 Found 444 hits with Last Name = 'prokopowicz' and Initial = 'a'
Found 444 hits with Last Name = 'prokopowicz' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50126732

(2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...)Show SMILES CCN(CC)CC=Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1C |w:6.5| Show InChI InChI=1S/C25H27Cl2N5O/c1-5-32(6-2)14-8-11-19-15(3)16-12-13-20-23(21(16)24(33)28-19)31(4)25(29-20)30-22-17(26)9-7-10-18(22)27/h7-13H,5-6,14H2,1-4H3,(H,28,33)(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Src protein tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50126732

(2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...)Show SMILES CCN(CC)CC=Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1C |w:6.5| Show InChI InChI=1S/C25H27Cl2N5O/c1-5-32(6-2)14-8-11-19-15(3)16-12-13-20-23(21(16)24(33)28-19)31(4)25(29-20)30-22-17(26)9-7-10-18(22)27/h7-13H,5-6,14H2,1-4H3,(H,28,33)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Brutons tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126732

(2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...)Show SMILES CCN(CC)CC=Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1C |w:6.5| Show InChI InChI=1S/C25H27Cl2N5O/c1-5-32(6-2)14-8-11-19-15(3)16-12-13-20-23(21(16)24(33)28-19)31(4)25(29-20)30-22-17(26)9-7-10-18(22)27/h7-13H,5-6,14H2,1-4H3,(H,28,33)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126732

(2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...)Show SMILES CCN(CC)CC=Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1C |w:6.5| Show InChI InChI=1S/C25H27Cl2N5O/c1-5-32(6-2)14-8-11-19-15(3)16-12-13-20-23(21(16)24(33)28-19)31(4)25(29-20)30-22-17(26)9-7-10-18(22)27/h7-13H,5-6,14H2,1-4H3,(H,28,33)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126751
