

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036037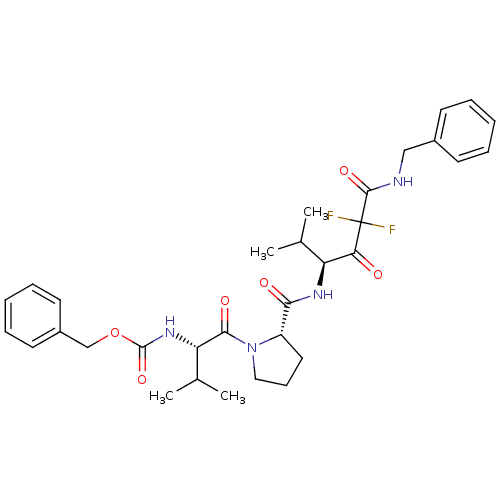 (CHEMBL165759 | {(S)-1-[(S)-2-((S)-3-Benzylcarbamoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala- Ala-Pro-Val-pN | J Med Chem 38: 76-85 (1995) BindingDB Entry DOI: 10.7270/Q2Z60N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036049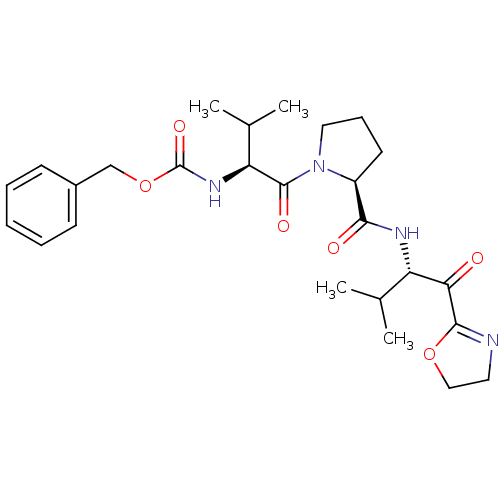 (((S)-1-{(S)-2-[(S)-1-(4,5-Dihydro-oxazole-2-carbon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala- Ala-Pro-Val-pN | J Med Chem 38: 76-85 (1995) BindingDB Entry DOI: 10.7270/Q2Z60N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036047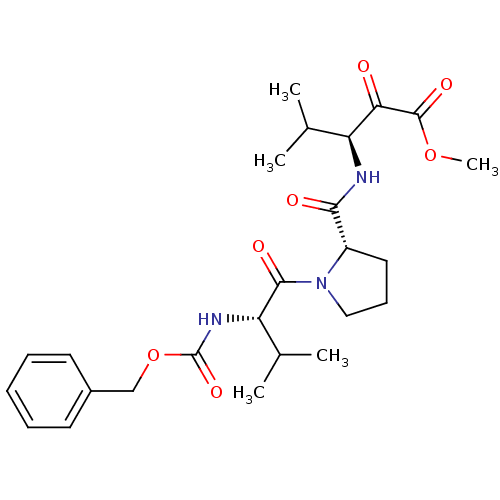 ((S)-3-{[(S)-1-((S)-2-Benzyloxycarbonylamino-3-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala- Ala-Pro-Val-pN | J Med Chem 38: 76-85 (1995) BindingDB Entry DOI: 10.7270/Q2Z60N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036044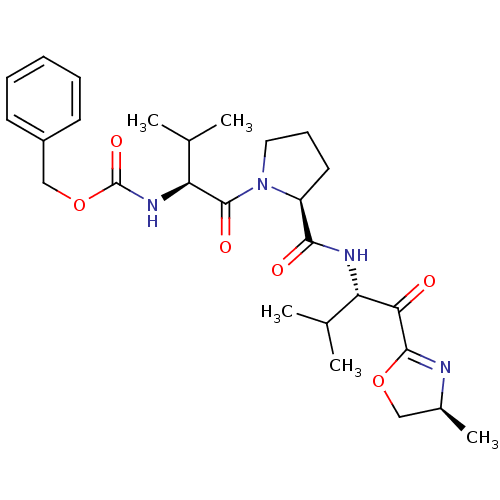 (((S)-2-Methyl-1-{(S)-2-[(S)-2-methyl-1-((S)-4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala- Ala-Pro-Val-pN | J Med Chem 38: 76-85 (1995) BindingDB Entry DOI: 10.7270/Q2Z60N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036058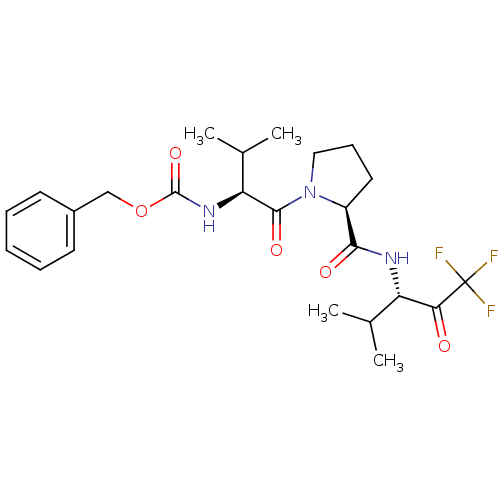 (CHEMBL354883 | benzyl (S)-1-((S)-2-(((S)-1,1,1-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala- Ala-Pro-Val-pN | J Med Chem 38: 76-85 (1995) BindingDB Entry DOI: 