

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326330 (4-(5-chloropyridin-3-yl)-6- [(1R or S)-1-fluoro-1-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326332 (3-{4-(5-chloropyridin-3- yl)-6-[(1R or S)-1-fluoro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326329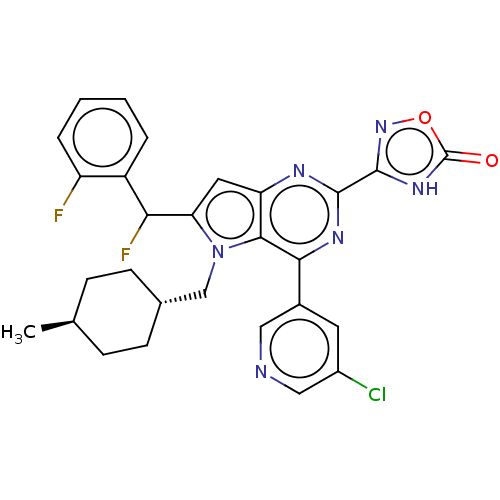 (3-{4-(5-chloropyridin-3- yl)-6-[fluoro(2- fluoroph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326328 (4-(5-chloropyridin-3-yl)-6- [fluoro(2- fluoropheny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326332 (3-{4-(5-chloropyridin-3- yl)-6-[(1R or S)-1-fluoro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326330 (4-(5-chloropyridin-3-yl)-6- [(1R or S)-1-fluoro-1-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326298 (5-[2-amino-4- (trifluoromethyl) benzyl]-4-{[(1R)-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326326 (3-[4-(5-chloropyridin-3- yl)-5-[(trans-4- methylcy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326309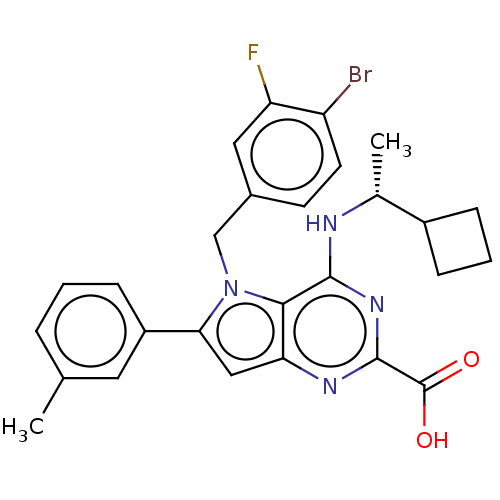 (5-(4-bromo-3- fluorobenzyl)-4-{[(1R)- 1- cyclobuty...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326295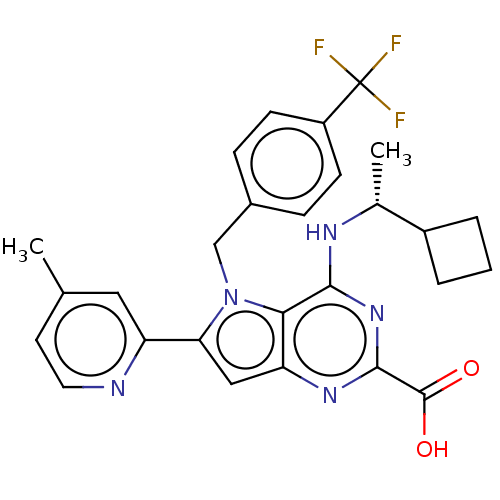 (4-{[(1R)-1- cyclobutylethyl]amino}- 6-(4-methylpyr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326306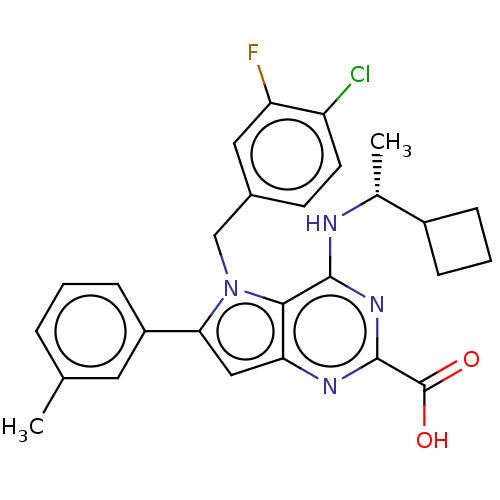 (5-(4-chloro-3- fluorobenzyl)-4-{[(1R)- 1- cyclobut...