| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Sucrase-isomaltase, intestinal |
|---|
| Ligand | BDBM18355 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_878845 (CHEMBL2184153) |
|---|
| IC50 | 58000±n/a nM |
|---|
| Citation |  Kato, A; Hayashi, E; Miyauchi, S; Adachi, I; Imahori, T; Natori, Y; Yoshimura, Y; Nash, RJ; Shimaoka, H; Nakagome, I; Koseki, J; Hirono, S; Takahata, H a-1-C-butyl-1,4-dideoxy-1,4-imino-l-arabinitol as a second-generation iminosugar-based orala-glucosidase inhibitor for improving postprandial hyperglycemia. J Med Chem55:10347-62 (2012) [PubMed] Article Kato, A; Hayashi, E; Miyauchi, S; Adachi, I; Imahori, T; Natori, Y; Yoshimura, Y; Nash, RJ; Shimaoka, H; Nakagome, I; Koseki, J; Hirono, S; Takahata, H a-1-C-butyl-1,4-dideoxy-1,4-imino-l-arabinitol as a second-generation iminosugar-based orala-glucosidase inhibitor for improving postprandial hyperglycemia. J Med Chem55:10347-62 (2012) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Sucrase-isomaltase, intestinal |
|---|
| Name: | Sucrase-isomaltase, intestinal |
|---|
| Synonyms: | SUIS_RAT | Si | Sucrase-isomaltase | alpha-Glucosidase (α-Glucosidase) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 210329.04 |
|---|
| Organism: | Rattus norvegicus (Rat) |
|---|
| Description: | P23739 |
|---|
| Residue: | 1841 |
|---|
| Sequence: | MAKKKFSALEISLIVLFIIVTAIAIALVTVLATKVPAVEEIKSPTPTSNSTPTSTPTSTS
TPTSTSTPSPGKCPPEQGEPINERINCIPEQHPTKAICEERGCCWRPWNNTVIPWCFFAD
NHGYNAESITNENAGLKATLNRIPSPTLFGEDIKSVILTTQTQTGNRFRFKITDPNNKRY
EVPHQFVKEETGIPAADTLYDVQVSENPFSIKVIRKSNNKVLCDTSVGPLLYSNQYLQIS
TRLPSEYIYGFGGHIHKRFRHDLYWKTWPIFTRDEIPGDNNHNLYGHQTFFMGIGDTSGK
SYGVFLMNSNAMEVFIQPTPIITYRVTGGILDFYIFLGDTPEQVVQQYQEVHWRPAMPAY
WNLGFQLSRWNYGSLDTVSEVVRRNREAGIPYDAQVTDIDYMEDHKEFTYDRVKFNGLPE
FAQDLHNHGKYIIILDPAISINKRANGAEYQTYVRGNEKNVWVNESDGTTPLIGEVWPGL
TVYPDFTNPQTIEWWANECNLFHQQVEYDGLWIDMNEVSSFIQGSLNLKGVLLIVLNYPP
FTPGILDKVMYSKTLCMDAVQHWGKQYDVHSLYGYSMAIATEQAVERVFPNKRSFILTRS
TFGGSGRHANHWLGDNTASWEQMEWSITGMLEFGIFGMPLVGATSCGFLADTTEELCRRW
MQLGAFYPFSRNHNAEGYMEQDPAYFGQDSSRHYLTIRYTLLPFLYTLFYRAHMFGETVA
RPFLYEFYDDTNSWIEDTQFLWGPALLITPVLRPGVENVSAYIPNATWYDYETGIKRPWR
KERINMYLPGDKIGLHLRGGYIIPTQEPDVTTTASRKNPLGLIVALDDNQAAKGELFWDD
