 Found 328 hits with Last Name = 'grist' and Initial = 'm'
Found 328 hits with Last Name = 'grist' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50322823

((S)-N-(4-(3-chloro-4-fluorophenylamino)-7-(tetrahy...)Show SMILES CN(C)C\C=C\C(=O)Nc1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2cc1O[C@H]1CCOC1 |r| Show InChI InChI=1S/C24H25ClFN5O3/c1-31(2)8-3-4-23(32)30-21-11-17-20(12-22(21)34-16-7-9-33-13-16)27-14-28-24(17)29-15-5-6-19(26)18(25)10-15/h3-6,10-12,14,16H,7-9,13H2,1-2H3,(H,30,32)(H,27,28,29)/b4-3+/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR exon 19 deletion activating mutant phosphorylation in human PC9 cells after 2 hrs by fluorescence assay |
J Med Chem 56: 7025-48 (2013)
Article DOI: 10.1021/jm400822z
BindingDB Entry DOI: 10.7270/Q2PN98KQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM112499
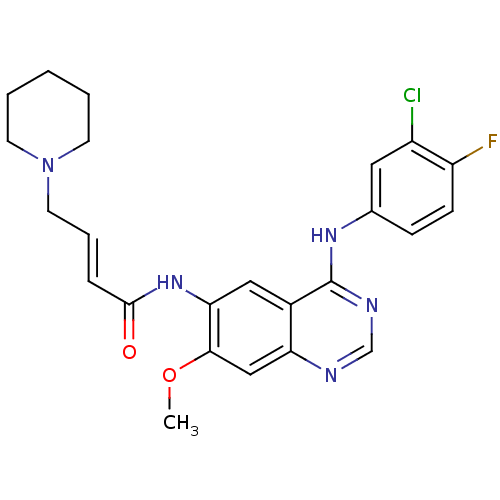
(DACOMITINIB | US8623883, No. 2 | WO2022090481, Exa...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1NC(=O)\C=C\CN1CCCCC1 Show InChI InChI=1S/C24H25ClFN5O2/c1-33-22-14-20-17(24(28-15-27-20)29-16-7-8-19(26)18(25)12-16)13-21(22)30-23(32)6-5-11-31-9-3-2-4-10-31/h5-8,12-15H,2-4,9-11H2,1H3,(H,30,32)(H,27,28,29)/b6-5+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR exon 19 deletion activating mutant phosphorylation in human PC9 cells after 2 hrs by fluorescence assay |
J Med Chem 56: 7025-48 (2013)
Article DOI: 10.1021/jm400822z
BindingDB Entry DOI: 10.7270/Q2PN98KQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50029670

(CHEMBL2426277 | US10227342, Example 26)Show SMILES COc1cc(N2CCN(C)CC2)c(NC(=O)C=C)cc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 Show InChI InChI=1S/C26H27ClN8O2/c1-4-24(36)30-19-13-20(23(37-3)14-22(19)34-11-9-33(2)10-12-34)31-26-28-16-18(27)25(32-26)17-15-29-35-8-6-5-7-21(17)35/h4-8,13-16H,1,9-12H2,2-3H3,(H,30,36)(H,28,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R (unknown origin) |
J Med Chem 57: 8249-67 (2014)
Article DOI: 10.1021/jm500973a
BindingDB Entry DOI: 10.7270/Q2ZS2Z4C |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50029667
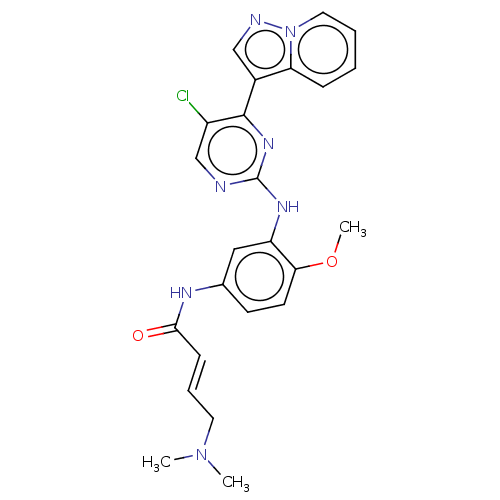
(CHEMBL2426288)Show SMILES COc1ccc(NC(=O)\C=C\CN(C)C)cc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 Show InChI InChI=1S/C24H24ClN7O2/c1-31(2)11-6-8-22(33)28-16-9-10-21(34-3)19(13-16)29-24-26-15-18(25)23(30-24)17-14-27-32-12-5-4-7-20(17)32/h4-10,12-15H,11H2,1-3H3,(H,28,33)(H,26,29,30)/b8-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R (unknown origin) |
J Med Chem 57: 8249-67 (2014)
Article DOI: 10.1021/jm500973a
BindingDB Entry DOI: 10.7270/Q2ZS2Z4C |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50493289
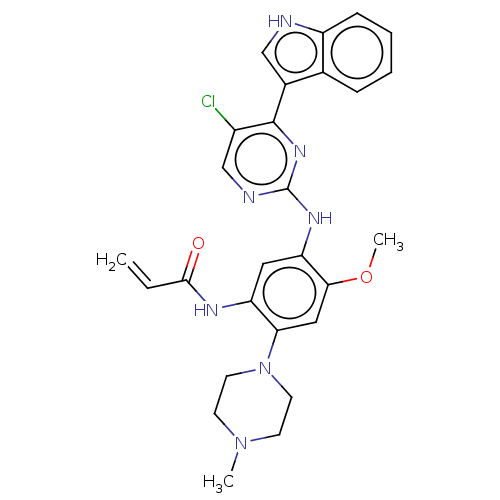
(CHEMBL2426278)Show SMILES COc1cc(N2CCN(C)CC2)c(NC(=O)C=C)cc1Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 Show InChI InChI=1S/C27H28ClN7O2/c1-4-25(36)31-21-13-22(24(37-3)14-23(21)35-11-9-34(2)10-12-35)32-27-30-16-19(28)26(33-27)18-15-29-20-8-6-5-7-17(18)20/h4-8,13-16,29H,1,9-12H2,2-3H3,(H,31,36)(H,30,32,33) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R/T970M double mutant phosphorylation in human NCI-H1975 cells after 2 hrs by fluorescence assay |
J Med Chem 56: 7025-48 (2013)
Article DOI: 10.1021/jm400822z
BindingDB Entry DOI: 10.7270/Q2PN98KQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR exon 19 deletion activating mutant phosphorylation in human PC9 cells after 2 hrs by fluorescence assay |
J Med Chem 56: 7025-48 (2013)
Article DOI: 10.1021/jm400822z
BindingDB Entry DOI: 10.7270/Q2PN98KQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50029685
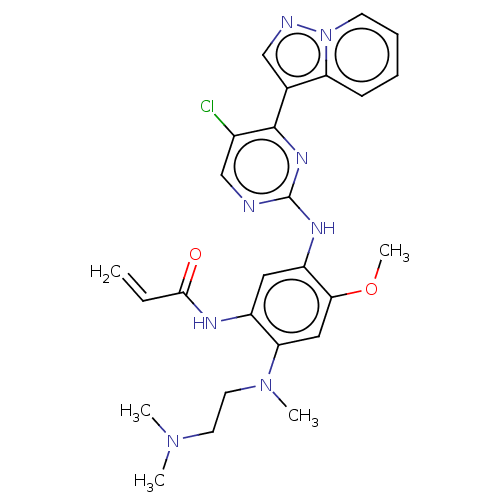
(CHEMBL3353404 | US10227342, Example 52)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 Show InChI InChI=1S/C26H29ClN8O2/c1-6-24(36)30-19-13-20(23(37-5)14-22(19)34(4)12-11-33(2)3)31-26-28-16-18(27)25(32-26)17-15-29-35-10-8-7-9-21(17)35/h6-10,13-16H,1,11-12H2,2-5H3,(H,30,36)(H,28,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R (unknown origin) |
J Med Chem 57: 8249-67 (2014)
Article DOI: 10.1021/jm500973a
BindingDB Entry DOI: 10.7270/Q2ZS2Z4C |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50029684