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326296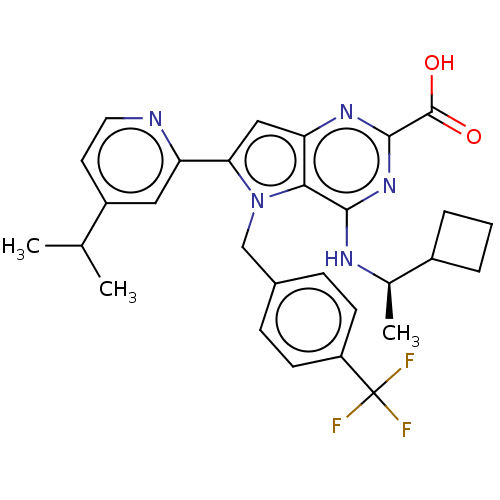 (4-{[(1R)-1- cyclobutylethyl]amino}- 6-[4-(1- methy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326324 (3-[4-(5-chloropyridin-3- yl)-5-[(trans-4- methylcy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326320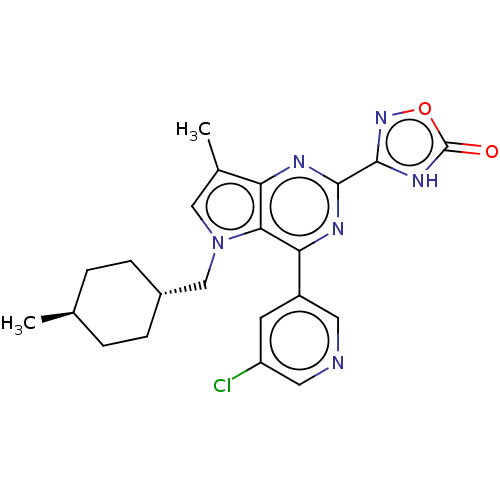 (3-{4-(5-chloropyridin-3- yl)-7-methyl-5-[(trans-4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326291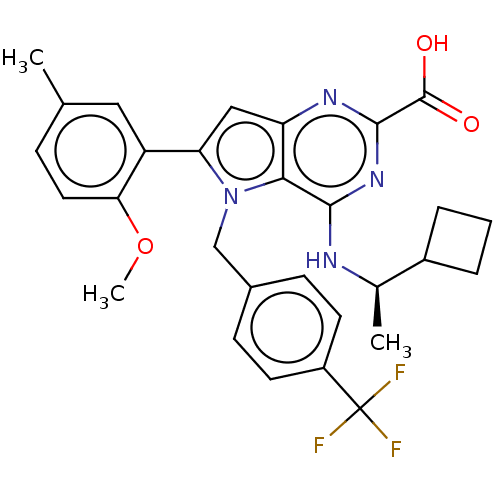 (4-{[(1R)-1- cyclobutylethyl]amino}- 6-(2-methoxy-5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326325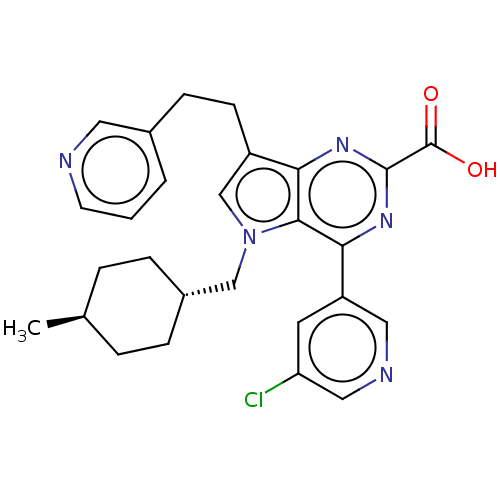 (4-(5-chloropyridin-3-yl)-5- [(trans-4- methylcyclo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326297 (4-{[(1R)-1- cyclobutylethyl]amino}- 7-methyl-6-[4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326327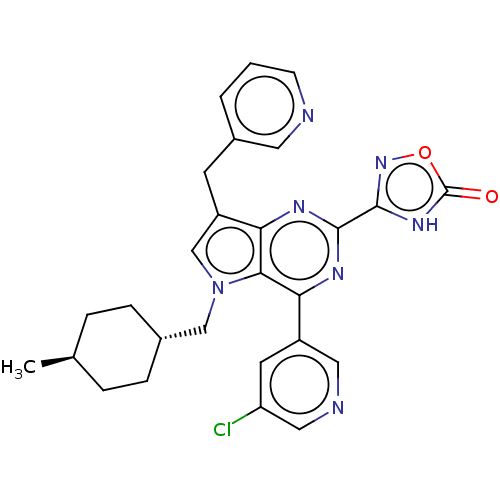 (3-[4-(5-chloropyridin-3- yl)-5-[(trans-4- methylcy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326322 (5-{7-benzyl-4-(5- chloropyridin-3-yl)-5- [(trans-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326315 (3-{4-(5-chloropyridin-3- yl)-5-[(trans-4- methylcy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326287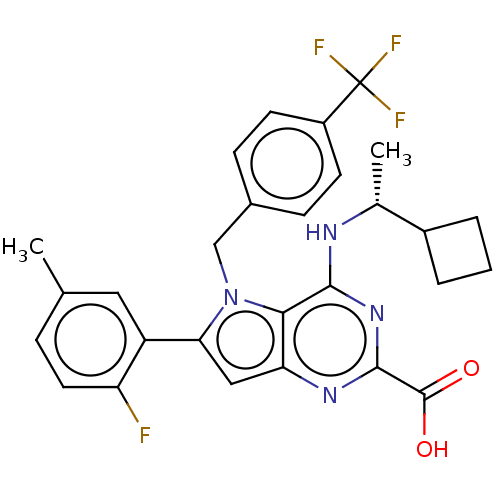 ((R)-4-((1- cyclobutylethyl)amino)- 6-(2-fluoro-5- ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326293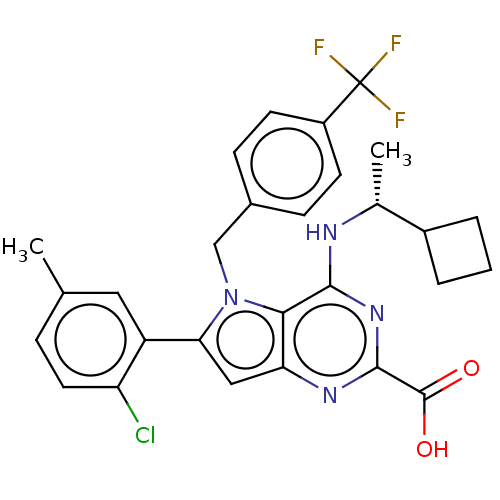 (6-(2-chloro-5- methylphenyl)-4-{[(1R)- 1- cyclobut...