

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50120491 (CHEMBL3617995) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Mixed-type inhibition of sEH (unknown origin) using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde measured during 30 mins by... | Bioorg Med Chem 23: 6659-65 (2015) Article DOI: 10.1016/j.bmc.2015.09.003 BindingDB Entry DOI: 10.7270/Q22F7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50120489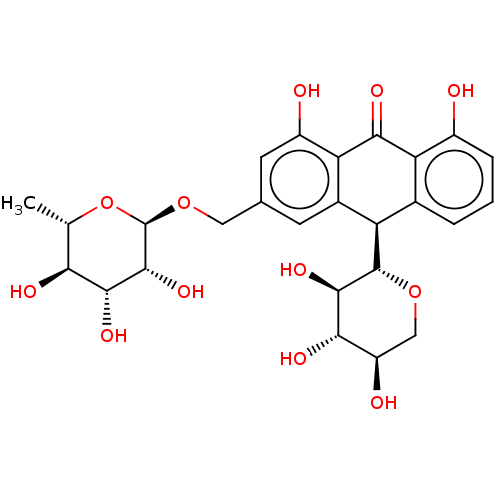 (CHEMBL3617997) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Non-competitive inhibition of sEH (unknown origin) using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde measured during 30 mi... | Bioorg Med Chem 23: 6659-65 (2015) Article DOI: 10.1016/j.bmc.2015.09.003 BindingDB Entry DOI: 10.7270/Q22F7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50120490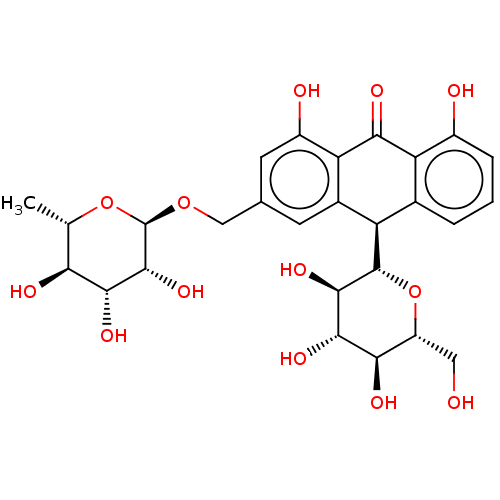 (CHEMBL3617996) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Mixed-type inhibition of sEH (unknown origin) using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde measured during 30 mins by... | Bioorg Med Chem 23: 6659-65 (2015) Article DOI: 10.1016/j.bmc.2015.09.003 BindingDB Entry DOI: 10.7270/Q22F7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50120493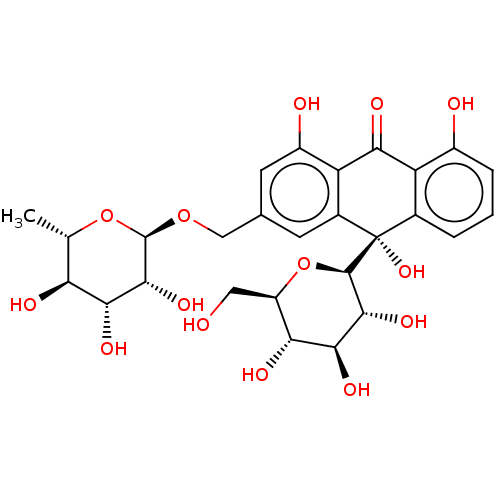 (CHEMBL3617998) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Mixed-type inhibition of sEH (unknown origin) using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde measured during 30 mins by... | Bioorg Med Chem 23: 6659-65 (2015) Article DOI: 10.1016/j.bmc.2015.09.003 BindingDB Entry DOI: 10.7270/Q22F7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50120494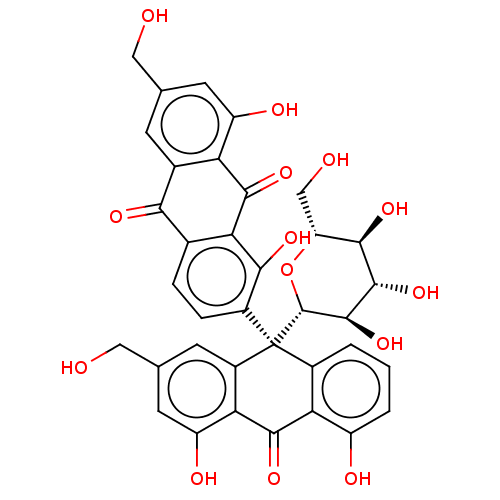 (CHEMBL3617999) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Non-competitive inhibition of sEH (unknown origin) using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde measured during 30 mi... | Bioorg Med Chem 23: 6659-65 (2015) Article DOI: 10.1016/j.bmc.2015.09.003 BindingDB Entry DOI: 10.7270/Q22F7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50120492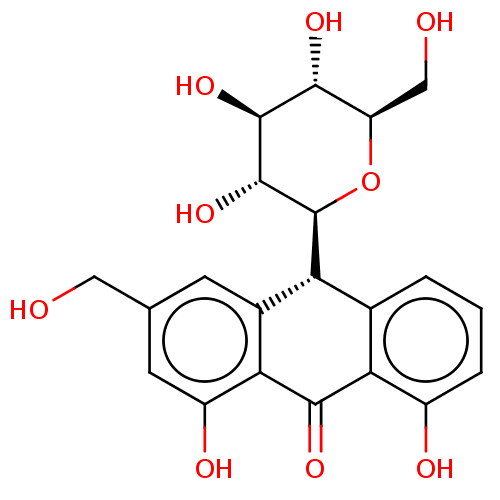 (CHEMBL3617994) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Mixed-type inhibition of sEH (unknown origin) using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde measured during 30 mins by... | Bioorg Med Chem 23: 6659-65 (2015) Article DOI: 10.1016/j.bmc.2015.09.003 BindingDB Entry DOI: 10.7270/Q22F7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50085551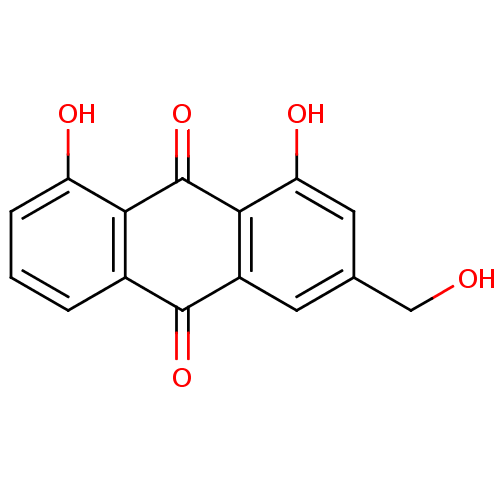 (1,8-Dihydroxy-3-hydroxymethyl-anthraquinone | 1,8-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 5.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Non-competitive inhibition of sEH (unknown origin) using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde measured during 30 mi... | Bioorg Med Chem 23: 6659-65 (2015) Article DOI: 10.1016/j.bmc.2015.09.003 BindingDB Entry DOI: 10.7270/Q22F7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50269016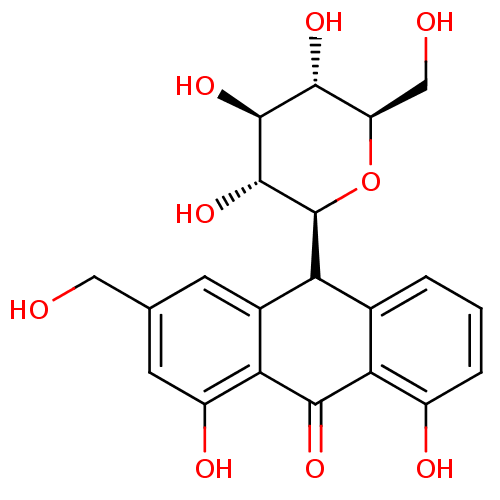 (CHEMBL497001 | aloin) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 5.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Mixed-type inhibition of sEH (unknown origin) using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde measured during 30 mins by... | Bioorg Med Chem 23: 6659-65 (2015) Article DOI: 10.1016/j.bmc.2015.09.003 BindingDB Entry DOI: 10.7270/Q22F7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM25737 (12-[(adamantan-1-ylcarbamoyl)amino]dodecanoic acid...