GESKDSIEKKMYILYTFSVSNNELVLNCTHSSYAEGTSLAFKTIKVLGLREDVRSITVGE
NDQQMATHTNFTFDSANKILSITALNFNLAGSFIVRWCRTFSDNEKFTCYPDVGTATEGT
CTQRGCLWQPVSGLSNVPPYYFPPENNPYTLTSIQPLPTGITAELQLNPPNARIKLPSNP
ISTLRVGVKYHPNDMLQFKIYDAQHKRYEVPVPLNIPDTPTSSNERLYDVEIKENPFGIQ
VRRRSSGKLIWDSRLPGFGFNDQFIQISTRLPSNYLYGFGEVEHTAFKRDLNWHTWGMFT
RDQPPGYKLNSYGFHPYYMALENEGNAHGVLLLNSNGMDVTFQPTPALTYRTIGGILDFY
MFLGPTPEIATRQYHEVIGFPVMPPYWALGFQLCRYGYRNTSEIEQLYNDMVAANIPYDV
QYTDINYMERQLDFTIGERFKTLPEFVDRIRKDGMKYIVILAPAISGNETQPYPAFERGI
QKDVFVKWPNTNDICWPKVWPDLPNVTIDETITEDEAVNASRAHVAFPDFFRNSTLEWWA
REIYDFYNEKMKFDGLWIDMNEPSSFGIQMGGKVLNECRRMMTLNYPPVFSPELRVKEGE
GASISEAMCMETEHILIDGSSVLQYDVHNLYGWSQVKPTLDALQNTTGLRGIVISRSTYP
TTGRWGGHWLGDNYTTWDNLEKSLIGMLELNLFGIPYIGADICGVFHDSGYPSLYFVGIQ
VGAFYPYPRESPTINFTRSQDPVSWMKLLLQMSKKVLEIRYTLLPYFYTQMHEAHAHGGT
VIRPLMHEFFDDKETWEIYKQFLWGPAFMVTPVVEPFRTSVTGYVPKARWFDYHTGADIK
LKGILHTFSAPFDTINLHVRGGYILPCQEPARNTHLSRQNYMKLIVAADDNQMAQGTLFG
DDGESIDTYERGQYTSIQFNLNQTTLTSTVLANGYKNKQEMRLGSIHIWGKGTLRISNAN
LVYGGRKHQPPFTQEEAKETLIFDLKNMNVTLDEPIQITWS
|
|
|
|---|
| BDBM18355 |
|---|
| n/a |
|---|
| Name | BDBM18355 |
|---|
| Synonyms: | (2R,3R,4R,5S)-1-butyl-2-(hydroxymethyl)piperidine-3,4,5-triol | CHEMBL1029 | MIGLUSTAT | N-Butyl-DNJ | US20230339856, Compound NB-DNJ | US9181184, 5 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C10H21NO4 |
|---|
| Mol. Mass. | 219.278 |
|---|
| SMILES | CCCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO |
|---|
| Structure | 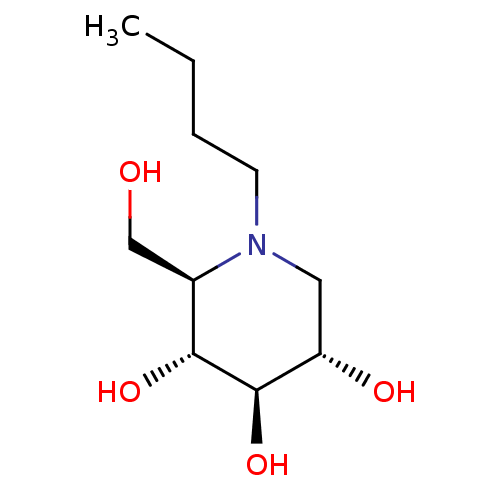
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Kato, A; Hayashi, E; Miyauchi, S; Adachi, I; Imahori, T; Natori, Y; Yoshimura, Y; Nash, RJ; Shimaoka, H; Nakagome, I; Koseki, J; Hirono, S; Takahata, H a-1-C-butyl-1,4-dideoxy-1,4-imino-l-arabinitol as a second-generation iminosugar-based orala-glucosidase inhibitor for improving postprandial hyperglycemia. J Med Chem55:10347-62 (2012) [PubMed] Article
Kato, A; Hayashi, E; Miyauchi, S; Adachi, I; Imahori, T; Natori, Y; Yoshimura, Y; Nash, RJ; Shimaoka, H; Nakagome, I; Koseki, J; Hirono, S; Takahata, H a-1-C-butyl-1,4-dideoxy-1,4-imino-l-arabinitol as a second-generation iminosugar-based orala-glucosidase inhibitor for improving postprandial hyperglycemia. J Med Chem55:10347-62 (2012) [PubMed] Article