10.7270/Q2Z60N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036041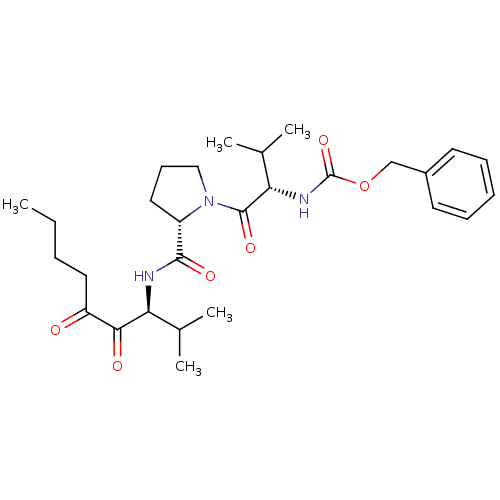 (CHEMBL169887 | {(S)-1-[(S)-2-((S)-1-Isopropyl-2,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala- Ala-Pro-Val-pN | J Med Chem 38: 76-85 (1995) BindingDB Entry DOI: 10.7270/Q2Z60N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036056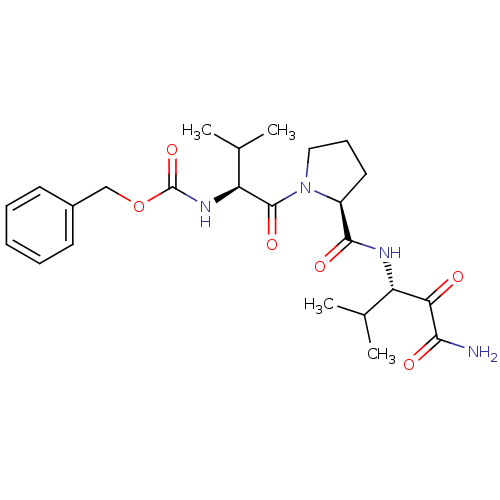 (CHEMBL424550 | {(S)-1-[(S)-2-((S)-1-Aminooxalyl-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala- Ala-Pro-Val-pN | J Med Chem 38: 76-85 (1995) BindingDB Entry DOI: 10.7270/Q2Z60N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50031199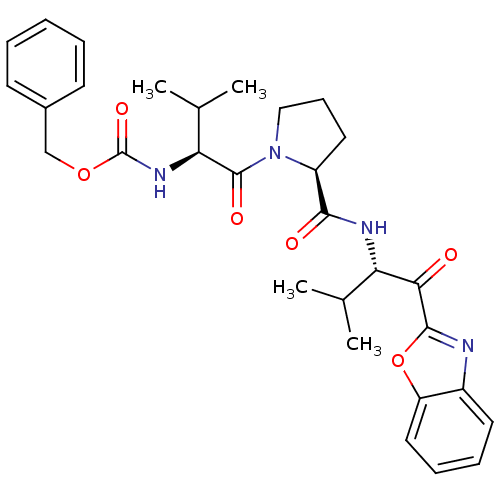 (((S)-1-{(S)-2-[(S)-1-(Benzooxazole-2-carbonyl)-2-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala- Ala-Pro-Val-pN | J Med Chem 38: 76-85 (1995) BindingDB Entry DOI: 10.7270/Q2Z60N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036051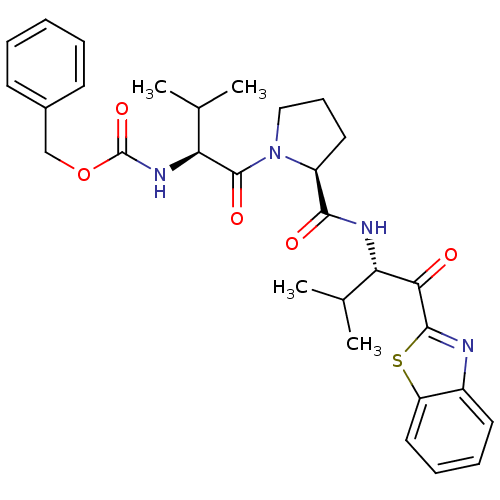 (((S)-1-{(S)-2-[(S)-1-(Benzothiazole-2-carbonyl)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala- Ala-Pro-Val-pN | J Med Chem 38: 76-85 (1995) BindingDB Entry DOI: 10.7270/Q2Z60N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036040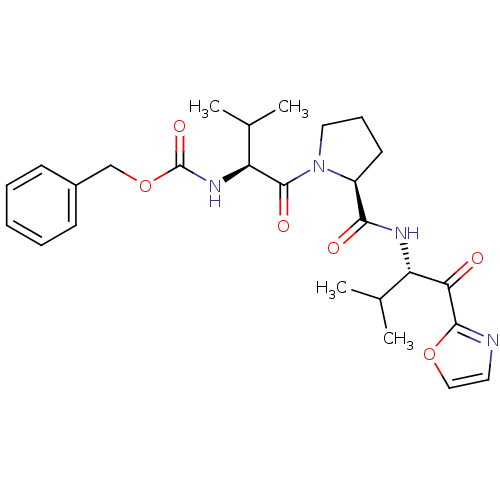 (((S)-2-Methyl-1-{(S)-2-[(S)-2-methyl-1-(oxazole-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala- Ala-Pro-Val-pN | J Med Chem 38: 76-85 (1995) BindingDB Entry DOI: 