(CHEMBL3353403)Show SMILES COc1cc(N2CC[C@H](C2)N(C)C)c(NC(=O)C=C)cc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 |r| Show InChI InChI=1S/C27H29ClN8O2/c1-5-25(37)31-20-12-21(24(38-4)13-23(20)35-11-9-17(16-35)34(2)3)32-27-29-15-19(28)26(33-27)18-14-30-36-10-7-6-8-22(18)36/h5-8,10,12-15,17H,1,9,11,16H2,2-4H3,(H,31,37)(H,29,32,33)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R (unknown origin) |
J Med Chem 57: 8249-67 (2014)
Article DOI: 10.1021/jm500973a
BindingDB Entry DOI: 10.7270/Q2ZS2Z4C |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5447

(CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR exon 19 deletion activating mutant phosphorylation in human PC9 cells after 2 hrs by fluorescence assay |
J Med Chem 56: 7025-48 (2013)
Article DOI: 10.1021/jm400822z
BindingDB Entry DOI: 10.7270/Q2PN98KQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Insulin receptor
(Homo sapiens (Human)) | BDBM50029685

(CHEMBL3353404 | US10227342, Example 52)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 Show InChI InChI=1S/C26H29ClN8O2/c1-6-24(36)30-19-13-20(23(37-5)14-22(19)34(4)12-11-33(2)3)31-26-28-16-18(27)25(32-26)17-15-29-35-10-8-7-9-21(17)35/h6-10,13-16H,1,11-12H2,2-5H3,(H,30,36)(H,28,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of INSR (unknown origin) |
J Med Chem 57: 8249-67 (2014)
Article DOI: 10.1021/jm500973a
BindingDB Entry DOI: 10.7270/Q2ZS2Z4C |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM112499

(DACOMITINIB | US8623883, No. 2 | WO2022090481, Exa...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1NC(=O)\C=C\CN1CCCCC1 Show InChI InChI=1S/C24H25ClFN5O2/c1-33-22-14-20-17(24(28-15-27-20)29-16-7-8-19(26)18(25)12-16)13-21(22)30-23(32)6-5-11-31-9-3-2-4-10-31/h5-8,12-15H,2-4,9-11H2,1H3,(H,30,32)(H,27,28,29)/b6-5+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR phosphorylation in human LoVo cells after 2 hrs by fluorescence assay |
J Med Chem 56: 7025-48 (2013)
Article DOI: 10.1021/jm400822z
BindingDB Entry DOI: 10.7270/Q2PN98KQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50322823

((S)-N-(4-(3-chloro-4-fluorophenylamino)-7-(tetrahy...)Show SMILES CN(C)C\C=C\C(=O)Nc1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2cc1O[C@H]1CCOC1 |r| Show InChI InChI=1S/C24H25ClFN5O3/c1-31(2)8-3-4-23(32)30-21-11-17-20(12-22(21)34-16-7-9-33-13-16)27-14-28-24(17)29-15-5-6-19(26)18(25)10-15/h3-6,10-12,14,16H,7-9,13H2,1-2H3,(H,30,32)(H,27,28,29)/b4-3+/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR phosphorylation in human LoVo cells after 2 hrs by fluorescence assay |
J Med Chem 56: 7025-48 (2013)
Article DOI: 10.1021/jm400822z
BindingDB Entry DOI: 10.7270/Q2PN98KQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Procathepsin L [114-333,T223A]
(Homo sapiens (Human)) | BDBM31992
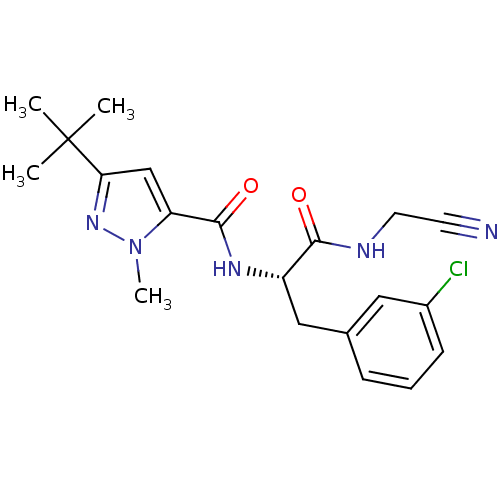
(Dipeptidyl nitrile inhibitor, 25)Show SMILES Cn1nc(cc1C(=O)N[C@@H](Cc1cccc(Cl)c1)C(=O)NCC#N)C(C)(C)C |r| Show InChI InChI=1S/C20H24ClN5O2/c1-20(2,3)17-12-16(26(4)25-17)19(28)24-15(18(27)23-9-8-22)11-13-6-5-7-14(21)10-13/h5-7,10,12,15H,9,11H2,1-4H3,(H,23,27)(H,24,28)/t15-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.0 | 37 |
AstraZeneca
| Assay Description
IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. |
Bioorg Med Chem Lett 19: 4280-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.071
BindingDB Entry DOI: 10.7270/Q2TT4PBM |
More data for this
Ligand-Target Pair | |
Procathepsin L [114-333,T223A]
(Homo sapiens (Human)) | BDBM31993

