

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50583383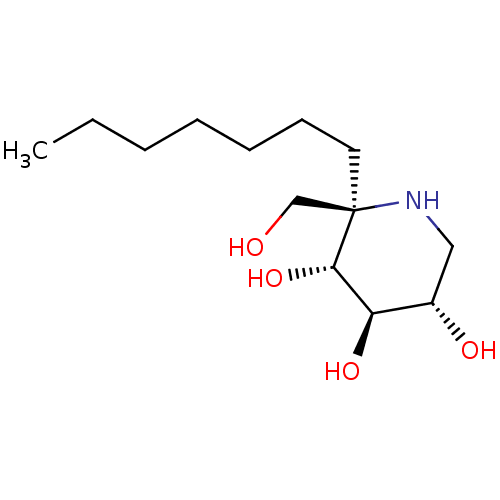 (CHEMBL5028005 | US20230339856, Compound (IIb3)) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Bos taurus) | BDBM50065257 ((2R,3R,4R,5R,6S)-2-Hydroxymethyl-6-methyl-piperidi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Inhibition of bovine epididymis alpha-L-fucosidase assessed as p-nitrophenol release by spectrophotometrically | J Nat Prod 65: 198-202 (2002) BindingDB Entry DOI: 10.7270/Q2ZW1KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50583382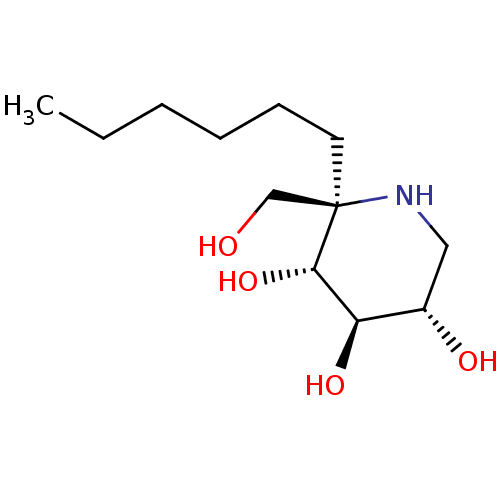 (CHEMBL5028265) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50583381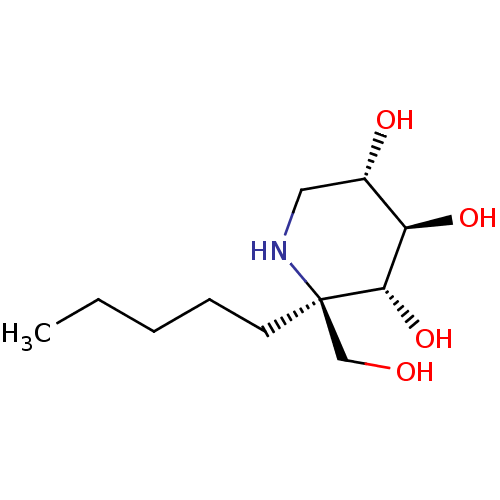 (CHEMBL5029066 | US20230339856, Compound (IIb1)) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50583380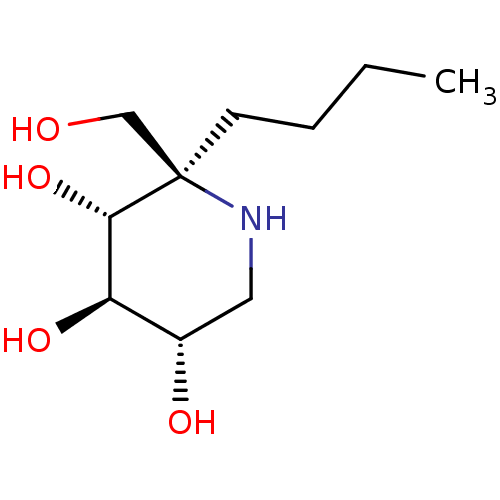 (CHEMBL5027974) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM18353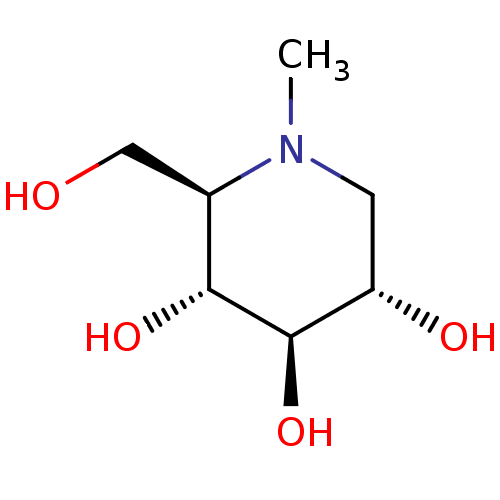 ((2R,3R,4R,5S)-2-(hydroxymethyl)-1-methylpiperidine...) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50583379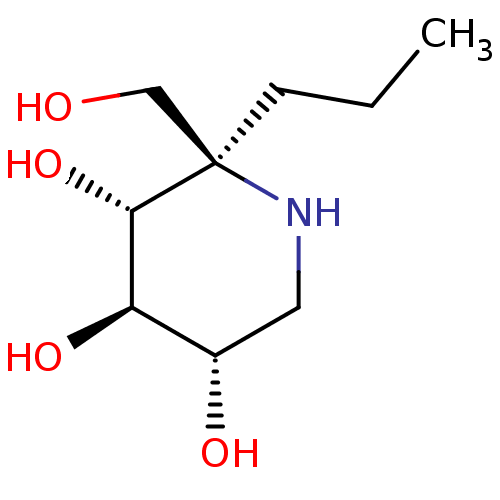 (CHEMBL5028138 | US20230339856, Compound (IIb)) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Bos taurus) | BDBM50242272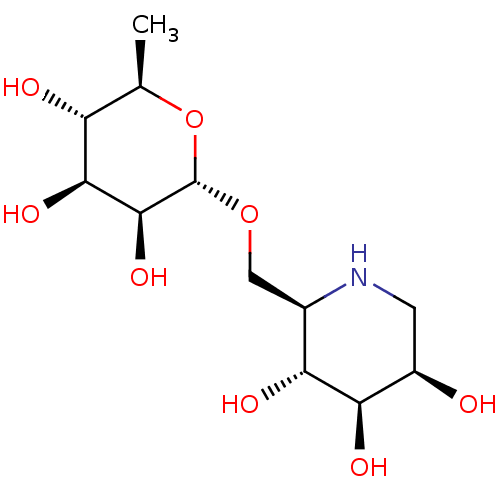 (6-O-alpha-rhamnopyranosyl-DMJ | CHEMBL469655) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Inhibition of bovine epididymis alpha-L-fucosidase assessed as p-nitrophenol release by spectrophotometrically | J Nat Prod 65: 198-202 (2002) BindingDB Entry DOI: 10.7270/Q2ZW1KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Homo sapiens (Human)) | BDBM50065257 ((2R,3R,4R,5R,6S)-2-Hydroxymethyl-6-methyl-piperidi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Inhibitory activity measured against alpha-L-fucosidase of bovine epididymis by colorimetric assay using the D-glucose oxidase-peroxidase method | J Med Chem 41: 2565-71 (1998) Article DOI: 10.1021/jm970836l BindingDB Entry DOI: 10.7270/Q25D8SHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Homo sapiens (Human)) | BDBM50065258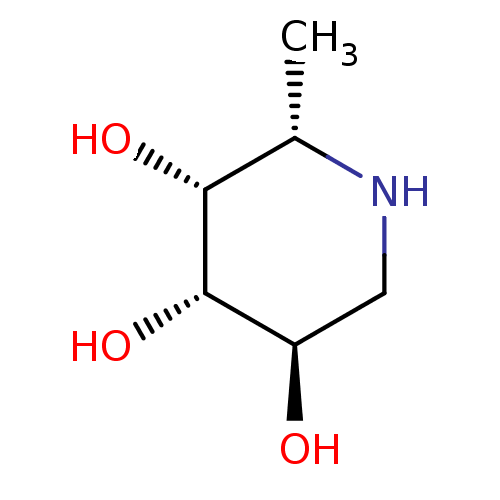 ((2S,3R,4S,5R)-2-Methyl-piperidine-3,4,5-triol | (2...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Inhibitory activity measured against alpha-L-fucosidase of bovine epididymis by colorimetric assay using the D-glucose oxidase-peroxidase method | J Med Chem 41: 2565-71 (1998) Article DOI: 10.1021/jm970836l BindingDB Entry DOI: 10.7270/Q25D8SHK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50333455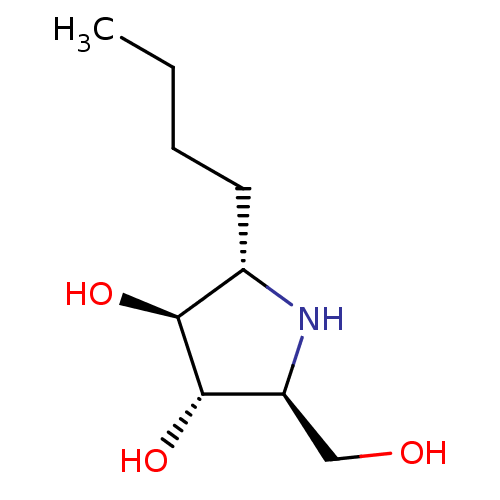 ((2S,3S,4S,5S)-2-butyl-5-(hydroxymethyl)pyrrolidine...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Competitive inhibition of rat intestinal maltase by Lineweaver-Burk plot analysis | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50335398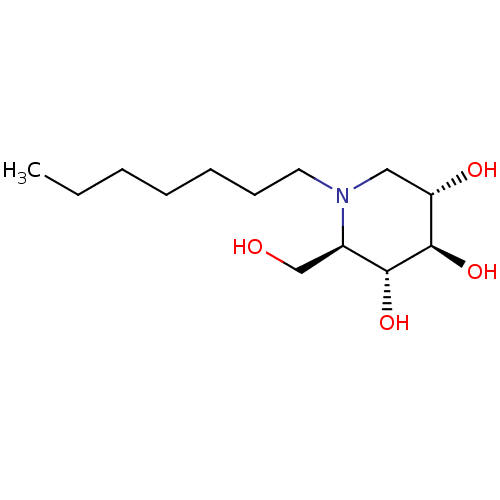 (CHEMBL1651551 | N-Heptyl-1-deoxynojirimycin) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM18356 ((2R,3R,4R,5S)-1-hexyl-2-(hydroxymethyl)piperidine-...) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM18355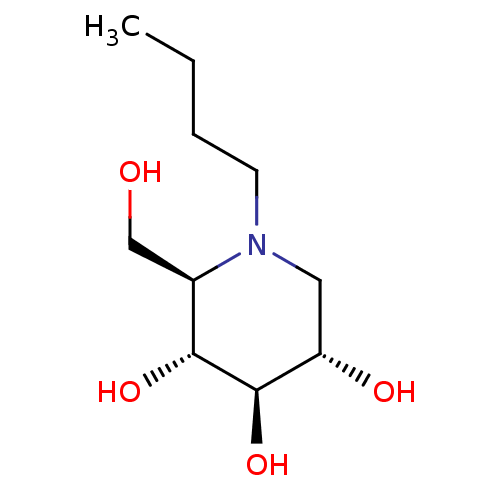 ((2R,3R,4R,5S)-1-butyl-2-(hydroxymethyl)piperidine-...) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50335399 (CHEMBL1651549 | N-Pentyl-1-deoxynojirimycin) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50583384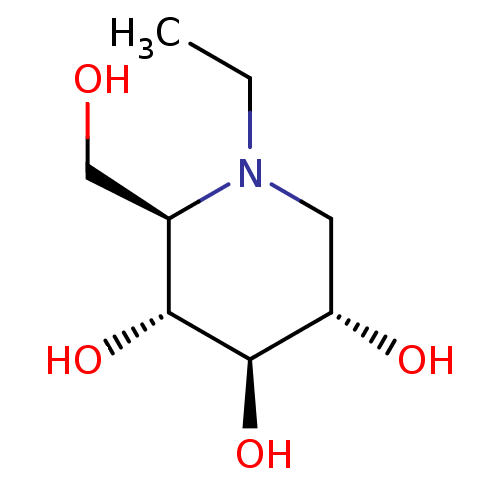 (CHEMBL5080975) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM18354 ((2R,3R,4R,5S)-2-(hydroxymethyl)-1-propylpiperidine...) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50408432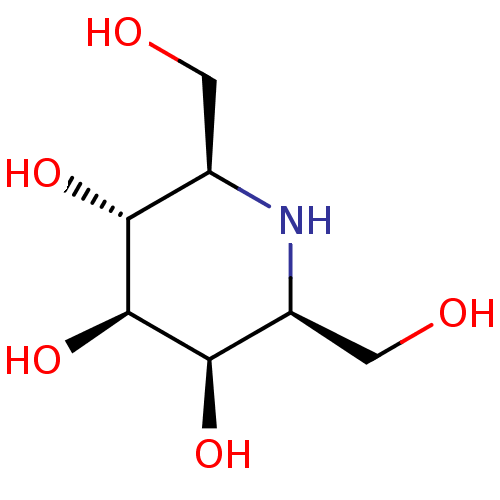 (CHEMBL2115215) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Compound was tested for binding affinity against alpha-glucosidase | J Med Chem 41: 2565-71 (1998) Article DOI: 10.1021/jm970836l BindingDB Entry DOI: 10.7270/Q25D8SHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50242271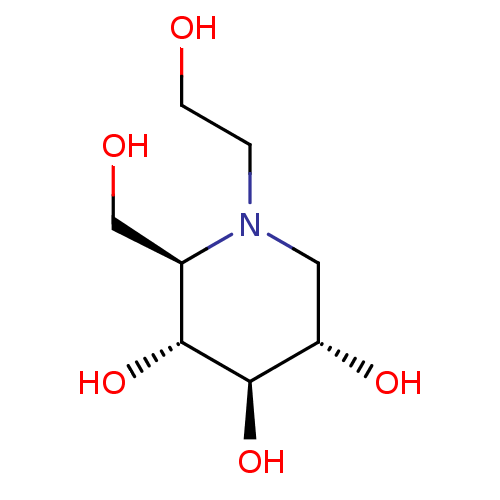 ((2R,3R,4R,5S)-1-(2-hydroxyethyl)-2-(hydroxymethyl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Competitive inhibition of rat intestinal maltase by Lineweaver-Burk plot analysis | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tissue alpha-L-fucosidase (Bos taurus) | BDBM50242268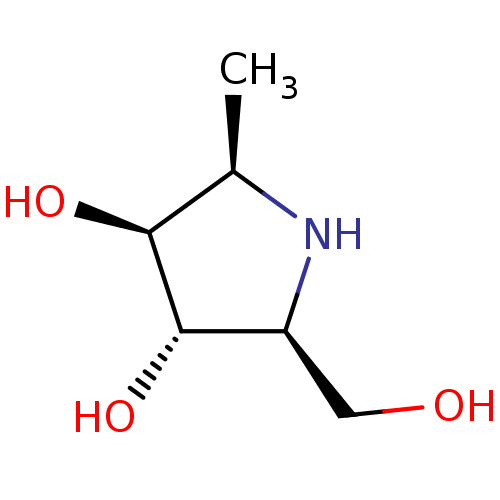 (2,5-Imino-1,2,5-trideoxy-L-glucitol | CHEMBL502230) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Inhibition of bovine epididymis alpha-L-fucosidase assessed as p-nitrophenol release by spectrophotometrically | J Nat Prod 65: 198-202 (2002) BindingDB Entry DOI: 10.7270/Q2ZW1KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-galactosidase A (Homo sapiens (Human)) | BDBM50241865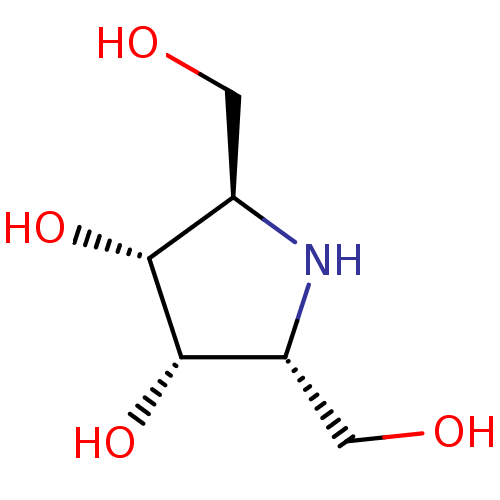 (2,5-dideoxy-2,5-imino-D-altritol | 2R,5R-dihydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Competitive inhibition of human lysosome alpha-galactosidase by Lineweaver-Burk plot analysis | Bioorg Med Chem 18: 3790-4 (2010) Article DOI: 10.1016/j.bmc.2010.04.048 BindingDB Entry DOI: 10.7270/Q2XK8FQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50583385 (CHEMBL5028105) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM36389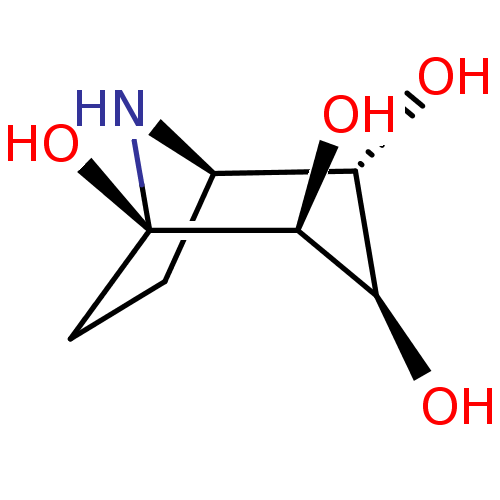 (8-Aza-bicyclo[3.2.1]octane-1,2,3,4-tetraol, 14 | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Competitive inhibition of human lysosomal beta-glucocerebrosidase using 4-methylumbelliferyl-beta-D-glucopyranoside as substrate preincubated for 45 ... | Bioorg Med Chem 22: 2435-41 (2014) Article DOI: 10.1016/j.bmc.2014.02.057 BindingDB Entry DOI: 10.7270/Q24X599T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Homo sapiens (Human)) | BDBM50065259 ((2R,3R,4R,5R)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Inhibitory activity measured against alpha-L-fucosidase of bovine epididymis by colorimetric assay using the D-glucose oxidase-peroxidase method | J Med Chem 41: 2565-71 (1998) Article DOI: 10.1021/jm970836l BindingDB Entry DOI: 10.7270/Q25D8SHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50583378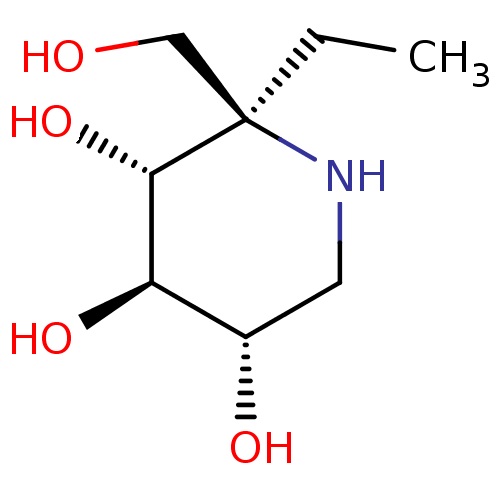 (CHEMBL5028084 | US20230339856, Compound (IIa)) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50408431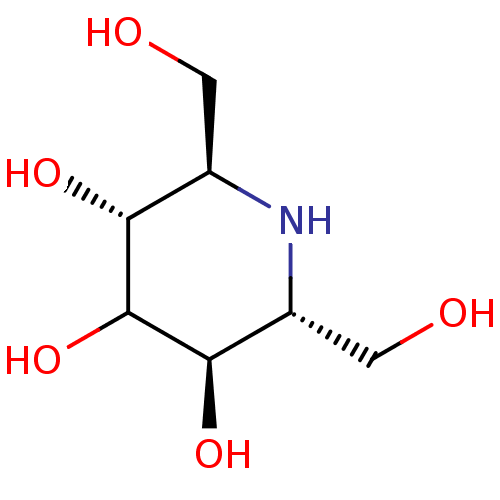 (CHEMBL2114210) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Compound was tested for binding affinity against alpha-glucosidase | J Med Chem 41: 2565-71 (1998) Article DOI: 10.1021/jm970836l BindingDB Entry DOI: 10.7270/Q25D8SHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50002856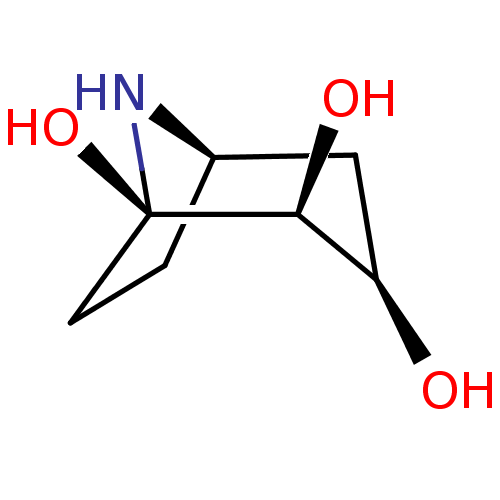 (CHEMBL3233942) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Competitive inhibition of human lysosomal beta-glucocerebrosidase using 4-methylumbelliferyl-beta-D-glucopyranoside as substrate preincubated for 45 ... | Bioorg Med Chem 22: 2435-41 (2014) Article DOI: 10.1016/j.bmc.2014.02.057 