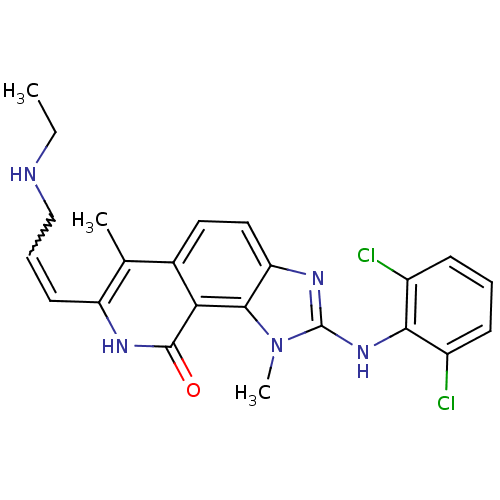
(2-(2,6-Dichloro-phenylamino)-7-(3-ethylamino-prope...)Show SMILES CCNCC=Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1C |w:4.3| Show InChI InChI=1S/C23H23Cl2N5O/c1-4-26-12-6-9-17-13(2)14-10-11-18-21(19(14)22(31)27-17)30(3)23(28-18)29-20-15(24)7-5-8-16(20)25/h5-11,26H,4,12H2,1-3H3,(H,27,31)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50126732

(2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...)Show SMILES CCN(CC)CC=Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1C |w:6.5| Show InChI InChI=1S/C25H27Cl2N5O/c1-5-32(6-2)14-8-11-19-15(3)16-12-13-20-23(21(16)24(33)28-19)31(4)25(29-20)30-22-17(26)9-7-10-18(22)27/h7-13H,5-6,14H2,1-4H3,(H,28,33)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Protein tyrosine kinase Lyn |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126735
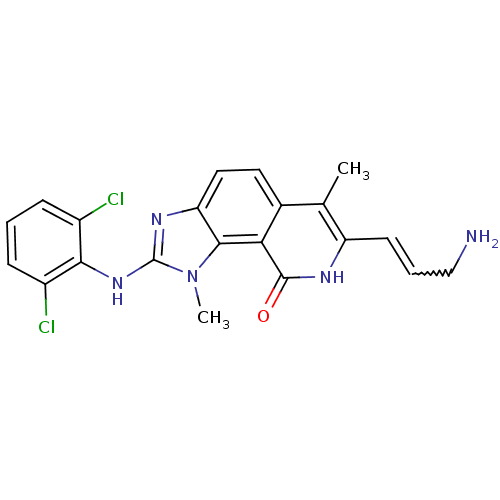
(7-(3-Amino-propenyl)-2-(2,6-dichloro-phenylamino)-...)Show SMILES Cc1c(C=CCN)[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc12 |w:4.4| Show InChI InChI=1S/C21H19Cl2N5O/c1-11-12-8-9-16-19(17(12)20(29)25-15(11)7-4-10-24)28(2)21(26-16)27-18-13(22)5-3-6-14(18)23/h3-9H,10,24H2,1-2H3,(H,25,29)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126739
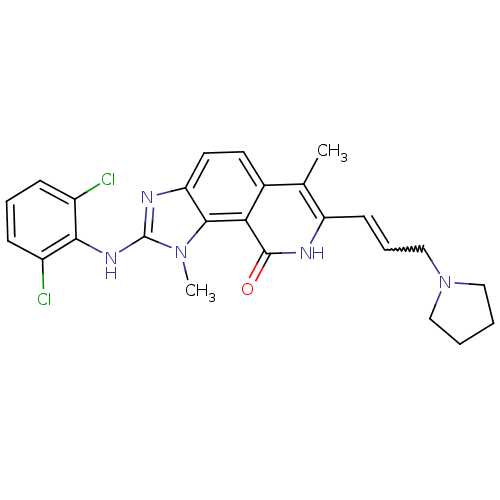