(Dipeptidyl nitrile inhibitor, 26)Show SMILES Cc1cccc(C[C@H](NC(=O)c2cc(nn2C)C(C)(C)C)C(=O)NCC#N)c1 |r| Show InChI InChI=1S/C21H27N5O2/c1-14-7-6-8-15(11-14)12-16(19(27)23-10-9-22)24-20(28)17-13-18(21(2,3)4)25-26(17)5/h6-8,11,13,16H,10,12H2,1-5H3,(H,23,27)(H,24,28)/t16-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.0 | 37 |
AstraZeneca
| Assay Description
IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. |
Bioorg Med Chem Lett 19: 4280-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.071
BindingDB Entry DOI: 10.7270/Q2TT4PBM |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029669
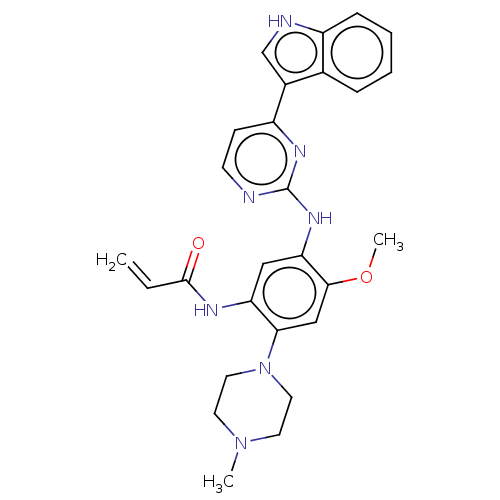
(CHEMBL2426279)Show SMILES COc1cc(N2CCN(C)CC2)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1c[nH]c2ccccc12 Show InChI InChI=1S/C27H29N7O2/c1-4-26(35)30-22-15-23(25(36-3)16-24(22)34-13-11-33(2)12-14-34)32-27-28-10-9-21(31-27)19-17-29-20-8-6-5-7-18(19)20/h4-10,15-17,29H,1,11-14H2,2-3H3,(H,30,35)(H,28,31,32) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR exon 19 deletion activating mutant phosphorylation in human PC9 cells after 2 hrs by fluorescence assay |
J Med Chem 57: 8249-67 (2014)
Article DOI: 10.1021/jm500973a
BindingDB Entry DOI: 10.7270/Q2ZS2Z4C |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029669

(CHEMBL2426279)Show SMILES COc1cc(N2CCN(C)CC2)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1c[nH]c2ccccc12 Show InChI InChI=1S/C27H29N7O2/c1-4-26(35)30-22-15-23(25(36-3)16-24(22)34-13-11-33(2)12-14-34)32-27-28-10-9-21(31-27)19-17-29-20-8-6-5-7-18(19)20/h4-10,15-17,29H,1,11-14H2,2-3H3,(H,30,35)(H,28,31,32) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR exon 19 deletion activating mutant phosphorylation in human PC9 cells after 2 hrs by fluorescence assay |
J Med Chem 56: 7025-48 (2013)
Article DOI: 10.1021/jm400822z
BindingDB Entry DOI: 10.7270/Q2PN98KQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029670

(CHEMBL2426277 | US10227342, Example 26)Show SMILES COc1cc(N2CCN(C)CC2)c(NC(=O)C=C)cc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 Show InChI InChI=1S/C26H27ClN8O2/c1-4-24(36)30-19-13-20(23(37-3)14-22(19)34-11-9-33(2)10-12-34)31-26-28-16-18(27)25(32-26)17-15-29-35-8-6-5-7-21(17)35/h4-8,13-16H,1,9-12H2,2-3H3,(H,30,36)(H,28,31,32) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R/T970M double mutant phosphorylation in human NCI-H1975 cells after 2 hrs by fluorescence assay |
J Med Chem 57: 8249-67 (2014)
Article DOI: 10.1021/jm500973a
BindingDB Entry DOI: 10.7270/Q2ZS2Z4C |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029670

(CHEMBL2426277 | US10227342, Example 26)Show SMILES COc1cc(N2CCN(C)CC2)c(NC(=O)C=C)cc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 Show InChI InChI=1S/C26H27ClN8O2/c1-4-24(36)30-19-13-20(23(37-3)14-22(19)34-11-9-33(2)10-12-34)31-26-28-16-18(27)25(32-26)17-15-29-35-8-6-5-7-21(17)35/h4-8,13-16H,1,9-12H2,2-3H3,(H,30,36)(H,28,31,32) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R/T970M double mutant phosphorylation in human NCI-H1975 cells after 2 hrs by fluorescence assay |
J Med Chem 56: 7025-48 (2013)
Article DOI: 10.1021/jm400822z
BindingDB Entry DOI: 10.7270/Q2PN98KQ |
More data for this
Ligand-Target Pair | |
Procathepsin L [114-333,T223A]
(Homo sapiens (Human)) | BDBM31984