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326323 (4-(5-chloropyridin-3-yl)-5- [(trans-4- methylcyclo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326321 (4-(5-chloropyridin-3-yl)-5- [(trans-4- methylcyclo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326304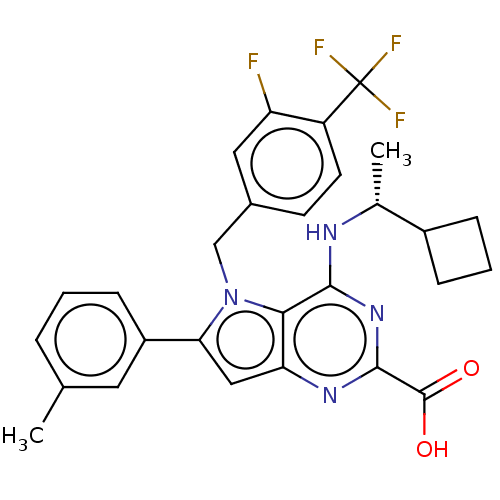 (4-{[(1R)-1- cyclobutylethyl] amino}-5-[3-fluoro-4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326319 (4-(5-chloropyridin-3-yl)-7- methyl-5-[(trans-4- me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326316 (5-{4-(5-chloropyridin-3- yl)-5-[(trans-4- methylcy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326292 (6-(1-benzofuran-5-yl)- 4-{[(1R)-1- cyclobutylethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326318 (3-{4-(3-chlorophenyl)-7- methyl-5-[(trans-4- methy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326311 (4-{[(1R)-1- cyclobutylethyl]amino}- 5-(3-fluoro-4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 124 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326310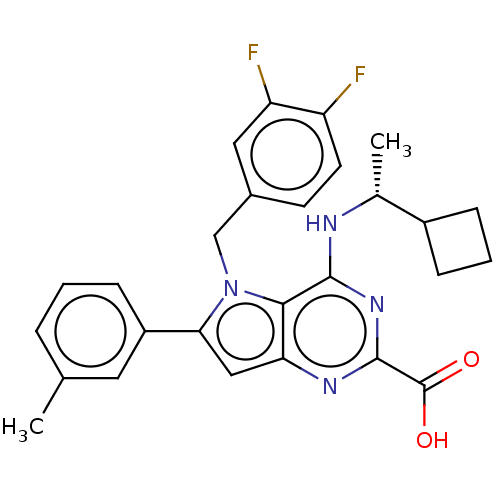 (4-{[(1R)-1- cyclobutylethyl]amino}- 5-(3,4-difluor...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326290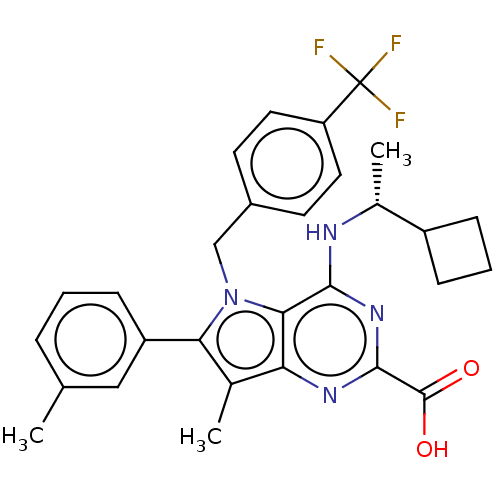 ((R)-4-((1- cyclobutylethyl)amino)- 7-methyl-6-(m-t...