

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50548115 (CHEMBL4778773) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01760 BindingDB Entry DOI: 10.7270/Q2TB1BHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50548110 (CHEMBL4747532) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01760 BindingDB Entry DOI: 10.7270/Q2TB1BHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50548117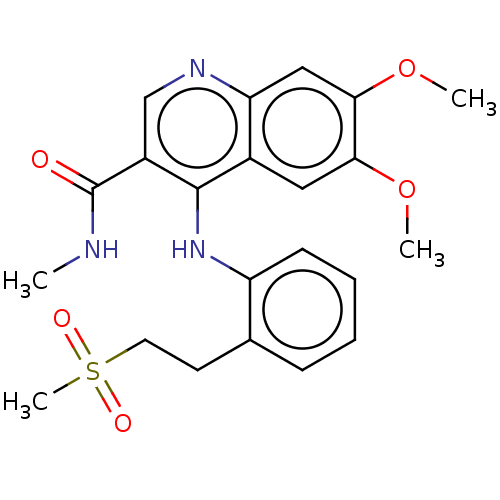 (CHEMBL4776026) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01760 BindingDB Entry DOI: 10.7270/Q2TB1BHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50162074 (CHEMBL3792684) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01760 BindingDB Entry DOI: 10.7270/Q2TB1BHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50162075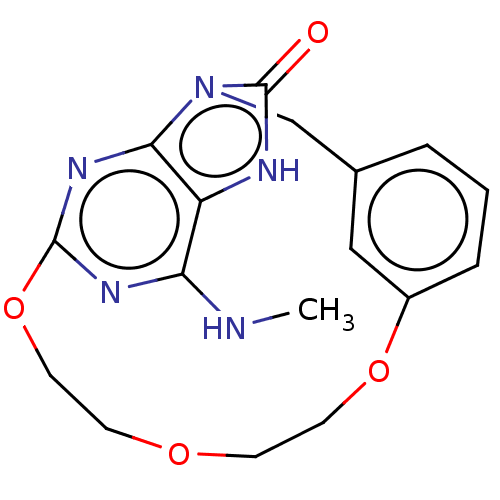 (CHEMBL3794167) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01760 BindingDB Entry DOI: 10.7270/Q2TB1BHR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50548124 (CHEMBL4784602) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01760 BindingDB Entry DOI: 10.7270/Q2TB1BHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50548109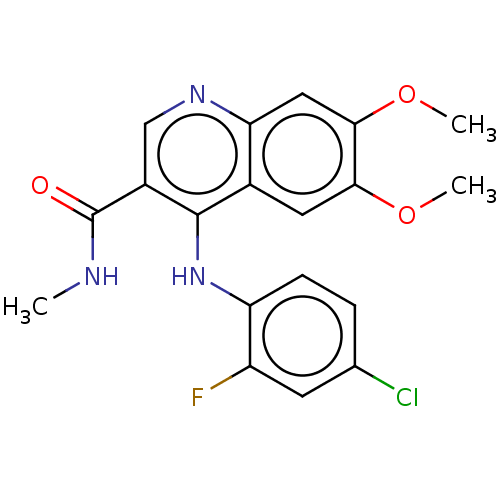 (CHEMBL4783492) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01760 BindingDB Entry DOI: 10.7270/Q2TB1BHR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1B (Homo sapiens (Human)) | BDBM50081185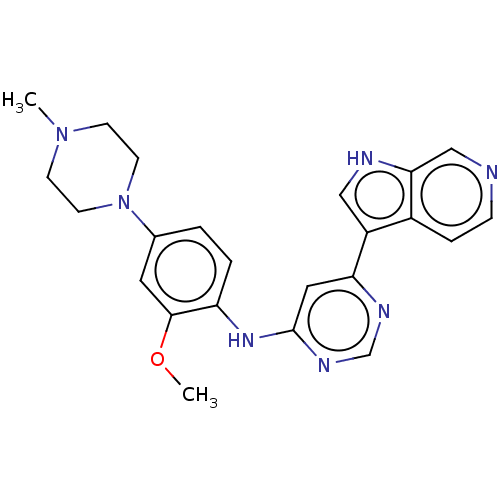 (CHEMBL3421963) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins | J Med Chem 58: 2834-44 (2015) Article DOI: 10.1021/acs.jmedchem.5b00098 BindingDB Entry DOI: 10.7270/Q23B61VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50548121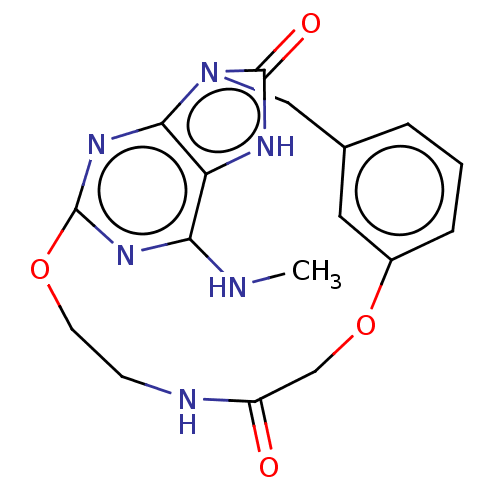 (CHEMBL4754128) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01760 BindingDB Entry DOI: 10.7270/Q2TB1BHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1B (Homo sapiens (Human)) | BDBM50081186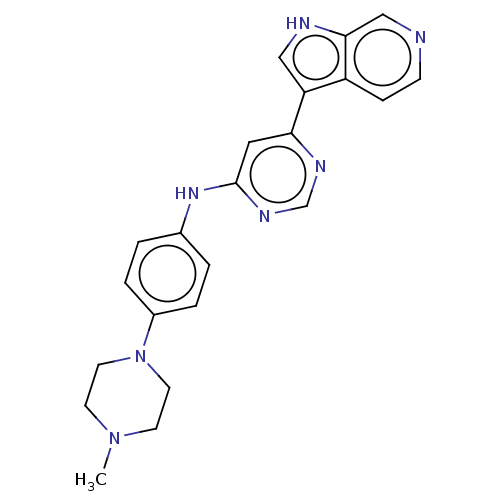 (CHEMBL3421962) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins | J Med Chem 58: 2834-44 (2015) Article DOI: 10.1021/acs.jmedchem.5b00098 BindingDB Entry DOI: 10.7270/Q23B61VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50548119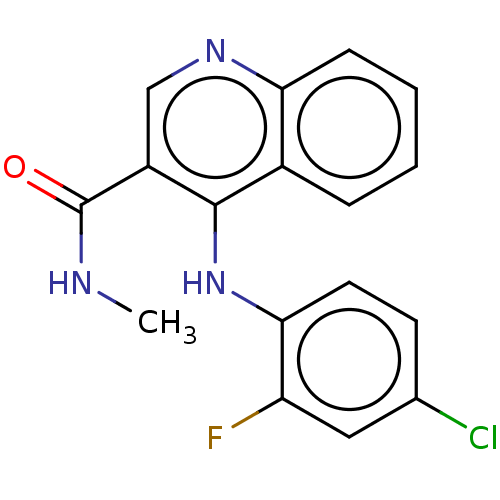 (CHEMBL4749068) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01760 BindingDB Entry DOI: 10.7270/Q2TB1BHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50575300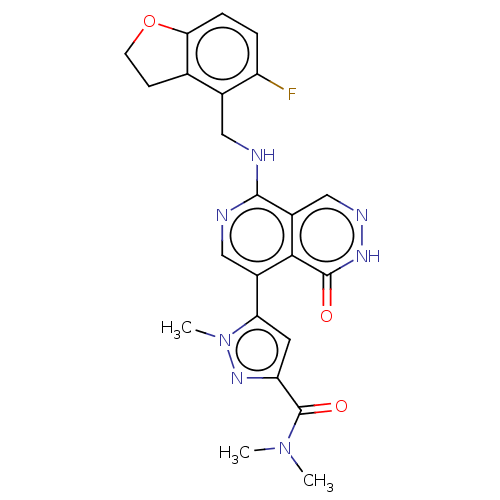 (CHEMBL4851414) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EED in human G-401 cells assessed as reduction in H3K27 trimethylation incubated for 48 hrs by HTRF assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01161 BindingDB Entry DOI: 10.7270/Q2MP5737 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50152124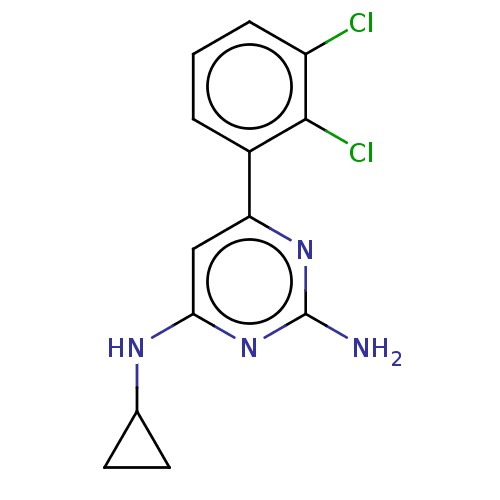 (CHEMBL3782004) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01760 BindingDB Entry DOI: 10.7270/Q2TB1BHR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| GTPase KRas (Homo sapiens (Human)) | BDBM50605537 (CHEMBL5183988) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00369 BindingDB Entry DOI: 10.7270/Q2W09B1P | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50427322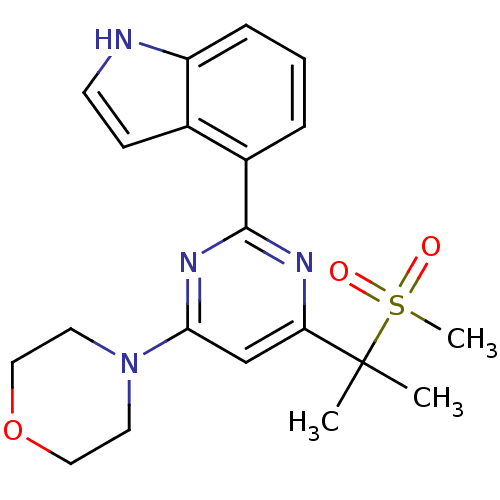 (CHEMBL2325703) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATR-mediated CHK1 phosphorylation at serine 345 in human HT29 cells after 1 hr in presence of 4-nitroquinoline 1-oxide | J Med Chem 56: 2125-38 (2013) Article DOI: 10.1021/jm301859s BindingDB Entry DOI: 10.7270/Q2VH5Q5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1B (Homo sapiens (Human)) | BDBM50081174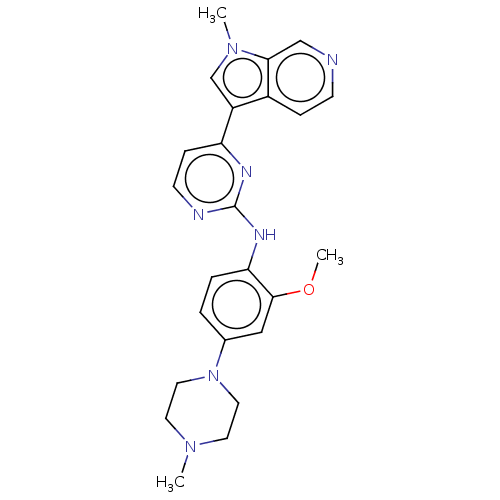 (CHEMBL3421968) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins | J Med Chem 58: 2834-44 (2015) Article DOI: 10.1021/acs.jmedchem.5b00098 BindingDB Entry DOI: 