(Dipeptidyl nitrile inhibitor, 17)Show SMILES Cc1ccc2cc(C)cc(C(=O)N[C@@H](Cc3cccc(Cl)c3)C(=O)NCC#N)c2c1 |r| Show InChI InChI=1S/C24H22ClN3O2/c1-15-6-7-18-10-16(2)12-21(20(18)11-15)23(29)28-22(24(30)27-9-8-26)14-17-4-3-5-19(25)13-17/h3-7,10-13,22H,9,14H2,1-2H3,(H,27,30)(H,28,29)/t22-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.0 | 37 |
AstraZeneca
| Assay Description
IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. |
Bioorg Med Chem Lett 19: 4280-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.071
BindingDB Entry DOI: 10.7270/Q2TT4PBM |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50029684

(CHEMBL3353403)Show SMILES COc1cc(N2CC[C@H](C2)N(C)C)c(NC(=O)C=C)cc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 |r| Show InChI InChI=1S/C27H29ClN8O2/c1-5-25(37)31-20-12-21(24(38-4)13-23(20)35-11-9-17(16-35)34(2)3)32-27-29-15-19(28)26(33-27)18-14-30-36-10-7-6-8-22(18)36/h5-8,10,12-15,17H,1,9,11,16H2,2-4H3,(H,31,37)(H,29,32,33)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of INSR (unknown origin) |
J Med Chem 57: 8249-67 (2014)
Article DOI: 10.1021/jm500973a
BindingDB Entry DOI: 10.7270/Q2ZS2Z4C |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50493288
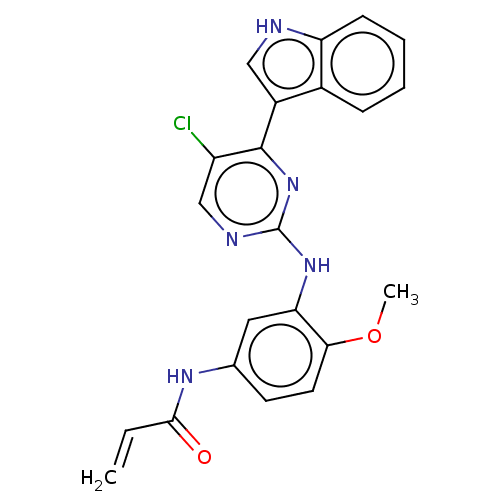
(CHEMBL2426282)Show SMILES COc1ccc(NC(=O)C=C)cc1Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 Show InChI InChI=1S/C22H18ClN5O2/c1-3-20(29)26-13-8-9-19(30-2)18(10-13)27-22-25-12-16(23)21(28-22)15-11-24-17-7-5-4-6-14(15)17/h3-12,24H,1H2,2H3,(H,26,29)(H,25,27,28) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R/T970M double mutant phosphorylation in human NCI-H1975 cells after 2 hrs by fluorescence assay |
J Med Chem 56: 7025-48 (2013)
Article DOI: 10.1021/jm400822z
BindingDB Entry DOI: 10.7270/Q2PN98KQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50493289

(CHEMBL2426278)Show SMILES COc1cc(N2CCN(C)CC2)c(NC(=O)C=C)cc1Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 Show InChI InChI=1S/C27H28ClN7O2/c1-4-25(36)31-21-13-22(24(37-3)14-23(21)35-11-9-34(2)10-12-35)32-27-30-16-19(28)26(33-27)18-15-29-20-8-6-5-7-17(18)20/h4-8,13-16,29H,1,9-12H2,2-3H3,(H,31,36)(H,30,32,33) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR exon 19 deletion activating mutant phosphorylation in human PC9 cells after 2 hrs by fluorescence assay |
J Med Chem 56: 7025-48 (2013)
Article DOI: 10.1021/jm400822z
BindingDB Entry DOI: 10.7270/Q2PN98KQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50383274
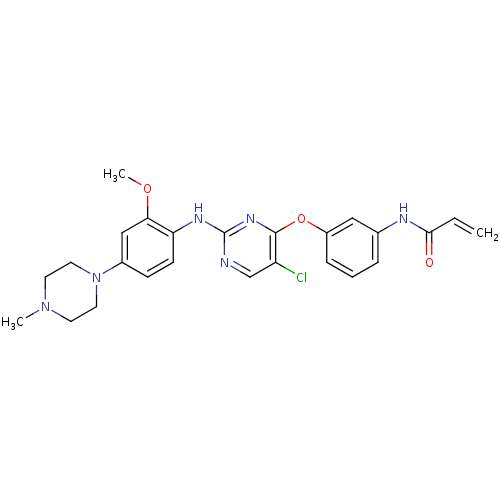
(CHEMBL1229592 | US10167264, WZ4002 | US9670213, WZ...)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Oc2cccc(NC(=O)C=C)c2)n1)N1CCN(C)CC1 Show InChI InChI=1S/C25H27ClN6O3/c1-4-23(33)28-17-6-5-7-19(14-17)35-24-20(26)16-27-25(30-24)29-21-9-8-18(15-22(21)34-3)32-12-10-31(2)11-13-32/h4-9,14-16H,1,10-13H2,2-3H3,(H,28,33)(H,27,29,30) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R/T970M double mutant phosphorylation in human NCI-H1975 cells after 2 hrs by fluorescence assay |
J Med Chem 56: 7025-48 (2013)
Article DOI: 10.1021/jm400822z
BindingDB Entry DOI: 10.7270/Q2PN98KQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50322823

((S)-N-(4-(3-chloro-4-fluorophenylamino)-7-(tetrahy...)Show SMILES CN(C)C\C=C\C(=O)Nc1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2cc1O[C@H]1CCOC1 |r| Show InChI InChI=1S/C24H25ClFN5O3/c1-31(2)8-3-4-23(32)30-21-11-17-20(12-22(21)34-16-7-9-33-13-16)27-14-28-24(17)29-15-5-6-19(26)18(25)10-15/h3-6,10-12,14,16H,7-9,13H2,1-2H3,(H,30,32)(H,27,28,29)/b4-3+/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R/T970M double mutant phosphorylation in human NCI-H1975 cells after 2 hrs by fluorescence assay |
J Med Chem 56: 7025-48 (2013)
Article DOI: 10.1021/jm400822z
BindingDB Entry DOI: 10.7270/Q2PN98KQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50029686