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of sEH (unknown origin) using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde measured during 1 hr by fluorescence ... | Bioorg Med Chem 23: 6659-65 (2015) Article DOI: 10.1016/j.bmc.2015.09.003 BindingDB Entry DOI: 10.7270/Q22F7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50120494 (CHEMBL3617999) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of sEH (unknown origin) using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde measured during 1 hr by fluorescence ... | Bioorg Med Chem 23: 6659-65 (2015) Article DOI: 10.1016/j.bmc.2015.09.003 BindingDB Entry DOI: 10.7270/Q22F7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50120493 (CHEMBL3617998) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of sEH (unknown origin) using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde measured during 1 hr by fluorescence ... | Bioorg Med Chem 23: 6659-65 (2015) Article DOI: 10.1016/j.bmc.2015.09.003 BindingDB Entry DOI: 10.7270/Q22F7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50120489 (CHEMBL3617997) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of sEH (unknown origin) using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde measured during 1 hr by fluorescence ... | Bioorg Med Chem 23: 6659-65 (2015) Article DOI: 10.1016/j.bmc.2015.09.003 BindingDB Entry DOI: 10.7270/Q22F7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50120491 (CHEMBL3617995) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of sEH (unknown origin) using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde measured during 1 hr by fluorescence ... | Bioorg Med Chem 23: 6659-65 (2015) Article DOI: 10.1016/j.bmc.2015.09.003 BindingDB Entry DOI: 10.7270/Q22F7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50120490 (CHEMBL3617996) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of sEH (unknown origin) using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde measured during 1 hr by fluorescence ... | Bioorg Med Chem 23: 6659-65 (2015) Article DOI: 10.1016/j.bmc.2015.09.003 BindingDB Entry DOI: 10.7270/Q22F7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50085551 (1,8-Dihydroxy-3-hydroxymethyl-anthraquinone | 1,8-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of sEH (unknown origin) using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde measured during 1 hr by fluorescence ... | Bioorg Med Chem 23: 6659-65 (2015) Article DOI: 10.1016/j.bmc.2015.09.003 BindingDB Entry DOI: 10.7270/Q22F7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50269016 (CHEMBL497001 | aloin) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of sEH (unknown origin) using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde measured during 1 hr by fluorescence ... | Bioorg Med Chem 23: 6659-65 (2015) Article DOI: 10.1016/j.bmc.2015.09.003 BindingDB Entry DOI: 10.7270/Q22F7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50120492 (CHEMBL3617994) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of sEH (unknown origin) using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde measured during 1 hr by fluorescence ... | Bioorg Med Chem 23: 6659-65 (2015) Article DOI: 10.1016/j.bmc.2015.09.003 BindingDB Entry DOI: 10.7270/Q22F7Q8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||