10.7270/Q23B61VW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1B (Homo sapiens (Human)) | BDBM50081188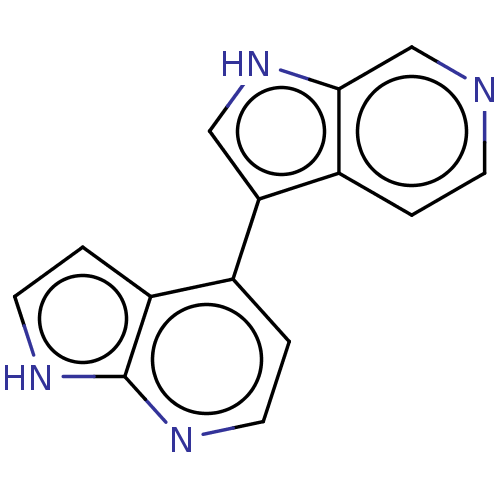 (CHEMBL3421981) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins | J Med Chem 58: 2834-44 (2015) Article DOI: 10.1021/acs.jmedchem.5b00098 BindingDB Entry DOI: 10.7270/Q23B61VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50575300 (CHEMBL4851414) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EED in human G-401 cells assessed as reduction in H3K27 trimethylation incubated for 48 hrs by HTRF assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01161 BindingDB Entry DOI: 10.7270/Q2MP5737 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50575298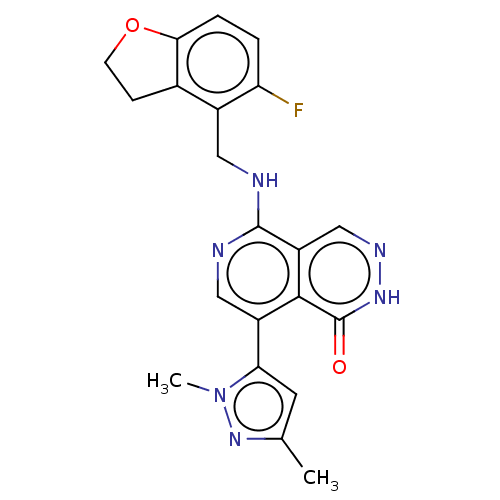 (CHEMBL4859723) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EED in human G-401 cells assessed as reduction in H3K27 trimethylation incubated for 48 hrs by HTRF assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01161 BindingDB Entry DOI: 10.7270/Q2MP5737 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50575298 (CHEMBL4859723) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EED in human G-401 cells assessed as reduction in H3K27 trimethylation incubated for 48 hrs by HTRF assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01161 BindingDB Entry DOI: 10.7270/Q2MP5737 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50427320 (CHEMBL2325705) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATR-mediated CHK1 phosphorylation at serine 345 in human HT29 cells after 1 hr in presence of 4-nitroquinoline 1-oxide | J Med Chem 56: 2125-38 (2013) Article DOI: 10.1021/jm301859s BindingDB Entry DOI: 10.7270/Q2VH5Q5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1B (Homo sapiens (Human)) | BDBM50081180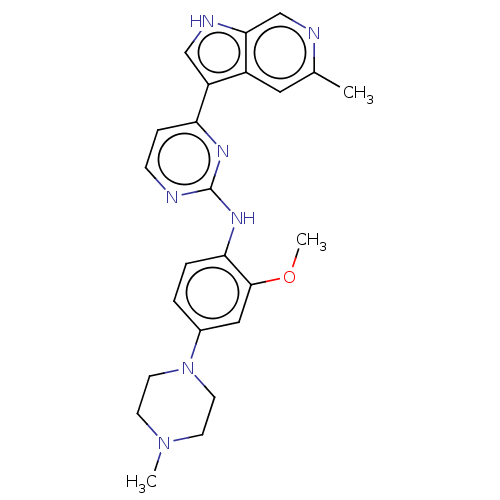 (CHEMBL3421969) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins | J Med Chem 58: 2834-44 (2015) Article DOI: 10.1021/acs.jmedchem.5b00098 BindingDB Entry DOI: 10.7270/Q23B61VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1B (Homo sapiens (Human)) | BDBM50081181 (CHEMBL3421967) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins | J Med Chem 58: 2834-44 (2015) Article DOI: 10.1021/acs.jmedchem.5b00098 BindingDB Entry DOI: 10.7270/Q23B61VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM291687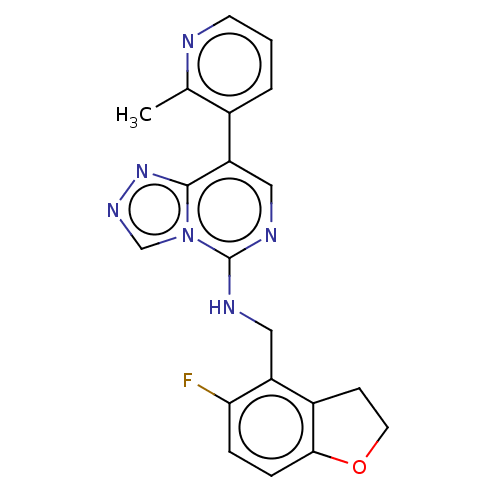 (N-((5-Fluoro-2,3-dihydrobenzofuran-4-yl)methyl)-8-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EED in human G-401 cells assessed as reduction in H3K27 trimethylation incubated for 48 hrs by HTRF assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01161 BindingDB Entry DOI: 10.7270/Q2MP5737 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| GTPase KRas (Homo sapiens (Human)) | BDBM50605543 (CHEMBL5207711) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00369 BindingDB Entry DOI: 10.7270/Q2W09B1P | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas (Homo sapiens (Human)) | BDBM50527057 (CHEMBL4461434) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GDP bound biotinylated human C-terminal Avi/His6-tagged KRAS G12C mutant (1 to 166 residues) expressed in Escherichia coli BL21 (DE3) a... | J Med Chem 63: 4468-4483 (2020) Article DOI: 10.1021/acs.jmedchem.9b01720 BindingDB Entry DOI: 10.7270/Q2F76H10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50427314 (CHEMBL2325711) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATR-mediated CHK1 phosphorylation at serine 345 in human HT29 cells after 1 hr in presence of 4-nitroquinoline 1-oxide | J Med Chem 56: 2125-38 (2013) Article DOI: 10.1021/jm301859s BindingDB Entry DOI: 10.7270/Q2VH5Q5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50427326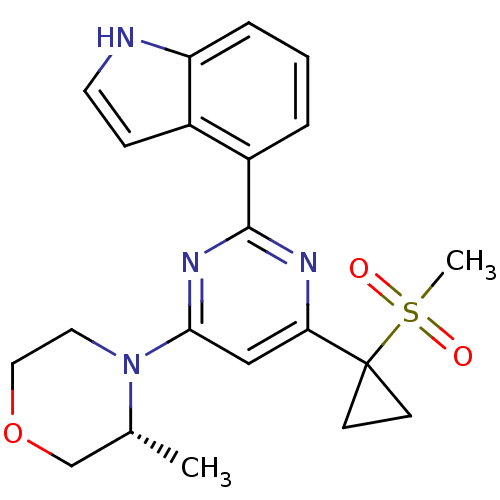 (CHEMBL2325697) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATR in human HeLa cell