(CHEMBL3353405 | US10227342, Example 25)Show SMILES COc1cc(N2CC(C2)N(C)C)c(NC(=O)C=C)cc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 Show InChI InChI=1S/C26H27ClN8O2/c1-5-24(36)30-19-10-20(23(37-4)11-22(19)34-14-16(15-34)33(2)3)31-26-28-13-18(27)25(32-26)17-12-29-35-9-7-6-8-21(17)35/h5-13,16H,1,14-15H2,2-4H3,(H,30,36)(H,28,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R (unknown origin) |
J Med Chem 57: 8249-67 (2014)
Article DOI: 10.1021/jm500973a
BindingDB Entry DOI: 10.7270/Q2ZS2Z4C |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50493288

(CHEMBL2426282)Show SMILES COc1ccc(NC(=O)C=C)cc1Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 Show InChI InChI=1S/C22H18ClN5O2/c1-3-20(29)26-13-8-9-19(30-2)18(10-13)27-22-25-12-16(23)21(28-22)15-11-24-17-7-5-4-6-14(15)17/h3-12,24H,1H2,2H3,(H,26,29)(H,25,27,28) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR exon 19 deletion activating mutant phosphorylation in human PC9 cells after 2 hrs by fluorescence assay |
J Med Chem 56: 7025-48 (2013)
Article DOI: 10.1021/jm400822z
BindingDB Entry DOI: 10.7270/Q2PN98KQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50493291
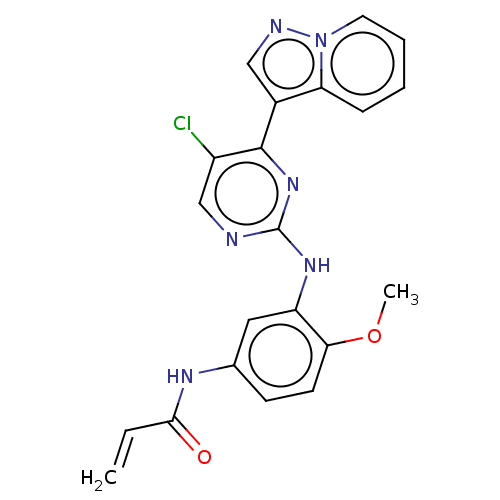
(CHEMBL2426286)Show SMILES COc1ccc(NC(=O)C=C)cc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 Show InChI InChI=1S/C21H17ClN6O2/c1-3-19(29)25-13-7-8-18(30-2)16(10-13)26-21-23-12-15(22)20(27-21)14-11-24-28-9-5-4-6-17(14)28/h3-12H,1H2,2H3,(H,25,29)(H,23,26,27) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R/T970M double mutant phosphorylation in human NCI-H1975 cells after 2 hrs by fluorescence assay |
J Med Chem 56: 7025-48 (2013)
Article DOI: 10.1021/jm400822z
BindingDB Entry DOI: 10.7270/Q2PN98KQ |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50029686

(CHEMBL3353405 | US10227342, Example 25)Show SMILES COc1cc(N2CC(C2)N(C)C)c(NC(=O)C=C)cc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 Show InChI InChI=1S/C26H27ClN8O2/c1-5-24(36)30-19-10-20(23(37-4)11-22(19)34-14-16(15-34)33(2)3)31-26-28-13-18(27)25(32-26)17-12-29-35-9-7-6-8-21(17)35/h5-13,16H,1,14-15H2,2-4H3,(H,30,36)(H,28,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of INSR (unknown origin) |
J Med Chem 57: 8249-67 (2014)
Article DOI: 10.1021/jm500973a
BindingDB Entry DOI: 10.7270/Q2ZS2Z4C |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM112499

(DACOMITINIB | US8623883, No. 2 | WO2022090481, Exa...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1NC(=O)\C=C\CN1CCCCC1 Show InChI InChI=1S/C24H25ClFN5O2/c1-33-22-14-20-17(24(28-15-27-20)29-16-7-8-19(26)18(25)12-16)13-21(22)30-23(32)6-5-11-31-9-3-2-4-10-31/h5-8,12-15H,2-4,9-11H2,1H3,(H,30,32)(H,27,28,29)/b6-5+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R/T970M double mutant phosphorylation in human NCI-H1975 cells after 2 hrs by fluorescence assay |
J Med Chem 56: 7025-48 (2013)
Article DOI: 10.1021/jm400822z
BindingDB Entry DOI: 10.7270/Q2PN98KQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50383274

(CHEMBL1229592 | US10167264, WZ4002 | US9670213, WZ...)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Oc2cccc(NC(=O)C=C)c2)n1)N1CCN(C)CC1 Show InChI InChI=1S/C25H27ClN6O3/c1-4-23(33)28-17-6-5-7-19(14-17)35-24-20(26)16-27-25(30-24)29-21-9-8-18(15-22(21)34-3)32-12-10-31(2)11-13-32/h4-9,14-16H,1,10-13H2,2-3H3,(H,28,33)(H,27,29,30) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR exon 19 deletion activating mutant phosphorylation in human PC9 cells after 2 hrs by fluorescence assay |
J Med Chem 56: 7025-48 (2013)
Article DOI: 10.1021/jm400822z
BindingDB Entry DOI: 10.7270/Q2PN98KQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Procathepsin L [114-333,T223A]
(Homo sapiens (Human)) | BDBM31980

(Dipeptidyl nitrile inhibitor, 13)Show SMILES Cc1cc(C)cc(c1)C(=O)N[C@@H](Cc1cccc(Cl)c1)C(=O)NCC#N |r| Show InChI InChI=1S/C20H20ClN3O2/c1-13-8-14(2)10-16(9-13)19(25)24-18(20(26)23-7-6-22)12-15-4-3-5-17(21)11-15/h3-5,8-11,18H,7,12H2,1-2H3,(H,23,26)(H,24,25)/t18-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.0 | 37 |
AstraZeneca
| Assay Description
IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. |
Bioorg Med Chem Lett 19: 4280-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.071
BindingDB Entry DOI: 10.7270/Q2TT4PBM |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50493298