BindingDB Entry DOI: 10.7270/Q24X599T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50065255 ((R)-2,6-Bis-hydroxymethyl-piperidine-3,4,5-triol |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Compound was tested for binding affinity against alpha-glucosidase | J Med Chem 41: 2565-71 (1998) Article DOI: 10.1021/jm970836l BindingDB Entry DOI: 10.7270/Q25D8SHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50583377 (CHEMBL5028072) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50002909 (CHEMBL3233945) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Competitive inhibition of human lysosomal beta-glucocerebrosidase using 4-methylumbelliferyl-beta-D-glucopyranoside as substrate preincubated for 45 ... | Bioorg Med Chem 22: 2435-41 (2014) Article DOI: 10.1016/j.bmc.2014.02.057 BindingDB Entry DOI: 10.7270/Q24X599T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50002857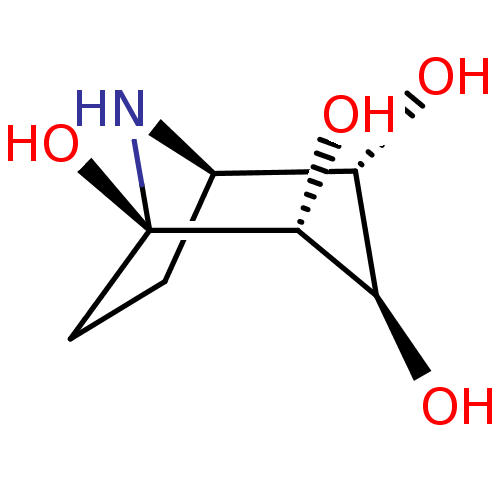 (CHEMBL3233944) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.99E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Competitive inhibition of human lysosomal beta-glucocerebrosidase using 4-methylumbelliferyl-beta-D-glucopyranoside as substrate preincubated for 45 ... | Bioorg Med Chem 22: 2435-41 (2014) Article DOI: 10.1016/j.bmc.2014.02.057 BindingDB Entry DOI: 10.7270/Q24X599T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-galactosidase (Coffea arabica (Coffee beans)) | BDBM50163440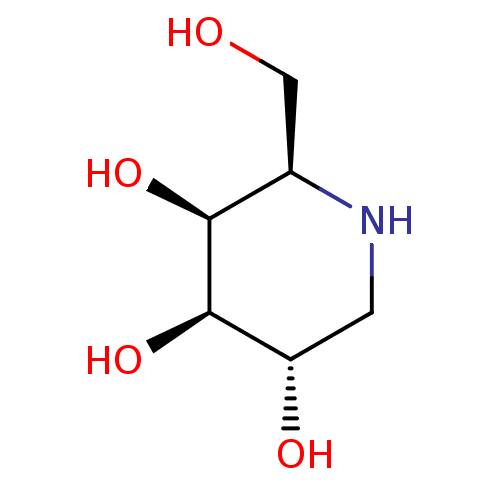 ((2R,3S,4R,5S)-2-(hydroxymethyl)piperidine-3,4,5-tr...