(2-(2,6-Dichloro-phenylamino)-1,6-dimethyl-7-(3-pyr...)Show SMILES Cc1c(C=CCN2CCCC2)[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc12 |w:4.4| Show InChI InChI=1S/C25H25Cl2N5O/c1-15-16-10-11-20-23(31(2)25(29-20)30-22-17(26)7-5-8-18(22)27)21(16)24(33)28-19(15)9-6-14-32-12-3-4-13-32/h5-11H,3-4,12-14H2,1-2H3,(H,28,33)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196941
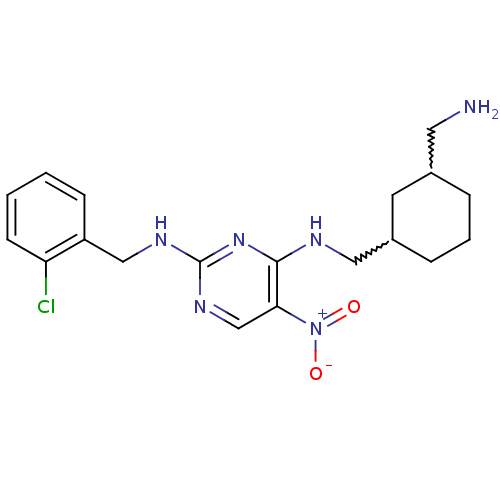
(CHEMBL246365 | N2-(2-chlorobenzyl)-N4-((3-(aminome...)Show SMILES NCC1CCCC(CNc2nc(NCc3ccccc3Cl)ncc2[N+]([O-])=O)C1 |w:6.6,2.1| Show InChI InChI=1S/C19H25ClN6O2/c20-16-7-2-1-6-15(16)11-23-19-24-12-17(26(27)28)18(25-19)22-10-14-5-3-4-13(8-14)9-21/h1-2,6-7,12-14H,3-5,8-11,21H2,(H2,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126746
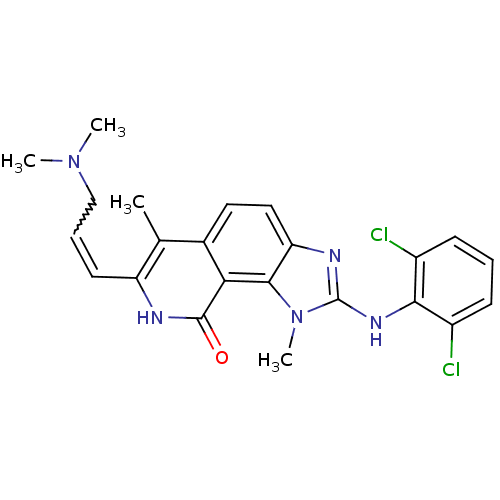
(2-(2,6-Dichloro-phenylamino)-7-(3-dimethylamino-pr...)Show SMILES CN(C)CC=Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1C |w:4.3| Show InChI InChI=1S/C23H23Cl2N5O/c1-13-14-10-11-18-21(19(14)22(31)26-17(13)9-6-12-29(2)3)30(4)23(27-18)28-20-15(24)7-5-8-16(20)25/h5-11H,12H2,1-4H3,(H,26,31)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126749
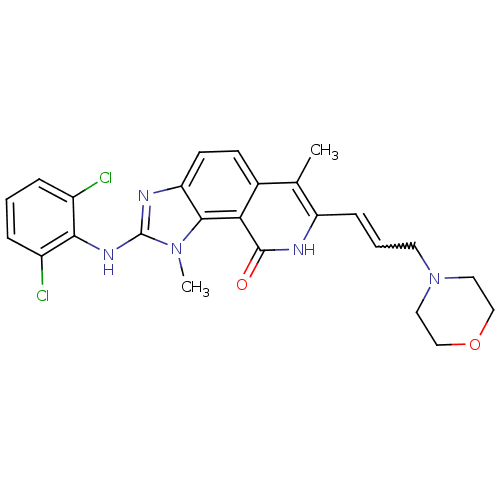
(2-(2,6-Dichloro-phenylamino)-1,6-dimethyl-7-(3-mor...)Show SMILES Cc1c(C=CCN2CCOCC2)[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc12 |w:4.4| Show InChI InChI=1S/C25H25Cl2N5O2/c1-15-16-8-9-20-23(31(2)25(29-20)30-22-17(26)5-3-6-18(22)27)21(16)24(33)28-19(15)7-4-10-32-11-13-34-14-12-32/h3-9H,10-14H2,1-2H3,(H,28,33)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196932

