| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 2C9 |
|---|
| Ligand | BDBM50186624 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_353249 (CHEMBL865088) |
|---|
| IC50 | >30000±n/a nM |
|---|
| Citation |  Morwick, T; Berry, A; Brickwood, J; Cardozo, M; Catron, K; DeTuri, M; Emeigh, J; Homon, C; Hrapchak, M; Jacober, S; Jakes, S; Kaplita, P; Kelly, TA; Ksiazek, J; Liuzzi, M; Magolda, R; Mao, C; Marshall, D; McNeil, D; Prokopowicz, A; Sarko, C; Scouten, E; Sledziona, C; Sun, S; Watrous, J; Wu, JP; Cywin, CL Evolution of the thienopyridine class of inhibitors of IkappaB kinase-beta: part I: hit-to-lead strategies. J Med Chem49:2898-908 (2006) [PubMed] Article Morwick, T; Berry, A; Brickwood, J; Cardozo, M; Catron, K; DeTuri, M; Emeigh, J; Homon, C; Hrapchak, M; Jacober, S; Jakes, S; Kaplita, P; Kelly, TA; Ksiazek, J; Liuzzi, M; Magolda, R; Mao, C; Marshall, D; McNeil, D; Prokopowicz, A; Sarko, C; Scouten, E; Sledziona, C; Sun, S; Watrous, J; Wu, JP; Cywin, CL Evolution of the thienopyridine class of inhibitors of IkappaB kinase-beta: part I: hit-to-lead strategies. J Med Chem49:2898-908 (2006) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 2C9 |
|---|
| Name: | Cytochrome P450 2C9 |
|---|
| Synonyms: | (R)-limonene 6-monooxygenase | (S)-limonene 6-monooxygenase | CP2C9_HUMAN | CYP2C10 | CYP2C9 | CYPIIC9 | Cytochrome P450 2C9 (CYP2C9 ) | Cytochrome P450 2C9 (CYP2C9) | P-450MP | P450 MP-4/MP-8 | P450 PB-1 | S-mephenytoin 4-hydroxylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 55636.33 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P11712 |
|---|
| Residue: | 490 |
|---|
| Sequence: | MDSLVVLVLCLSCLLLLSLWRQSSGRGKLPPGPTPLPVIGNILQIGIKDISKSLTNLSKV
YGPVFTLYFGLKPIVVLHGYEAVKEALIDLGEEFSGRGIFPLAERANRGFGIVFSNGKKW
KEIRRFSLMTLRNFGMGKRSIEDRVQEEARCLVEELRKTKASPCDPTFILGCAPCNVICS
IIFHKRFDYKDQQFLNLMEKLNENIKILSSPWIQICNNFSPIIDYFPGTHNKLLKNVAFM
KSYILEKVKEHQESMDMNNPQDFIDCFLMKMEKEKHNQPSEFTIESLENTAVDLFGAGTE
TTSTTLRYALLLLLKHPEVTAKVQEEIERVIGRNRSPCMQDRSHMPYTDAVVHEVQRYID
LLPTSLPHAVTCDIKFRNYLIPKGTTILISLTSVLHDNKEFPNPEMFDPHHFLDEGGNFK
KSKYFMPFSAGKRICVGEALAGMELFLFLTSILQNFNLKSLVDPKNLDTTPVVNGFASVP
PFYQLCFIPV
|
|
|
|---|
| BDBM50186624 |
|---|
| n/a |
|---|
| Name | BDBM50186624 |
|---|
| Synonyms: | 3-amino-4,6-dimethylthieno[2,3-b]pyridine-2-carboxamide | CHEMBL377085 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C10H11N3OS |
|---|
| Mol. Mass. | 221.279 |
|---|
| SMILES | Cc1cc(C)c2c(N)c(sc2n1)C(N)=O |
|---|
| Structure | 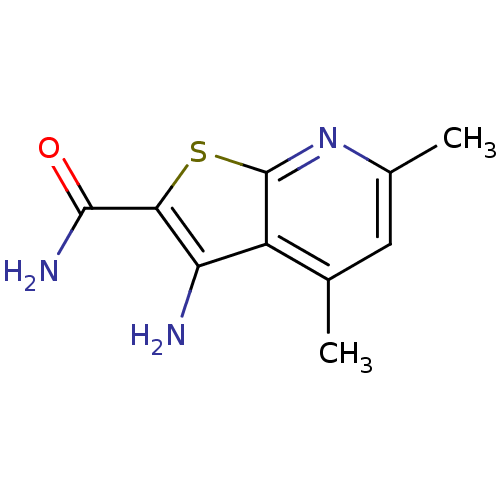
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Morwick, T; Berry, A; Brickwood, J; Cardozo, M; Catron, K; DeTuri, M; Emeigh, J; Homon, C; Hrapchak, M; Jacober, S; Jakes, S; Kaplita, P; Kelly, TA; Ksiazek, J; Liuzzi, M; Magolda, R; Mao, C; Marshall, D; McNeil, D; Prokopowicz, A; Sarko, C; Scouten, E; Sledziona, C; Sun, S; Watrous, J; Wu, JP; Cywin, CL Evolution of the thienopyridine class of inhibitors of IkappaB kinase-beta: part I: hit-to-lead strategies. J Med Chem49:2898-908 (2006) [PubMed] Article
Morwick, T; Berry, A; Brickwood, J; Cardozo, M; Catron, K; DeTuri, M; Emeigh, J; Homon, C; Hrapchak, M; Jacober, S; Jakes, S; Kaplita, P; Kelly, TA; Ksiazek, J; Liuzzi, M; Magolda, R; Mao, C; Marshall, D; McNeil, D; Prokopowicz, A; Sarko, C; Scouten, E; Sledziona, C; Sun, S; Watrous, J; Wu, JP; Cywin, CL Evolution of the thienopyridine class of inhibitors of IkappaB kinase-beta: part I: hit-to-lead strategies. J Med Chem49:2898-908 (2006) [PubMed] Article