)26-18-13-19(22-14-18)20(25)24-9-3-8-23(10-11-24)16-5-2-6-16/h1,4,7,12,16,18-19,22H,2-3,5-6,8-11,13-14H2/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Displacement of labeled dofetilide human ERG |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50317700

((4-cyclobutyl-1,4-diazepan-1-yl)((2R,4S)-4-(4-fluo...)Show SMILES Fc1ccc(O[C@@H]2CN[C@H](C2)C(=O)N2CCCN(CC2)C2CCC2)cc1 |r| Show InChI InChI=1S/C20H28FN3O2/c21-15-5-7-17(8-6-15)26-18-13-19(22-14-18)20(25)24-10-2-9-23(11-12-24)16-3-1-4-16/h5-8,16,18-19,22H,1-4,9-14H2/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp assay |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50317699

((4-cyclobutyl-1,4-diazepan-1-yl)((2R,4S)-4-(3-fluo...)Show SMILES Fc1cccc(O[C@@H]2CN[C@H](C2)C(=O)N2CCCN(CC2)C2CCC2)c1 |r| Show InChI InChI=1S/C20H28FN3O2/c21-15-4-1-7-17(12-15)26-18-13-19(22-14-18)20(25)24-9-3-8-23(10-11-24)16-5-2-6-16/h1,4,7,12,16,18-19,22H,2-3,5-6,8-11,13-14H2/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Displacement of labeled dofetilide human ERG |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50317703

(4-((3S,5R)-5-(4-cyclobutyl-1,4-diazepane-1-carbony...)Show SMILES O=C([C@H]1C[C@@H](CN1)Oc1ccc(cc1)C#N)N1CCCN(CC1)C1CCC1 |r| Show InChI InChI=1S/C21H28N4O2/c22-14-16-5-7-18(8-6-16)27-19-13-20(23-15-19)21(26)25-10-2-9-24(11-12-25)17-3-1-4-17/h5-8,17,19-20,23H,1-4,9-13,15H2/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Displacement of labeled dofetilide human ERG |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50317698

(1-((2R,4S)-2-(4-cyclobutyl-1,4-diazepane-1-carbony...)Show SMILES CC(=O)N1C[C@H](C[C@@H]1C(=O)N1CCCN(CC1)C1CCC1)Oc1cccc(F)c1 |r| Show InChI InChI=1S/C22H30FN3O3/c1-16(27)26-15-20(29-19-8-2-5-17(23)13-19)14-21(26)22(28)25-10-4-9-24(11-12-25)18-6-3-7-18/h2,5,8,13,18,20-21H,3-4,6-7,9-12,14-15H2,1H3/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Displacement of labeled dofetilide human ERG |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50317706

(1-((2R,4S)-2-(4-cyclobutyl-1,4-diazepane-1-carbony...)Show SMILES CC(=O)N1C[C@H](C[C@@H]1C(=O)N1CCCN(CC1)C1CCC1)Oc1ccc(F)cc1 |r| Show InChI InChI=1S/C22H30FN3O3/c1-16(27)26-15-20(29-19-8-6-17(23)7-9-19)14-21(26)22(28)25-11-3-10-24(12-13-25)18-4-2-5-18/h6-9,18,20-21H,2-5,10-15H2,1H3/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Displacement of labeled dofetilide human ERG |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50317700

((4-cyclobutyl-1,4-diazepan-1-yl)((2R,4S)-4-(4-fluo...)Show SMILES Fc1ccc(O[C@@H]2CN[C@H](C2)C(=O)N2CCCN(CC2)C2CCC2)cc1 |r| Show InChI InChI=1S/C20H28FN3O2/c21-15-5-7-17(8-6-15)26-18-13-19(22-14-18)20(25)24-10-2-9-23(11-12-24)16-3-1-4-16/h5-8,16,18-19,22H,1-4,9-14H2/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C.
Curated by ChEMBL
| Assay Description
Displacement of labeled dofetilide human ERG |
Bioorg Med Chem Lett 20: 2755-60 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.071
BindingDB Entry DOI: 10.7270/Q2Z60P7S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data