10.7270/Q2Z60N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036057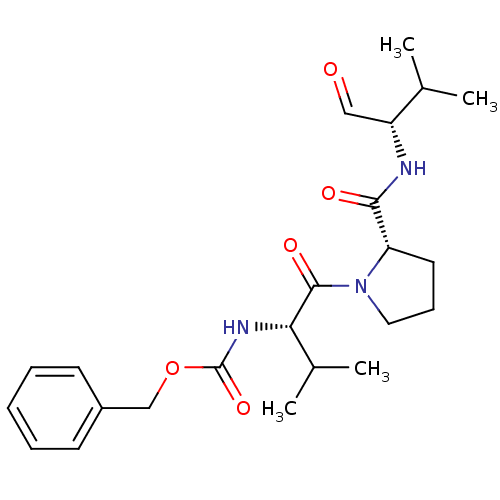 (CHEMBL165525 | benzyl (S)-1-((S)-2-(((S)-3-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala- Ala-Pro-Val-pN | J Med Chem 38: 76-85 (1995) BindingDB Entry DOI: 10.7270/Q2Z60N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036039 (((S)-2-Methyl-1-{(S)-2-[(S)-2-methyl-1-(2-methyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala- Ala-Pro-Val-pN | J Med Chem 38: 76-85 (1995) BindingDB Entry DOI: 10.7270/Q2Z60N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036059 (((S)-2-Methyl-1-{(S)-2-[(S)-2-methyl-1-(thiazole-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala- Ala-Pro-Val-pN | J Med Chem 38: 76-85 (1995) BindingDB Entry DOI: 10.7270/Q2Z60N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036045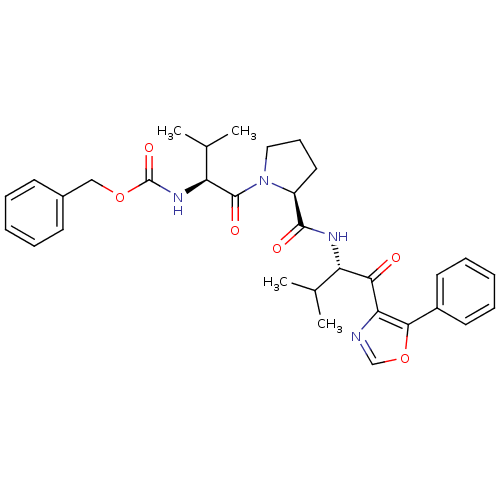 (((S)-2-Methyl-1-{(S)-2-[(S)-2-methyl-1-(5-phenyl-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala- Ala-Pro-Val-pN | J Med Chem 38: 76-85 (1995) BindingDB Entry DOI: 10.7270/Q2Z60N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036036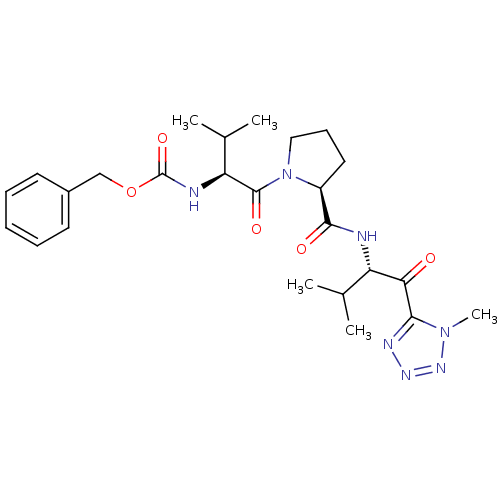 (((S)-2-Methyl-1-{(S)-2-[(S)-2-methyl-1-(1-methyl-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala- Ala-Pro-Val-pN | J Med Chem 38: 76-85 (1995) BindingDB Entry DOI: 10.7270/Q2Z60N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036043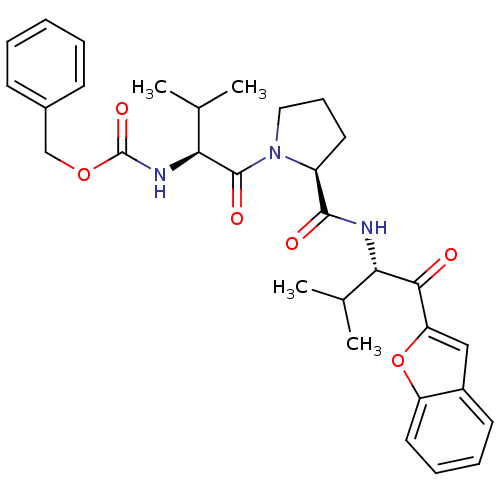 (((S)-1-{(S)-2-[(S)-1-(Benzofuran-2-carbonyl)-2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala- Ala-Pro-Val-pN | J Med Chem 38: 76-85 (1995) BindingDB Entry DOI: 10.7270/Q2Z60N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036038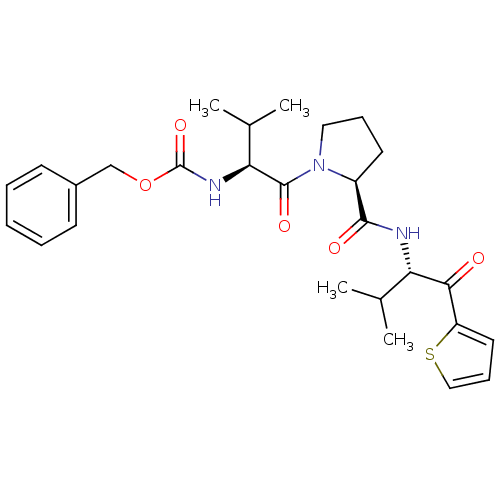 (((S)-2-Methyl-1-{(S)-2-[(S)-2-methyl-1-(thiophene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala- Ala-Pro-Val-pN | J Med Chem 38: 76-85 (1995) BindingDB Entry DOI: 10.7270/Q2Z60N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036042 (((S)-1-{(S)-2-[(S)-1-(1H-Benzoimidazole-2-carbonyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala- Ala-Pro-Val-pN | J Med Chem 38: 76-85 (1995) BindingDB Entry DOI: 10.7270/Q2Z60N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036055 (CHEMBL355477 | benzyl (S)-1-((S)-2-(((S)-4-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala- Ala-Pro-Val-pN | J Med Chem 38: 76-85 (1995) BindingDB Entry DOI: 10.7270/Q2Z60N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036052 (((S)-2-Methyl-1-{(S)-2-[(S)-2-methyl-1-(1-methyl-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala- Ala-Pro-Val-pN | J Med Chem 38: 76-85 (1995) BindingDB Entry DOI: 10.7270/Q2Z60N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036053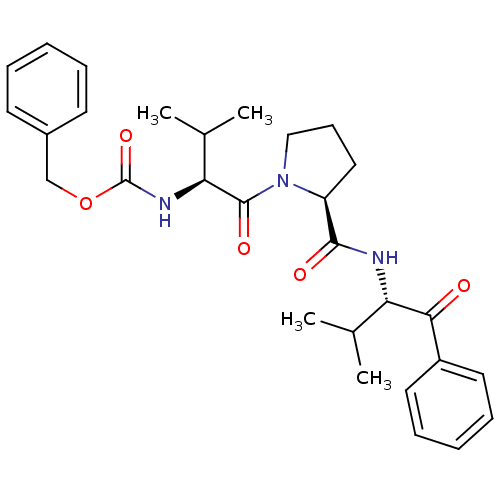 (CHEMBL355765 | {(S)-1-[(S)-2-((S)-1-Benzoyl-2-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE-catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala- Ala-Pro-Val-pN) | J