(CHEMBL2426287)Show SMILES COc1ccc(NC(=O)\C=C\CN(C)C)cc1Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 Show InChI InChI=1S/C25H25ClN6O2/c1-32(2)12-6-9-23(33)29-16-10-11-22(34-3)21(13-16)30-25-28-15-19(26)24(31-25)18-14-27-20-8-5-4-7-17(18)20/h4-11,13-15,27H,12H2,1-3H3,(H,29,33)(H,28,30,31)/b9-6+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R/T970M double mutant phosphorylation in human NCI-H1975 cells after 2 hrs by fluorescence assay |
J Med Chem 56: 7025-48 (2013)
Article DOI: 10.1021/jm400822z
BindingDB Entry DOI: 10.7270/Q2PN98KQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5447

(CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR phosphorylation in human LoVo cells after 2 hrs by fluorescence assay |
J Med Chem 56: 7025-48 (2013)
Article DOI: 10.1021/jm400822z
BindingDB Entry DOI: 10.7270/Q2PN98KQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Procathepsin L [114-333,T223A]
(Homo sapiens (Human)) | BDBM31990
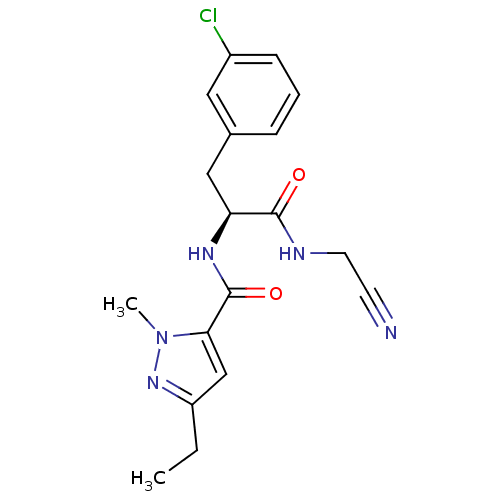
(Dipeptidyl nitrile inhibitor, 23)Show SMILES CCc1cc(C(=O)N[C@@H](Cc2cccc(Cl)c2)C(=O)NCC#N)n(C)n1 |r| Show InChI InChI=1S/C18H20ClN5O2/c1-3-14-11-16(24(2)23-14)18(26)22-15(17(25)21-8-7-20)10-12-5-4-6-13(19)9-12/h4-6,9,11,15H,3,8,10H2,1-2H3,(H,21,25)(H,22,26)/t15-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | 7.0 | 37 |
AstraZeneca
| Assay Description
IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. |
Bioorg Med Chem Lett 19: 4280-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.071
BindingDB Entry DOI: 10.7270/Q2TT4PBM |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029669

(CHEMBL2426279)Show SMILES COc1cc(N2CCN(C)CC2)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1c[nH]c2ccccc12 Show InChI InChI=1S/C27H29N7O2/c1-4-26(35)30-22-15-23(25(36-3)16-24(22)34-13-11-33(2)12-14-34)32-27-28-10-9-21(31-27)19-17-29-20-8-6-5-7-18(19)20/h4-10,15-17,29H,1,11-14H2,2-3H3,(H,30,35)(H,28,31,32) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R/T970M double mutant phosphorylation in human NCI-H1975 cells after 2 hrs by fluorescence assay |
J Med Chem 57: 8249-67 (2014)
Article DOI: 10.1021/jm500973a
BindingDB Entry DOI: 10.7270/Q2ZS2Z4C |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029669

(CHEMBL2426279)Show SMILES COc1cc(N2CCN(C)CC2)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1c[nH]c2ccccc12 Show InChI InChI=1S/C27H29N7O2/c1-4-26(35)30-22-15-23(25(36-3)16-24(22)34-13-11-33(2)12-14-34)32-27-28-10-9-21(31-27)19-17-29-20-8-6-5-7-18(19)20/h4-10,15-17,29H,1,11-14H2,2-3H3,(H,30,35)(H,28,31,32) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R/T970M double mutant phosphorylation in human NCI-H1975 cells after 2 hrs by fluorescence assay |
J Med Chem 56: 7025-48 (2013)
Article DOI: 10.1021/jm400822z
BindingDB Entry DOI: 10.7270/Q2PN98KQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50493285

(CHEMBL2424676)Show SMILES COc1cc(N2CCN(C)CC2)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cnn2ccccc12 Show InChI InChI=1S/C26H28N8O2/c1-4-25(35)29-20-15-21(24(36-3)16-23(20)33-13-11-32(2)12-14-33)31-26-27-9-8-19(30-26)18-17-28-34-10-6-5-7-22(18)34/h4-10,15-17H,1,11-14H2,2-3H3,(H,29,35)(H,27,30,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R/T970M double mutant phosphorylation in human NCI-H1975 cells after 2 hrs by fluorescence assay |
J Med Chem 56: 7025-48 (2013)
Article DOI: 10.1021/jm400822z
BindingDB Entry DOI: 10.7270/Q2PN98KQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR phosphorylation in human LoVo cells after 2 hrs by fluorescence assay |
J Med Chem 56: 7025-48 (2013)
Article DOI: 10.1021/jm400822z
BindingDB Entry DOI: 10.7270/Q2PN98KQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
B-cell lymphoma 6 protein
(Homo sapiens) | BDBM50239369