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326313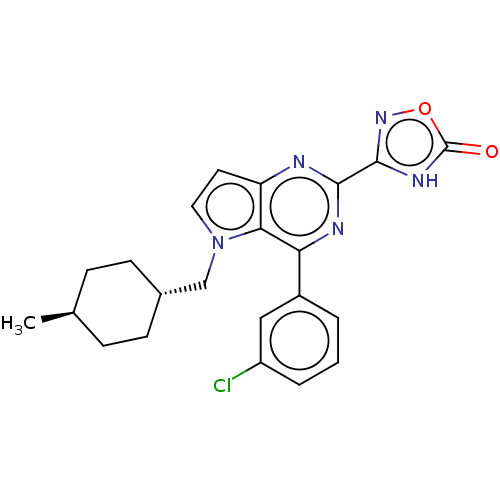 (3-{4-(3-chlorophenyl)-5- [(trans-4- methylcyclohex...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326289 ((R)-4-((1- cyclobutylethyl) amino)- 6-(m-tolyl)-5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326308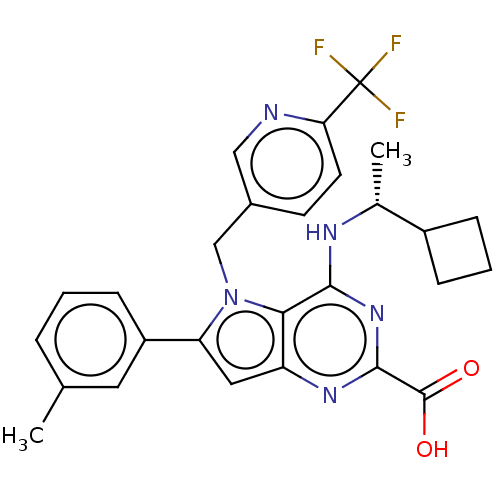 (4-{[(1R)-1- cyclobutylethyl]amino}- 6-(3-methylphe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 178 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326317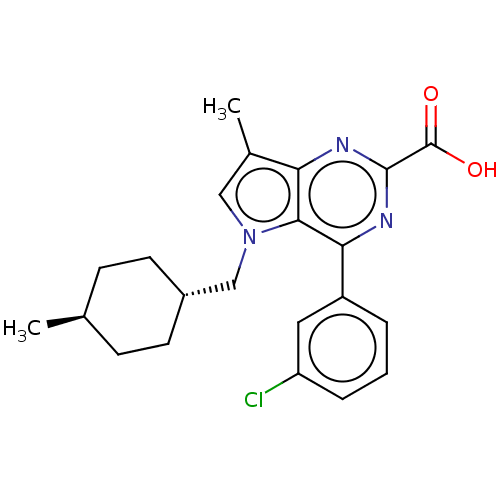 (4-(3-chlorophenyl)-7- methyl-5-[(trans-4- methylcy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 226 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326314 (4-(5-chloropyridin-3-yl)-5- [(trans-4- methylcyclo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 247 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326301 (4-{[(1R)-1- cyclobutylethyl] amino}-5-[3-fluoro-4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 267 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326312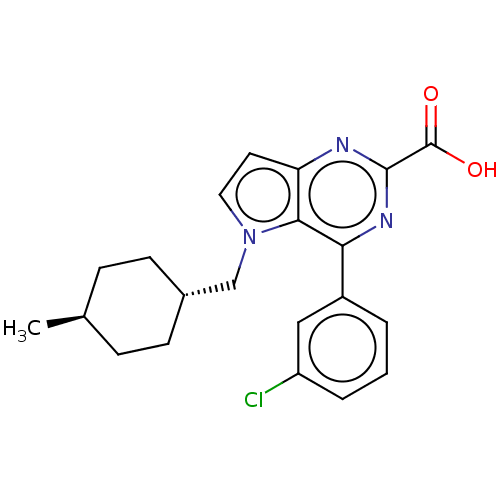 (4-(3-chlorophenyl)-5- [(trans-4- methylcyclohexyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 294 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326300 (4-{[(1R)-1- cyclobutylethyl] amino}-6-(3- methylph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 348 