nuclear extracts using glutathione S-transferase-p53N66 and ATP as substrate incubated for 10 mins prior to ATP ... | J Med Chem 56: 2125-38 (2013) Article DOI: 10.1021/jm301859s BindingDB Entry DOI: 10.7270/Q2VH5Q5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1B (Homo sapiens (Human)) | BDBM50081179 (CHEMBL3421970) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins | J Med Chem 58: 2834-44 (2015) Article DOI: 10.1021/acs.jmedchem.5b00098 BindingDB Entry DOI: 10.7270/Q23B61VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM291687 (N-((5-Fluoro-2,3-dihydrobenzofuran-4-yl)methyl)-8-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EED in human G-401 cells assessed as reduction in H3K27 trimethylation incubated for 48 hrs by HTRF assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01161 BindingDB Entry DOI: 10.7270/Q2MP5737 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50575293 (CHEMBL4855253) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EED in human G-401 cells assessed as reduction in H3K27 trimethylation incubated for 48 hrs by HTRF assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01161 BindingDB Entry DOI: 10.7270/Q2MP5737 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50575293 (CHEMBL4855253) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EED in human G-401 cells assessed as reduction in H3K27 trimethylation incubated for 48 hrs by HTRF assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01161 BindingDB Entry DOI: 10.7270/Q2MP5737 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATPase family AAA domain-containing protein 2 (Homo sapiens (Human)) | BDBM50584925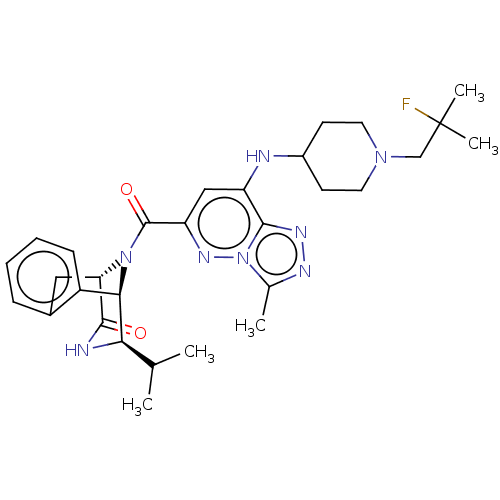 (CHEMBL5079885) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of GST-ATAD2 BD (981 to 1108 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) and biotinylated tetra-acetylated histone... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01871 BindingDB Entry DOI: 10.7270/Q2280CH8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1B (Homo sapiens (Human)) | BDBM50081189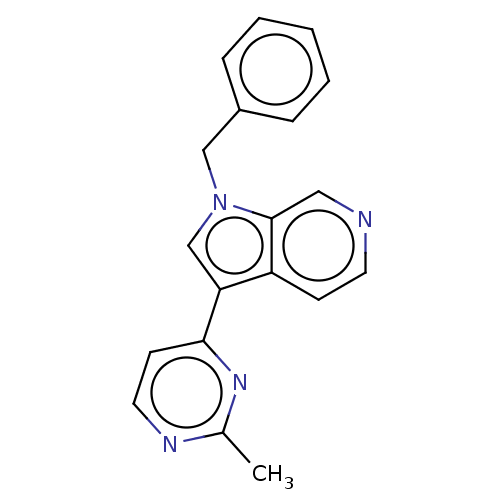 (CHEMBL3421980) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins | J Med Chem 58: 2834-44 (2015) Article DOI: 10.1021/acs.jmedchem.5b00098 BindingDB Entry DOI: 10.7270/Q23B61VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATPase family AAA domain-containing protein 2 (Homo sapiens (Human)) | BDBM50584925 (CHEMBL5079885) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of GST-ATAD2 BD (951 to 1132 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) and biotinylated tetra-acetylated histone... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01871 BindingDB Entry DOI: 10.7270/Q2280CH8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50427327 (CHEMBL2325699) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATR-mediated CHK1 phosphorylation at serine 345 in human HT29 cells after 1 hr in presence of 4-nitroquinoline 1-oxide | J Med Chem 56: 2125-38 (2013) Article DOI: 10.1021/jm301859s BindingDB Entry DOI: 10.7270/Q2VH5Q5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1B (Homo sapiens (Human)) | BDBM50081191 (CHEMBL3421978) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins | J Med Chem 58: 2834-44 (2015) Article DOI: 10.1021/acs.jmedchem.5b00098 BindingDB Entry DOI: 10.7270/Q23B61VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50575302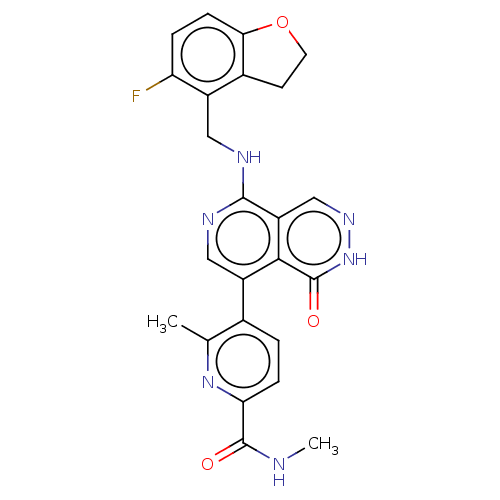 (CHEMBL4847216) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EED in human G-401 cells assessed as reduction in H3K27 trimethylation incubated for 48 hrs by HTRF assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01161 BindingDB Entry DOI: 10.7270/Q2MP5737 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50575302 (CHEMBL4847216) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EED in human G-401 