(CHEMBL392642 | N2-(2-(methylthio)benzyl)-N4-((4-(a...)Show SMILES CSc1ccccc1CNc1ncc(c(NCC2CCC(CN)CC2)n1)[N+]([O-])=O |(25.65,-13.62,;25.64,-12.08,;24.31,-11.32,;22.98,-12.09,;21.64,-11.32,;21.64,-9.78,;22.97,-9.01,;24.3,-9.79,;25.64,-9.02,;26.97,-9.79,;28.31,-9.02,;28.31,-7.47,;29.64,-6.7,;30.97,-7.47,;30.98,-9.02,;32.31,-9.79,;32.32,-11.33,;33.65,-12.09,;33.64,-13.63,;34.97,-14.4,;36.31,-13.63,;37.64,-14.4,;37.64,-15.94,;36.31,-12.09,;34.97,-11.31,;29.64,-9.79,;32.31,-6.69,;33.64,-7.45,;32.3,-5.15,)| Show InChI InChI=1S/C20H28N6O2S/c1-29-18-5-3-2-4-16(18)12-23-20-24-13-17(26(27)28)19(25-20)22-11-15-8-6-14(10-21)7-9-15/h2-5,13-15H,6-12,21H2,1H3,(H2,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196957
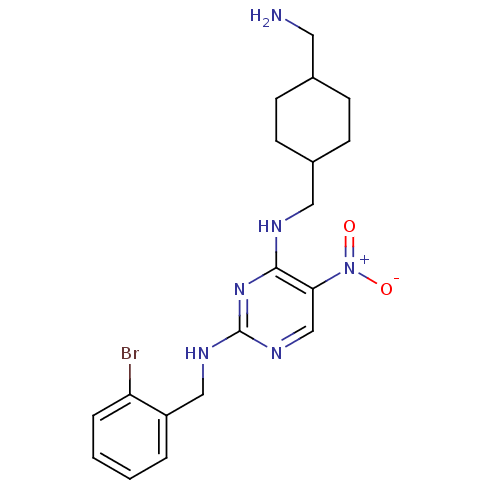
(CHEMBL246175 | N2-(2-bromobenzyl)-N4-((4-(aminomet...)Show SMILES NCC1CCC(CNc2nc(NCc3ccccc3Br)ncc2[N+]([O-])=O)CC1 |(5.93,-24.27,;5.93,-22.73,;4.6,-21.95,;3.26,-22.72,;1.93,-21.95,;1.94,-20.42,;.61,-19.65,;.6,-18.11,;-.73,-17.34,;-2.07,-18.11,;-3.4,-17.34,;-4.74,-18.11,;-6.07,-17.34,;-7.41,-18.11,;-8.74,-17.33,;-10.07,-18.1,;-10.07,-19.64,;-8.73,-20.41,;-7.4,-19.64,;-6.07,-20.41,;-3.4,-15.8,;-2.07,-15.03,;-.74,-15.79,;.6,-15.01,;1.93,-15.78,;.59,-13.47,;3.26,-19.63,;4.6,-20.41,)| Show InChI InChI=1S/C19H25BrN6O2/c20-16-4-2-1-3-15(16)11-23-19-24-12-17(26(27)28)18(25-19)22-10-14-7-5-13(9-21)6-8-14/h1-4,12-14H,5-11,21H2,(H2,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196961
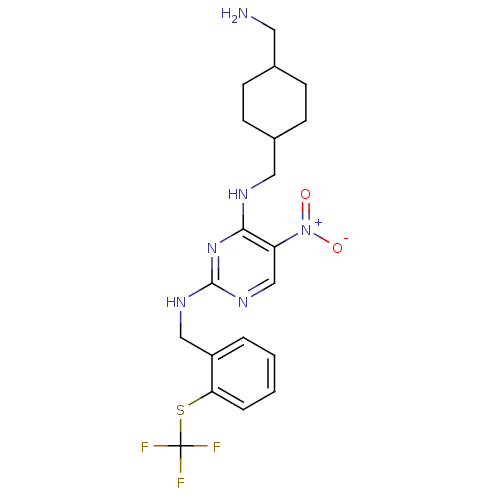
(CHEMBL246174 | N2-(2-(trifluoromethylthio)benzyl)-...)Show SMILES NCC1CCC(CNc2nc(NCc3ccccc3SC(F)(F)F)ncc2[N+]([O-])=O)CC1 |(21.67,-14.36,;21.67,-12.82,;20.34,-12.05,;19,-12.82,;17.68,-12.05,;17.68,-10.51,;16.35,-9.75,;16.35,-8.21,;15.01,-7.44,;13.67,-8.21,;12.34,-7.44,;11,-8.21,;9.67,-7.44,;8.34,-8.21,;7.01,-7.43,;5.67,-8.2,;5.67,-9.74,;7.01,-10.51,;8.34,-9.74,;9.68,-10.51,;9.68,-12.05,;9.67,-13.58,;11.22,-12.05,;8.14,-12.05,;12.34,-5.9,;13.67,-5.12,;15,-5.89,;16.34,-5.11,;17.68,-5.88,;16.33,-3.57,;19.01,-9.73,;20.34,-10.51,)| Show