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326288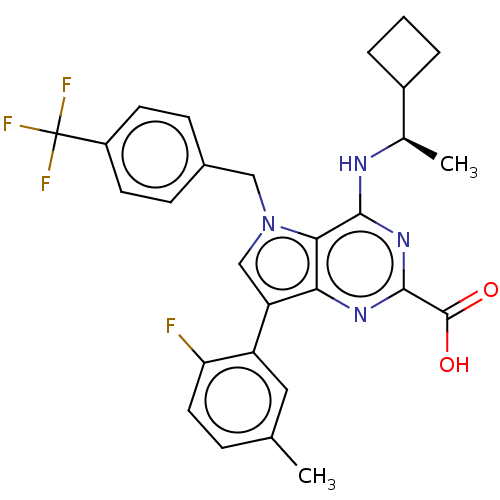 ((R)-4-((1- cyclobutylethyl)amino)- 7-(2-fluoro-5- ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 353 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326307 (4-{[(1R)-1- cyclobutylethyl]amino}- 5-[4- (difluor...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326294 (7-(2-chloro-5- methylphenyl)-4-{[(1R)- 1- cyclobut...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 712 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326299 (4-{[(1R)-1- cyclobutylethyl] amino}-6-(3- methylph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 844 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326303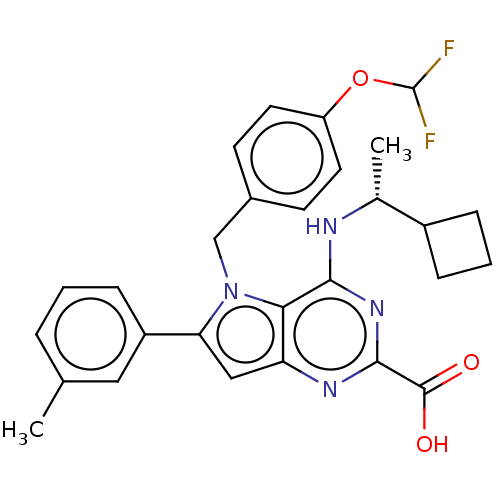 (4-{[(1R)-1- cyclobutylethyl] amino}-5-[4- (difluor...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326302 (4-{[(1R)-1- cyclobutylethyl]amino}- 5-[2-methoxy-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 [17-125] (Homo sapiens (Human)) | BDBM326305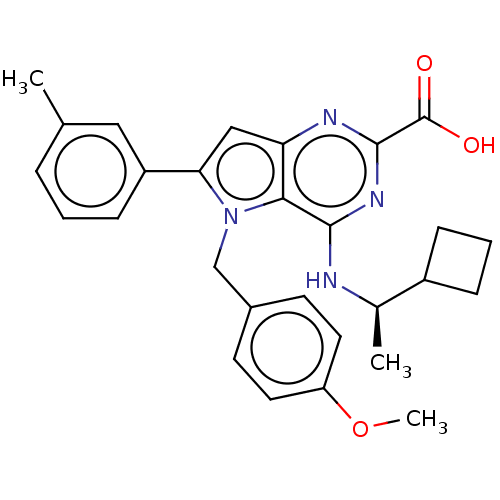 (4-{[(1R)-1- cyclobutylethyl] amino}-5-(4- methoxyb...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Methods: An HDM2 FRET assay was developed to assess the compounds' inhibitory activity towards binding of p53 protein. A truncated version of HDM... | US Patent US9637493 (2017) BindingDB Entry DOI: 10.7270/Q28917Z1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||