Med Chem 38: 76-85 (1995) BindingDB Entry DOI: 10.7270/Q2Z60N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036046 (((S)-1-{(S)-2-[(S)-1-(Benzooxazol-2-yl-hydroxy-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala- Ala-Pro-Val-pN | J Med Chem 38: 76-85 (1995) BindingDB Entry DOI: 10.7270/Q2Z60N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036054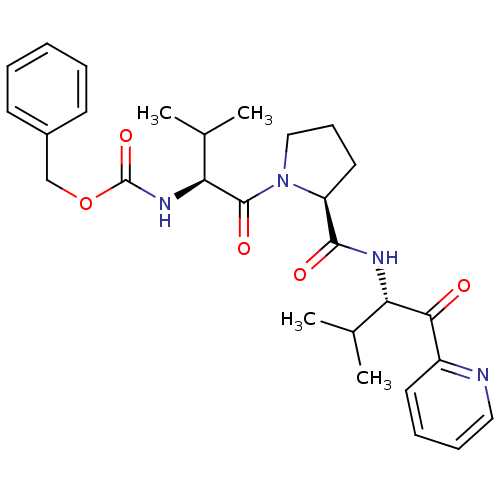 (((S)-2-Methyl-1-{(S)-2-[(S)-2-methyl-1-(pyridine-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala- Ala-Pro-Val-pN | J Med Chem 38: 76-85 (1995) BindingDB Entry DOI: 10.7270/Q2Z60N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036050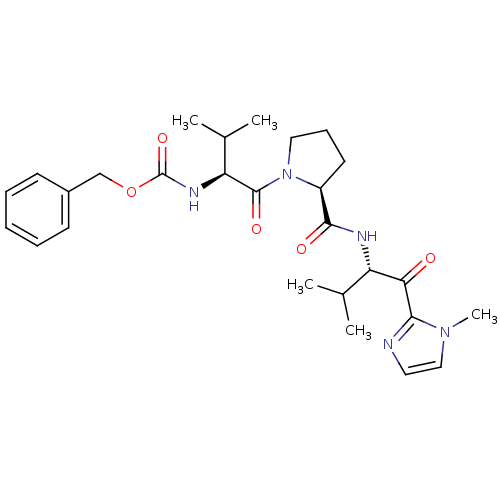 (((S)-2-Methyl-1-{(S)-2-[(S)-2-methyl-1-(1-methyl-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala- Ala-Pro-Val-pN | J Med Chem 38: 76-85 (1995) BindingDB Entry DOI: 10.7270/Q2Z60N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50036048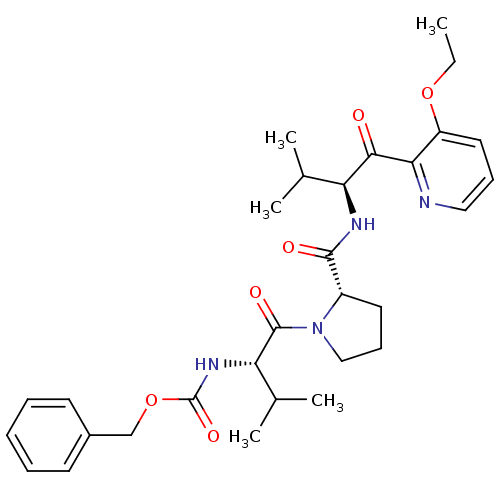 (((S)-1-{(S)-2-[(S)-1-(3-Ethoxy-pyridine-2-carbonyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 8.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human neutrophil elastase (HNE) catalyzed hydrolysis of the synthetic substrate MeO-Suc-Ala- Ala-Pro-Val-pN | J Med Chem 38: 76-85 (1995) BindingDB Entry DOI: 10.7270/Q2Z60N41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||