(CHEMBL4100303)Show SMILES [H][C@]12C[C@@H](CO)N(C1)c1cc(Nc3cc4CCC(=O)N(Cc5ccccc5C)c4c(OC\C=C\CO2)c3)n2ncc(Cl)c2n1 |r,t:35| Show InChI InChI=1S/C32H33ClN6O4/c1-20-6-2-3-7-22(20)17-38-30(41)9-8-21-12-23-13-27(31(21)38)43-11-5-4-10-42-25-14-24(19-40)37(18-25)28-15-29(35-23)39-32(36-28)26(33)16-34-39/h2-7,12-13,15-16,24-25,35,40H,8-11,14,17-19H2,1H3/b5-4+/t24-,25-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... |
J Med Chem 60: 4386-4402 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00359
BindingDB Entry DOI: 10.7270/Q2ZW1P1C |
More data for this
Ligand-Target Pair | |
Procathepsin L [114-333,T223A]
(Homo sapiens (Human)) | BDBM31991
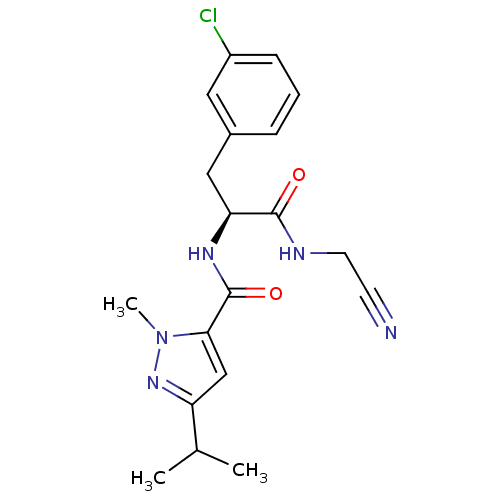
(Dipeptidyl nitrile inhibitor, 24)Show SMILES CC(C)c1cc(C(=O)N[C@@H](Cc2cccc(Cl)c2)C(=O)NCC#N)n(C)n1 |r| Show InChI InChI=1S/C19H22ClN5O2/c1-12(2)15-11-17(25(3)24-15)19(27)23-16(18(26)22-8-7-21)10-13-5-4-6-14(20)9-13/h4-6,9,11-12,16H,8,10H2,1-3H3,(H,22,26)(H,23,27)/t16-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | 7.0 | 37 |
AstraZeneca
| Assay Description
IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. |
Bioorg Med Chem Lett 19: 4280-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.071
BindingDB Entry DOI: 10.7270/Q2TT4PBM |
More data for this
Ligand-Target Pair | |
Procathepsin L [114-333,T223A]
(Homo sapiens (Human)) | BDBM31989

(Dipeptidyl nitrile inhibitor, 22)Show SMILES Cc1cc(C(=O)N[C@@H](Cc2cccc(Cl)c2)C(=O)NCC#N)c(C)s1 |r| Show InChI InChI=1S/C18H18ClN3O2S/c1-11-8-15(12(2)25-11)17(23)22-16(18(24)21-7-6-20)10-13-4-3-5-14(19)9-13/h3-5,8-9,16H,7,10H2,1-2H3,(H,21,24)(H,22,23)/t16-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | 7.0 | 37 |
AstraZeneca
| Assay Description
IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. |
Bioorg Med Chem Lett 19: 4280-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.071
BindingDB Entry DOI: 10.7270/Q2TT4PBM |
More data for this
Ligand-Target Pair | |
Procathepsin L [114-333,T223A]
(Homo sapiens (Human)) | BDBM31977

(Dipeptidyl nitrile inhibitor, 10)Show SMILES Clc1cccc(C[C@H](NC(=O)c2cccc(Cl)c2)C(=O)NCC#N)c1 |r| Show InChI InChI=1S/C18H15Cl2N3O2/c19-14-5-1-3-12(9-14)10-16(18(25)22-8-7-21)23-17(24)13-4-2-6-15(20)11-13/h1-6,9,11,16H,8,10H2,(H,22,25)(H,23,24)/t16-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | 7.0 | 37 |
AstraZeneca
| Assay Description
IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. |
Bioorg Med Chem Lett 19: 4280-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.071
BindingDB Entry DOI: 10.7270/Q2TT4PBM |
More data for this
Ligand-Target Pair | |
Procathepsin L [114-333,T223A]
(Homo sapiens (Human)) | BDBM31978

(Dipeptidyl nitrile inhibitor, 11)Show SMILES Cc1ccc(C)c(c1)C(=O)N[C@@H](Cc1cccc(Cl)c1)C(=O)NCC#N |r| Show InChI InChI=1S/C20H20ClN3O2/c1-13-6-7-14(2)17(10-13)19(25)24-18(20(26)23-9-8-22)12-15-4-3-5-16(21)11-15/h3-7,10-11,18H,9,12H2,1-2H3,(H,23,26)(H,24,25)/t18-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | 7.0 | 37 |
AstraZeneca
| Assay Description
IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. |
Bioorg Med Chem Lett 19: 4280-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.071
BindingDB Entry DOI: 10.7270/Q2TT4PBM |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50493293
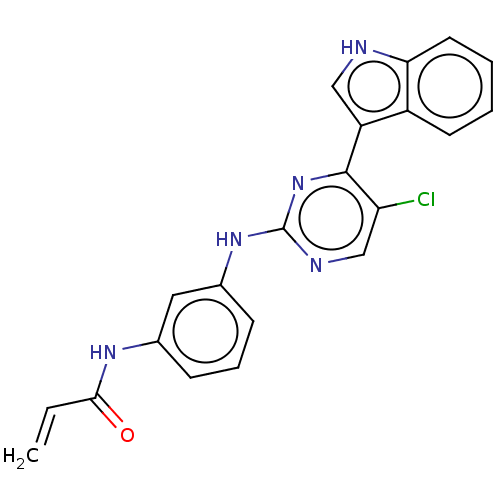
(CHEMBL2426281)Show SMILES Clc1cnc(Nc2cccc(NC(=O)C=C)c2)nc1-c1c[nH]c2ccccc12 Show InChI InChI=1S/C21H16ClN5O/c1-2-19(28)25-13-6-5-7-14(10-13)26-21-24-12-17(22)20(27-21)16-11-23-18-9-4-3-8-15(16)18/h2-12,23H,1H2,(H,25,28)(H,24,26,27) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R/T970M double mutant phosphorylation in human NCI-H1975 cells after 2 hrs by fluorescence assay |
J Med Chem 56: 7025-48 (2013)
Article DOI: 10.1021/jm400822z
BindingDB Entry DOI: 10.7270/Q2PN98KQ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50493298

