| Reaction Details |
|---|
 | Report a problem with these data |
| Target | 5-hydroxytryptamine receptor 1B |
|---|
| Ligand | BDBM50239362 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1666005 (CHEMBL4015801) |
|---|
| EC50 | 570±n/a nM |
|---|
| Citation |  McCoull, W; Abrams, RD; Anderson, E; Blades, K; Barton, P; Box, M; Burgess, J; Byth, K; Cao, Q; Chuaqui, C; Carbajo, RJ; Cheung, T; Code, E; Ferguson, AD; Fillery, S; Fuller, NO; Gangl, E; Gao, N; Grist, M; Hargreaves, D; Howard, MR; Hu, J; Kemmitt, PD; Nelson, JE; O'Connell, N; Prince, DB; Raubo, P; Rawlins, PB; Robb, GR; Shi, J; Waring, MJ; Whittaker, D; Wylot, M; Zhu, X Discovery of Pyrazolo[1,5-a]pyrimidine B-Cell Lymphoma 6 (BCL6) Binders and Optimization to High Affinity Macrocyclic Inhibitors. J Med Chem60:4386-4402 (2017) [PubMed] Article McCoull, W; Abrams, RD; Anderson, E; Blades, K; Barton, P; Box, M; Burgess, J; Byth, K; Cao, Q; Chuaqui, C; Carbajo, RJ; Cheung, T; Code, E; Ferguson, AD; Fillery, S; Fuller, NO; Gangl, E; Gao, N; Grist, M; Hargreaves, D; Howard, MR; Hu, J; Kemmitt, PD; Nelson, JE; O'Connell, N; Prince, DB; Raubo, P; Rawlins, PB; Robb, GR; Shi, J; Waring, MJ; Whittaker, D; Wylot, M; Zhu, X Discovery of Pyrazolo[1,5-a]pyrimidine B-Cell Lymphoma 6 (BCL6) Binders and Optimization to High Affinity Macrocyclic Inhibitors. J Med Chem60:4386-4402 (2017) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| 5-hydroxytryptamine receptor 1B |
|---|
| Name: | 5-hydroxytryptamine receptor 1B |
|---|
| Synonyms: | 5-HT-1B | 5-HT-1D-beta | 5-HT1B | 5-hydroxytryptamine receptor 1B (5-HT1B) | 5HT1B_HUMAN | HTR1B | HTR1DB | S12 | Serotonin (5-HT) receptor | Serotonin 1D beta receptor | Serotonin Receptor 1B |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 43579.17 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Receptor binding assays were performed using human clone stably expressed in CHO cells |
|---|
| Residue: | 390 |
|---|
| Sequence: | MEEPGAQCAPPPPAGSETWVPQANLSSAPSQNCSAKDYIYQDSISLPWKVLLVMLLALIT
LATTLSNAFVIATVYRTRKLHTPANYLIASLAVTDLLVSILVMPISTMYTVTGRWTLGQV
VCDFWLSSDITCCTASILHLCVIALDRYWAITDAVEYSAKRTPKRAAVMIALVWVFSISI
SLPPFFWRQAKAEEEVSECVVNTDHILYTVYSTVGAFYFPTLLLIALYGRIYVEARSRIL
KQTPNRTGKRLTRAQLITDSPGSTSSVTSINSRVPDVPSESGSPVYVNQVKVRVSDALLE
KKKLMAARERKATKTLGIILGAFIVCWLPFFIISLVMPICKDACWFHLAIFDFFTWLGYL
NSLINPIIYTMSNEDFKQAFHKLIRFKCTS
|
|
|
|---|
| BDBM50239362 |
|---|
| n/a |
|---|
| Name | BDBM50239362 |
|---|
| Synonyms: | CHEMBL4064865 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C31H31ClN6O4 |
|---|
| Mol. Mass. | 587.069 |
|---|
| SMILES | [H][C@]12C[C@@H](CO)N(C1)c1cc(Nc3cc4CCC(=O)N(Cc5ccccc5)c4c(OC\C=C\CO2)c3)n2ncc(Cl)c2n1 |r,t:34| |
|---|
| Structure | 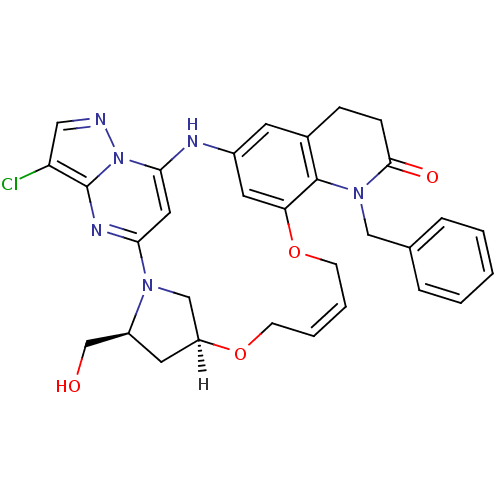
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 McCoull, W; Abrams, RD; Anderson, E; Blades, K; Barton, P; Box, M; Burgess, J; Byth, K; Cao, Q; Chuaqui, C; Carbajo, RJ; Cheung, T; Code, E; Ferguson, AD; Fillery, S; Fuller, NO; Gangl, E; Gao, N; Grist, M; Hargreaves, D; Howard, MR; Hu, J; Kemmitt, PD; Nelson, JE; O'Connell, N; Prince, DB; Raubo, P; Rawlins, PB; Robb, GR; Shi, J; Waring, MJ; Whittaker, D; Wylot, M; Zhu, X Discovery of Pyrazolo[1,5-a]pyrimidine B-Cell Lymphoma 6 (BCL6) Binders and Optimization to High Affinity Macrocyclic Inhibitors. J Med Chem60:4386-4402 (2017) [PubMed] Article
McCoull, W; Abrams, RD; Anderson, E; Blades, K; Barton, P; Box, M; Burgess, J; Byth, K; Cao, Q; Chuaqui, C; Carbajo, RJ; Cheung, T; Code, E; Ferguson, AD; Fillery, S; Fuller, NO; Gangl, E; Gao, N; Grist, M; Hargreaves, D; Howard, MR; Hu, J; Kemmitt, PD; Nelson, JE; O'Connell, N; Prince, DB; Raubo, P; Rawlins, PB; Robb, GR; Shi, J; Waring, MJ; Whittaker, D; Wylot, M; Zhu, X Discovery of Pyrazolo[1,5-a]pyrimidine B-Cell Lymphoma 6 (BCL6) Binders and Optimization to High Affinity Macrocyclic Inhibitors. J Med Chem60:4386-4402 (2017) [PubMed] Article