cells assessed as reduction in H3K27 trimethylation incubated for 48 hrs by HTRF assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01161 BindingDB Entry DOI: 10.7270/Q2MP5737 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50427321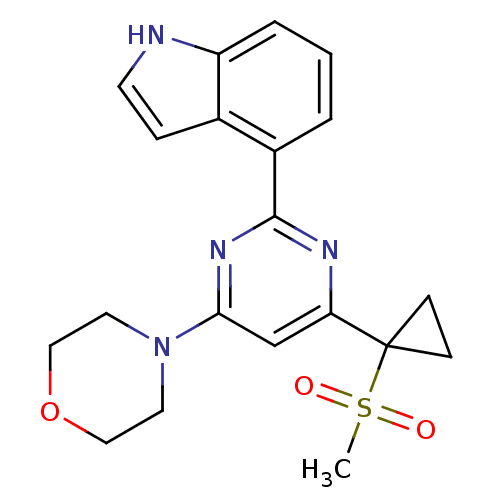 (CHEMBL2325704) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATR-mediated CHK1 phosphorylation at serine 345 in human HT29 cells after 1 hr in presence of 4-nitroquinoline 1-oxide | J Med Chem 56: 2125-38 (2013) Article DOI: 10.1021/jm301859s BindingDB Entry DOI: 10.7270/Q2VH5Q5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50427305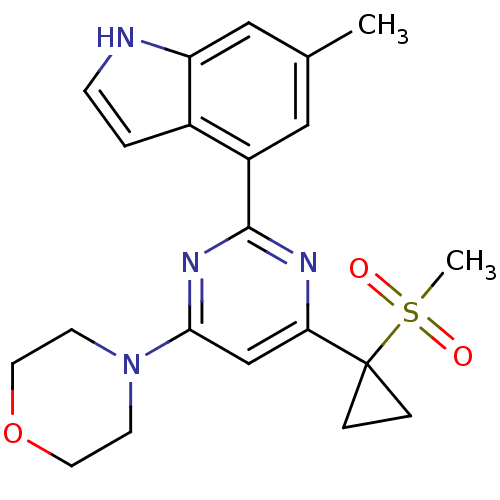 (CHEMBL2325720) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATR in human HeLa cell nuclear extracts using glutathione S-transferase-p53N66 and ATP as substrate incubated for 10 mins prior to ATP ... | J Med Chem 56: 2125-38 (2013) Article DOI: 10.1021/jm301859s BindingDB Entry DOI: 10.7270/Q2VH5Q5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50575303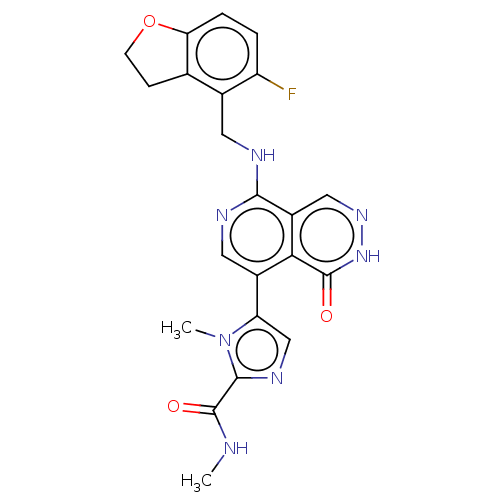 (CHEMBL4850478) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EED in human G-401 cells assessed as reduction in H3K27 trimethylation incubated for 48 hrs by HTRF assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01161 BindingDB Entry DOI: 10.7270/Q2MP5737 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50575303 (CHEMBL4850478) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of EED in human G-401 cells assessed as reduction in H3K27 trimethylation incubated for 48 hrs by HTRF assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01161 BindingDB Entry DOI: 10.7270/Q2MP5737 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas (Homo sapiens (Human)) | BDBM50605540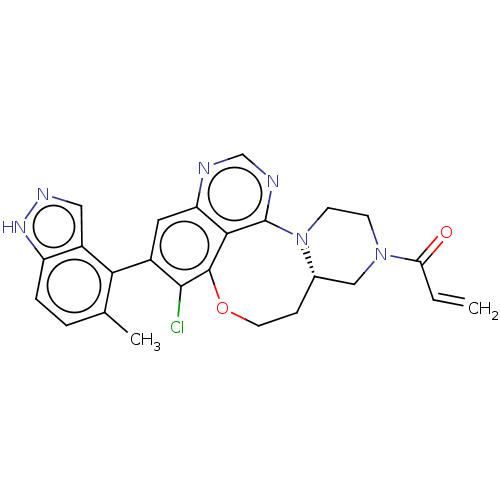 (CHEMBL5171553) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00369 BindingDB Entry DOI: 10.7270/Q2W09B1P | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50427319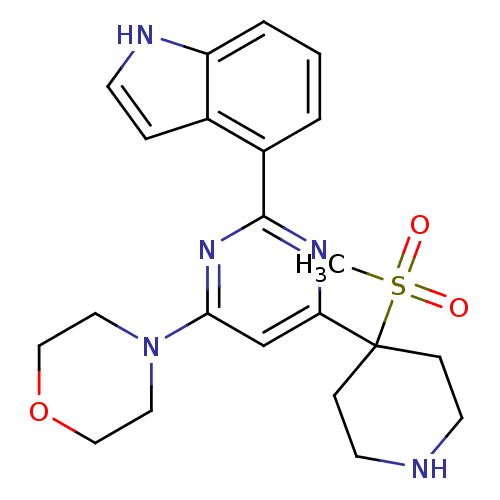 (CHEMBL2325706) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATR-mediated CHK1 phosphorylation at serine 345 in human HT29 cells after 1 hr in presence of 4-nitroquinoline 1-oxide | J Med Chem 56: 2125-38 (2013) Article DOI: 10.1021/jm301859s BindingDB Entry DOI: 10.7270/Q2VH5Q5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50548118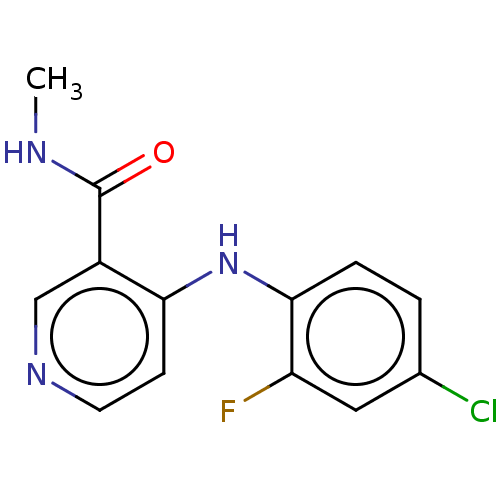 (CHEMBL4758926) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01760 BindingDB Entry DOI: 10.7270/Q2TB1BHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1B (Homo sapiens (Human)) | BDBM50081178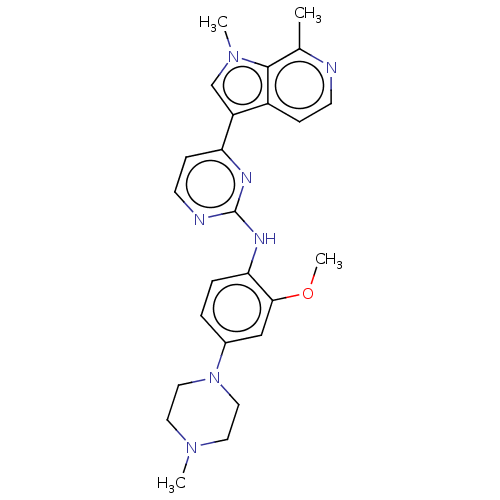 (CHEMBL3421971) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins | J Med Chem 58: 2834-44 (2015) Article DOI: 10.1021/acs.jmedchem.5b00098 BindingDB Entry DOI: 10.7270/Q23B61VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1B (Homo sapiens (Human)) | BDBM50081182 (CHEMBL3421966) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins | J Med Chem 58: 2834-44 (2015) Article DOI: 10.1021/acs.jmedchem.5b00098 BindingDB Entry DOI: 10.7270/Q23B61VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50548107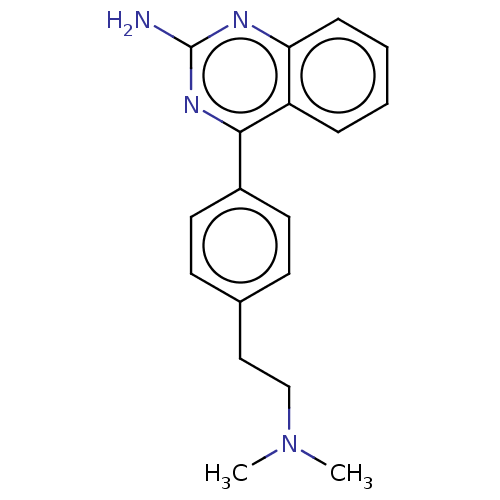 (CHEMBL4799076) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MTH1 (unknown origin) using 8-oxo-dGTP as substrate preincubated for 15 mins followed by substrate addition and measured after 20 mins ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01760 BindingDB Entry DOI: 10.7270/Q2TB1BHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1B (Homo sapiens (Human)) | BDBM50081193 (CHEMBL3421976) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins | J Med Chem 58: 2834-44 (2015) Article DOI: 10.1021/acs.jmedchem.5b00098 BindingDB Entry DOI: 10.7270/Q23B61VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 691 total ) | Next | Last >> |