InChI InChI=1S/C20H25F3N6O2S/c21-20(22,23)32-17-4-2-1-3-15(17)11-26-19-27-12-16(29(30)31)18(28-19)25-10-14-7-5-13(9-24)6-8-14/h1-4,12-14H,5-11,24H2,(H2,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196936
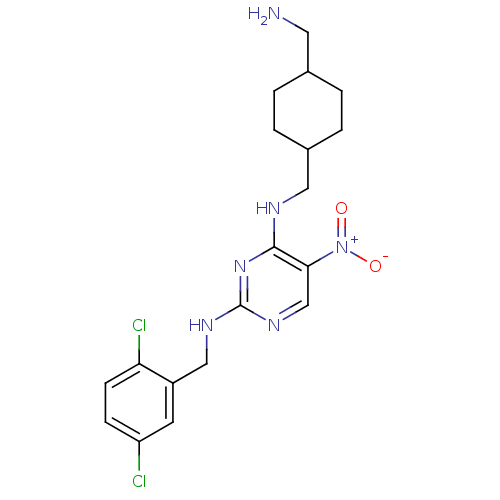
(CHEMBL246386 | N2-(2,5-dichlorobenzyl)-N4-((4-(ami...)Show SMILES NCC1CCC(CNc2nc(NCc3cc(Cl)ccc3Cl)ncc2[N+]([O-])=O)CC1 |(6.39,-4.5,;6.4,-2.96,;5.07,-2.19,;3.73,-2.95,;2.4,-2.19,;2.41,-.65,;1.07,.12,;1.07,1.66,;-.27,2.42,;-1.6,1.65,;-2.94,2.42,;-4.27,1.65,;-5.6,2.42,;-6.94,1.66,;-8.27,2.43,;-9.6,1.66,;-10.94,2.43,;-9.61,.12,;-8.26,-.65,;-6.94,.12,;-5.6,-.64,;-2.93,3.97,;-1.61,4.74,;-.27,3.97,;1.06,4.75,;2.4,3.99,;1.06,6.29,;3.73,.13,;5.07,-.65,)| Show InChI InChI=1S/C19H24Cl2N6O2/c20-15-5-6-16(21)14(7-15)10-24-19-25-11-17(27(28)29)18(26-19)23-9-13-3-1-12(8-22)2-4-13/h5-7,11-13H,1-4,8-10,22H2,(H2,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50325446
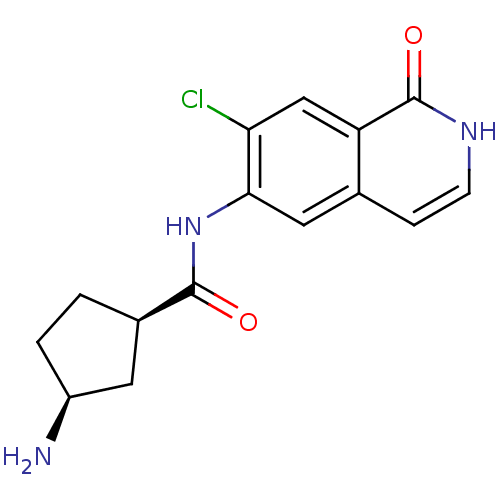
(CHEMBL1222573 | cis-3-amino-N-(7-chloro-1-oxo-1,2-...)Show SMILES N[C@H]1CC[C@H](C1)C(=O)Nc1cc2cc[nH]c(=O)c2cc1Cl |r| Show InChI InChI=1S/C15H16ClN3O2/c16-12-7-11-8(3-4-18-15(11)21)6-13(12)19-14(20)9-1-2-10(17)5-9/h3-4,6-7,9-10H,1-2,5,17H2,(H,18,21)(H,19,20)/t9-,10+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 |
Bioorg Med Chem Lett 20: 5153-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.014
