

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM50029090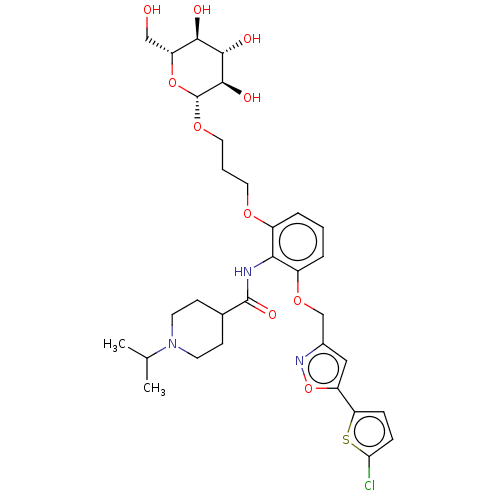 (CHEMBL3343301) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of human factor10a assessed as reduction in hydrolysis of chromogenic substrate S-2765 by Cheng-Prusoff equation analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50029090 (CHEMBL3343301) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of bovine thrombin assessed as reduction in hydrolysis of chromogenic substrate S-2238 by Lineweaver-Burk analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50029091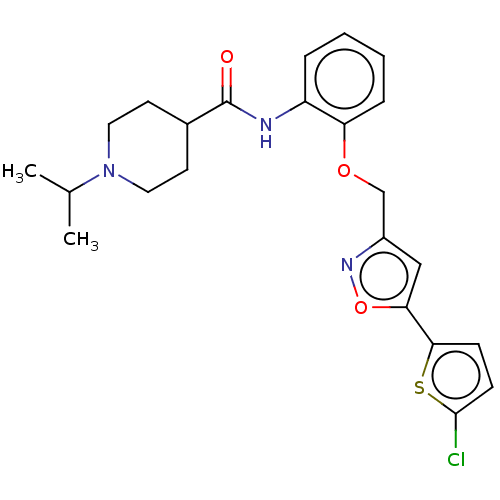 (CHEMBL3343299) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of human factor10a assessed as reduction in hydrolysis of chromogenic substrate S-2765 by Lineweaver-Burk analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50029095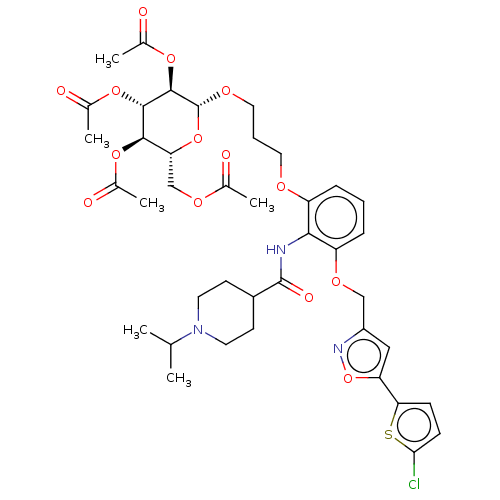 (CHEMBL3343300) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of human factor10a assessed as reduction in hydrolysis of chromogenic substrate S-2765 by Cheng-Prusoff equation analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50029091 (CHEMBL3343299) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of human factor10a assessed as reduction in hydrolysis of chromogenic substrate S-2765 by Cheng-Prusoff equation analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50029090 (CHEMBL3343301) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of human factor10a assessed as reduction in hydrolysis of chromogenic substrate S-2765 by Lineweaver-Burk analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50029091 (CHEMBL3343299) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of bovine thrombin assessed as reduction in hydrolysis of chromogenic substrate S-2238 by Lineweaver-Burk analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50029090 (CHEMBL3343301) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of bovine thrombin assessed as reduction in hydrolysis of chromogenic substrate S-2238 by Cheng-Prusoff equation analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50029095 (CHEMBL3343300) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of bovine thrombin assessed as reduction in hydrolysis of chromogenic substrate S-2238 by Cheng-Prusoff equation analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50029092 (CHEMBL3343304) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of human factor10a assessed as reduction in hydrolysis of chromogenic substrate S-2765 by Cheng-Prusoff equation analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50029091 (CHEMBL3343299) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of bovine thrombin assessed as reduction in hydrolysis of chromogenic substrate S-2238 by Cheng-Prusoff equation analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50029094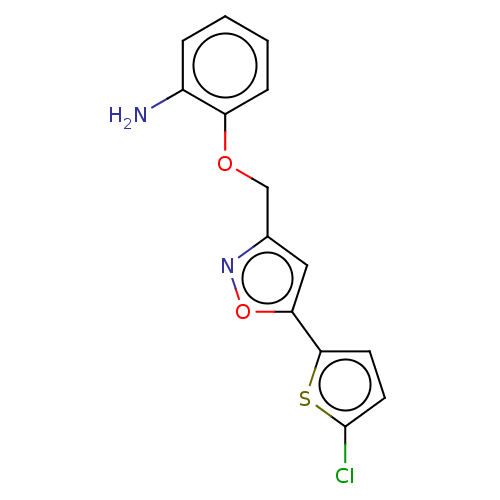 (CHEMBL3343302) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of human factor10a assessed as reduction in hydrolysis of chromogenic substrate S-2765 by Cheng-Prusoff equation analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50029093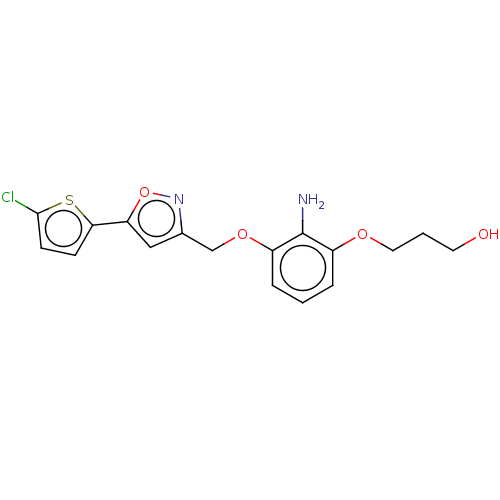 (CHEMBL3343303) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of human factor10a assessed as reduction in hydrolysis of chromogenic substrate S-2765 by Cheng-Prusoff equation analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50029093 (CHEMBL3343303) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of bovine thrombin assessed as reduction in hydrolysis of chromogenic substrate S-2238 by Cheng-Prusoff equation analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50029094 (CHEMBL3343302) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of bovine thrombin assessed as reduction in hydrolysis of chromogenic substrate S-2238 by Cheng-Prusoff equation analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50029092 (CHEMBL3343304) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Consiglio Nazionale delle Ricerche Curated by ChEMBL | Assay Description Inhibition of bovine thrombin assessed as reduction in hydrolysis of chromogenic substrate S-2238 by Cheng-Prusoff equation analysis | J Med Chem 57: 8563-75 (2014) Article DOI: 10.1021/jm5010754 BindingDB Entry DOI: 10.7270/Q2GT5PSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||