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 6.5 | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of coffee bean alpha-galactosidase assessed as p-nitrophenol release at pH 6.5 by spectrometric analysis | Bioorg Med Chem 18: 3790-4 (2010) Article DOI: 10.1016/j.bmc.2010.04.048 BindingDB Entry DOI: 10.7270/Q2XK8FQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50065252 (2,6-Bis-hydroxymethyl-1-methyl-piperidine-3,4,5-tr...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Inhibitory activity measured against alpha-glucosidase of rat intestinal sucrase by colorimetric assay using the D-glucose oxidase-peroxidase method | J Med Chem 41: 2565-71 (1998) Article DOI: 10.1021/jm970836l BindingDB Entry DOI: 10.7270/Q25D8SHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50333455 ((2S,3S,4S,5S)-2-butyl-5-(hydroxymethyl)pyrrolidine...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using sucrose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50259956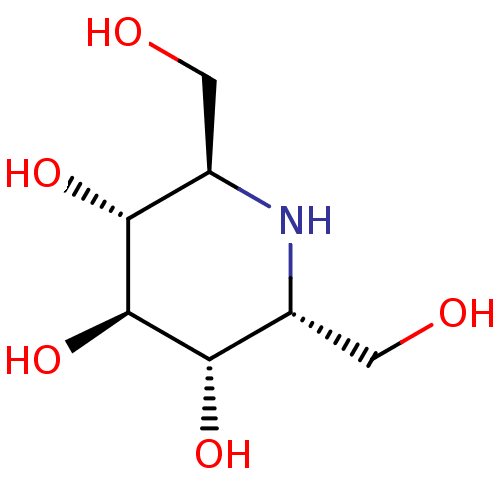 (2,6-Bis-hydroxymethyl-piperidine-3,4,5-triol | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Inhibitory activity measured against alpha-glucosidase of rice by colorimetric assay using the D-glucose oxidase-peroxidase method | J Med Chem 41: 2565-71 (1998) Article DOI: 10.1021/jm970836l BindingDB Entry DOI: 10.7270/Q25D8SHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50182801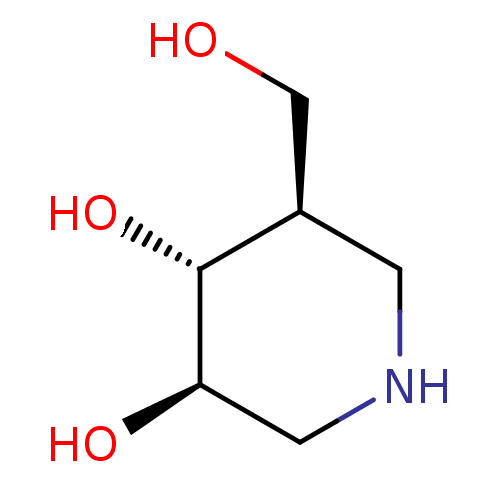 ((3R,4R,5R)-5-(Hydroxymethyl)piperidine-3,4-diol, 8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of human lysosome beta-glucosidase assessed as production of 4-methylumbelliferone using 4-methylumbelliferyl beta-D-glucoside as substrat... | Bioorg Med Chem 19: 3558-68 (2011) Article DOI: 10.1016/j.bmc.2011.04.011 BindingDB Entry DOI: 10.7270/Q2BC3ZWW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Alpha-galactosidase A (Homo