(CHEMBL2426287)Show SMILES COc1ccc(NC(=O)\C=C\CN(C)C)cc1Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 Show InChI InChI=1S/C25H25ClN6O2/c1-32(2)12-6-9-23(33)29-16-10-11-22(34-3)21(13-16)30-25-28-15-19(26)24(31-25)18-14-27-20-8-5-4-7-17(18)20/h4-11,13-15,27H,12H2,1-3H3,(H,29,33)(H,28,30,31)/b9-6+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR exon 19 deletion activating mutant phosphorylation in human PC9 cells after 2 hrs by fluorescence assay |
J Med Chem 56: 7025-48 (2013)
Article DOI: 10.1021/jm400822z
BindingDB Entry DOI: 10.7270/Q2PN98KQ |
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein
(Homo sapiens) | BDBM50239369

(CHEMBL4100303)Show SMILES [H][C@]12C[C@@H](CO)N(C1)c1cc(Nc3cc4CCC(=O)N(Cc5ccccc5C)c4c(OC\C=C\CO2)c3)n2ncc(Cl)c2n1 |r,t:35| Show InChI InChI=1S/C32H33ClN6O4/c1-20-6-2-3-7-22(20)17-38-30(41)9-8-21-12-23-13-27(31(21)38)43-11-5-4-10-42-25-14-24(19-40)37(18-25)28-15-29(35-23)39-32(36-28)26(33)16-34-39/h2-7,12-13,15-16,24-25,35,40H,8-11,14,17-19H2,1H3/b5-4+/t24-,25-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... |
J Med Chem 60: 4386-4402 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00359
BindingDB Entry DOI: 10.7270/Q2ZW1P1C |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029667

(CHEMBL2426288)Show SMILES COc1ccc(NC(=O)\C=C\CN(C)C)cc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 Show InChI InChI=1S/C24H24ClN7O2/c1-31(2)11-6-8-22(33)28-16-9-10-21(34-3)19(13-16)29-24-26-15-18(25)23(30-24)17-14-27-32-12-5-4-7-20(17)32/h4-10,12-15H,11H2,1-3H3,(H,28,33)(H,26,29,30)/b8-6+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R/T970M double mutant phosphorylation in human NCI-H1975 cells after 2 hrs by fluorescence assay |
J Med Chem 57: 8249-67 (2014)
Article DOI: 10.1021/jm500973a
BindingDB Entry DOI: 10.7270/Q2ZS2Z4C |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029667

(CHEMBL2426288)Show SMILES COc1ccc(NC(=O)\C=C\CN(C)C)cc1Nc1ncc(Cl)c(n1)-c1cnn2ccccc12 Show InChI InChI=1S/C24H24ClN7O2/c1-31(2)11-6-8-22(33)28-16-9-10-21(34-3)19(13-16)29-24-26-15-18(25)23(30-24)17-14-27-32-12-5-4-7-20(17)32/h4-10,12-15H,11H2,1-3H3,(H,28,33)(H,26,29,30)/b8-6+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR L858R/T970M double mutant phosphorylation in human NCI-H1975 cells after 2 hrs by fluorescence assay |
J Med Chem 56: 7025-48 (2013)
Article DOI: 10.1021/jm400822z
BindingDB Entry DOI: 10.7270/Q2PN98KQ |
More data for this
Ligand-Target Pair | |
Procathepsin L [114-333,T223A]
(Homo sapiens (Human)) | BDBM31983

(Dipeptidyl nitrile inhibitor, 16)Show SMILES Clc1cccc(C[C@H](NC(=O)c2ccnc3ccccc23)C(=O)NCC#N)c1 |r| Show InChI InChI=1S/C21H17ClN4O2/c22-15-5-3-4-14(12-15)13-19(21(28)25-11-9-23)26-20(27)17-8-10-24-18-7-2-1-6-16(17)18/h1-8,10,12,19H,11,13H2,(H,25,28)(H,26,27)/t19-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.0 | 37 |
AstraZeneca
| Assay Description
IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. |
Bioorg Med Chem Lett 19: 4280-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.071
BindingDB Entry DOI: 10.7270/Q2TT4PBM |
More data for this
Ligand-Target Pair | |
B-cell lymphoma 6 protein
(Homo sapiens) | BDBM50239385
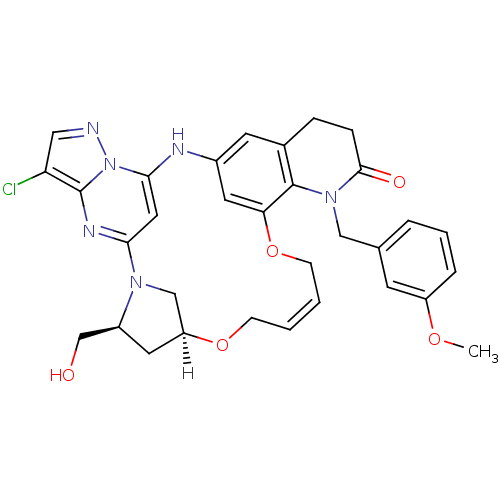
(CHEMBL4092565)Show SMILES [H][C@]12C[C@@H](CO)N(C1)c1cc(Nc3cc4CCC(=O)N(Cc5cccc(OC)c5)c4c(OC\C=C\CO2)c3)n2ncc(Cl)c2n1 |r,t:36| Show InChI InChI=1S/C32H33ClN6O5/c1-42-24-6-4-5-20(11-24)17-38-30(41)8-7-21-12-22-13-27(31(21)38)44-10-3-2-9-43-25-14-23(19-40)37(18-25)28-15-29(35-22)39-32(36-28)26(33)16-34-39/h2-6,11-13,15-16,23,25,35,40H,7-10,14,17-19H2,1H3/b3-2+/t23-,25-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity against alpha-1 adrenergic receptor in guinea pig cerebral cortical membranes by displacement of [3H]- WB-4101 |
J Med Chem 60: 4386-4402 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00359
BindingDB Entry DOI: 10.7270/Q2ZW1P1C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data