sapiens (Human)) | BDBM50163440 ((2R,3S,4R,5S)-2-(hydroxymethyl)piperidine-3,4,5-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of human lysosome alpha-galactosidase assessed as p-nitrophenol release by spectrometric analysis | Bioorg Med Chem 18: 3790-4 (2010) Article DOI: 10.1016/j.bmc.2010.04.048 BindingDB Entry DOI: 10.7270/Q2XK8FQP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50333457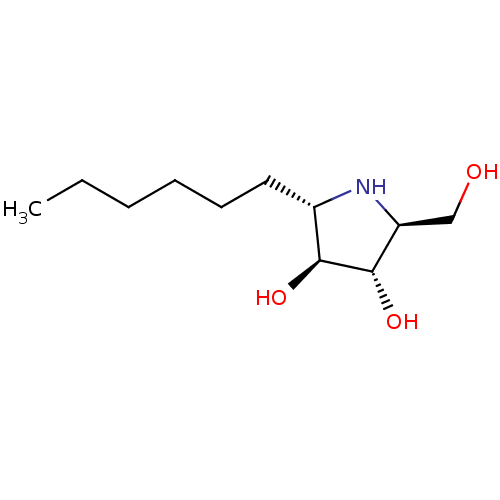 ((2S,3S,4S,5S)-2-hexyl-5-(hydroxymethyl)pyrrolidine...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using sucrose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50263044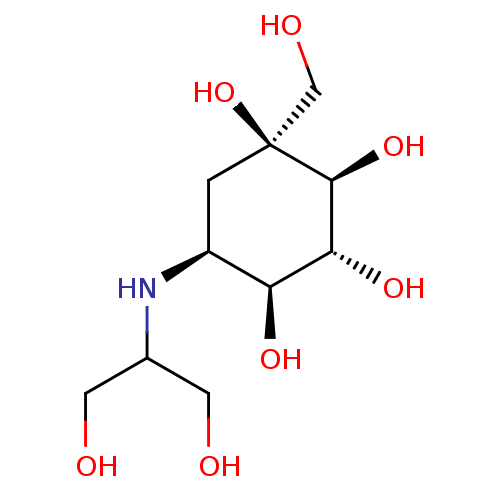 (CHEMBL476960 | Voglibose) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal maltase using moltose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactase/phlorizin hydrolase (Rattus norvegicus) | BDBM50182801 ((3R,4R,5R)-5-(Hydroxymethyl)piperidine-3,4-diol, 8...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal lactase assessed as production of p-nitrophenol by spectrophotometry | Bioorg Med Chem 19: 3558-68 (2011) Article DOI: 10.1016/j.bmc.2011.04.011 BindingDB Entry DOI: 10.7270/Q2BC3ZWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM18363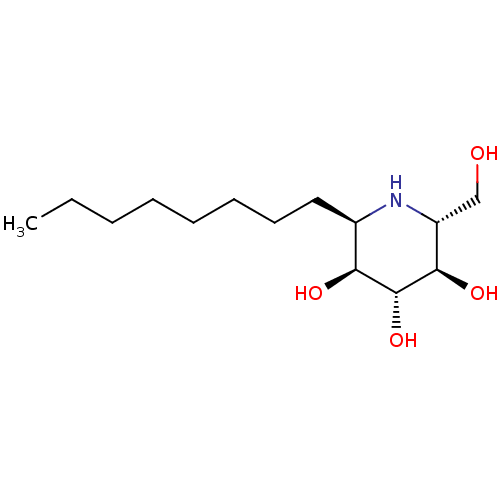 ((2R,3R,4R,5S,6R)-2-(hydroxymethyl)-6-octylpiperidi...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal isomaltase using isomoltose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50333455 ((2S,3S,4S,5S)-2-butyl-5-(hydroxymethyl)pyrrolidine...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal maltase using moltose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Homo sapiens (Human)) | BDBM50065258 ((2S,3R,4S,5R)-2-Methyl-piperidine-3,4,5-triol | (2...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of placental alpha-L-fucosidase (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50259956 (2,6-Bis-hydroxymethyl-piperidine-3,4,5-triol | CHE...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Inhibitory activity measured against alpha-glucosidase of rat intestinal sucrase by colorimetric assay using the D-glucose oxidase-peroxidase method | J Med Chem 41: 2565-71 (1998) Article DOI: 10.1021/jm970836l BindingDB Entry DOI: 10.7270/Q25D8SHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50333465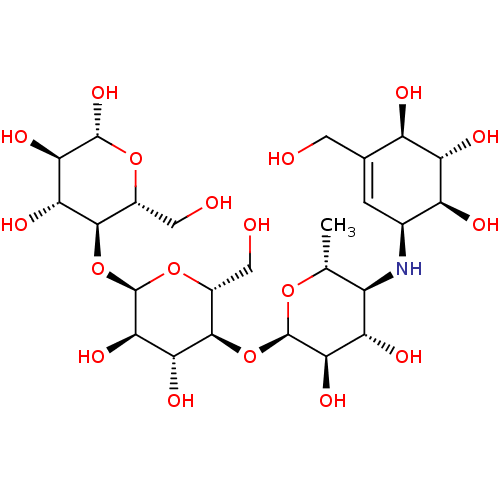 ((2R,3R,4R,5R,6R)-5-((2R,3R,4R,5S,6R)-5-((2R,3R,4S,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal maltase using moltose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50333456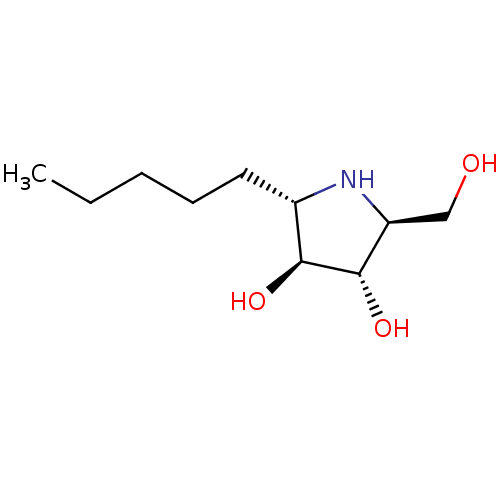 ((2S,3S,4S,5S)-2-(hydroxymethyl)-5-pentylpyrrolidin...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using sucrose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50399654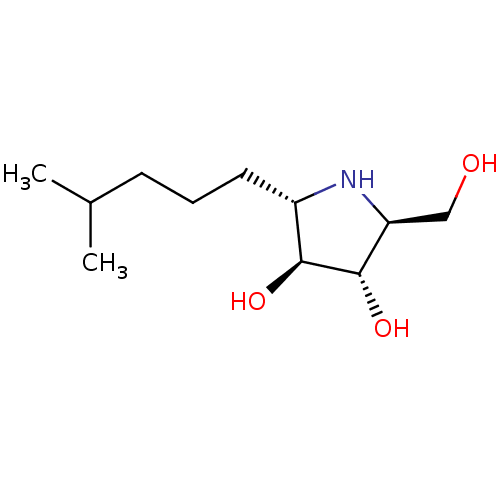 (CHEMBL2177691) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal maltase using moltose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using sucrose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Rattus norvegicus) | BDBM50399655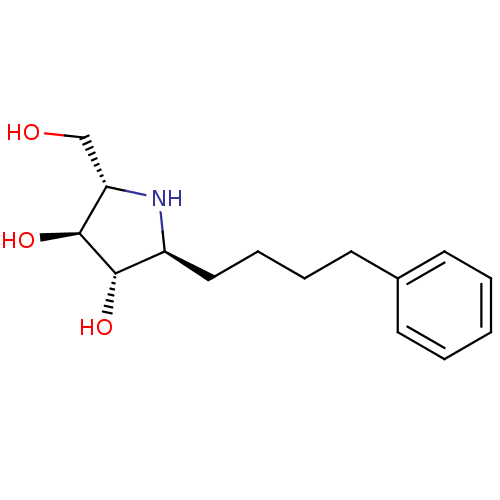 (CHEMBL2177690) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toyama Curated by ChEMBL | Assay Description Inhibition of rat intestinal maltase using moltose as substrate | J Med Chem 55: 10347-62 (2012) Article DOI: 10.1021/jm301304e BindingDB Entry DOI: 10.7270/Q2K35VTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 362 total ) | Next | Last >> |