BindingDB Entry DOI: 10.7270/Q2RR1ZDC |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196978

(CHEMBL246183 | N2-(2,3-dichlorobenzyl)-N4-((4-(ami...)Show SMILES NCC1CCC(CNc2nc(NCc3cccc(Cl)c3Cl)ncc2[N+]([O-])=O)CC1 |(21.13,-47,;21.14,-45.46,;19.8,-44.69,;18.47,-45.45,;17.14,-44.68,;17.15,-43.15,;15.81,-42.38,;15.81,-40.84,;14.47,-40.07,;13.14,-40.85,;11.8,-40.08,;10.47,-40.84,;9.13,-40.07,;7.8,-40.84,;6.47,-40.07,;5.14,-40.83,;5.13,-42.38,;6.48,-43.15,;6.48,-44.69,;7.8,-42.38,;9.14,-43.14,;11.8,-38.53,;13.13,-37.76,;14.47,-38.52,;15.8,-37.75,;17.14,-38.51,;15.8,-36.21,;18.47,-42.37,;19.81,-43.14,)| Show InChI InChI=1S/C19H24Cl2N6O2/c20-15-3-1-2-14(17(15)21)10-24-19-25-11-16(27(28)29)18(26-19)23-9-13-6-4-12(8-22)5-7-13/h1-3,11-13H,4-10,22H2,(H2,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50126413
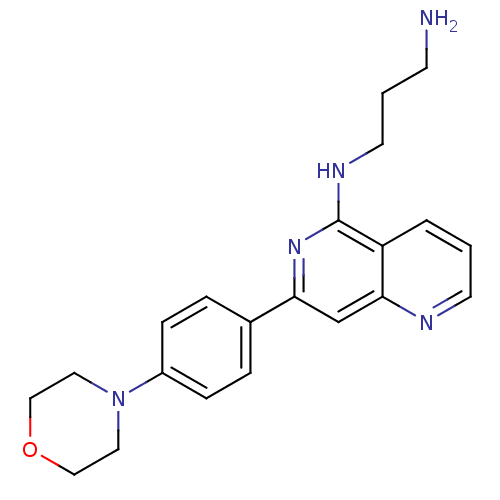
(CHEMBL287478 | N*1*-[7-(4-Morpholin-4-yl-phenyl)-[...)Show InChI InChI=1S/C21H25N5O/c22-8-2-10-24-21-18-3-1-9-23-20(18)15-19(25-21)16-4-6-17(7-5-16)26-11-13-27-14-12-26/h1,3-7,9,15H,2,8,10-14,22H2,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against spleen tyrosine kinase (SYK) |
Bioorg Med Chem Lett 13: 1415-8 (2003)
BindingDB Entry DOI: 10.7270/Q2MC8ZCJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126752
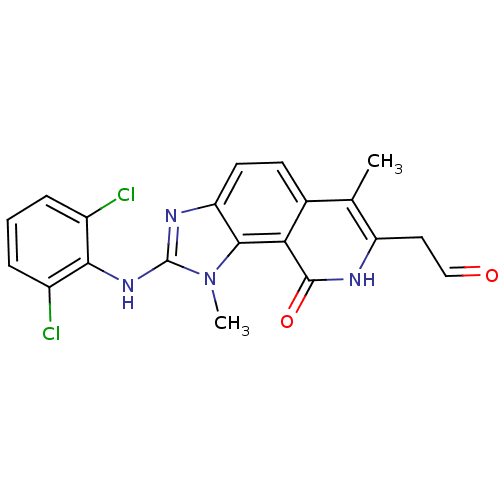
(2-(2,6-Dichloro-phenylamino)-7-(2-hydroxy-vinyl)-1...)Show SMILES Cc1c(CC=O)[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc12 Show InChI InChI=1S/C20H16Cl2N4O2/c1-10-11-6-7-15-18(16(11)19(28)23-14(10)8-9-27)26(2)20(24-15)25-17-12(21)4-3-5-13(17)22/h3-7,9H,8H2,1-2H3,(H,23,28)(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126743
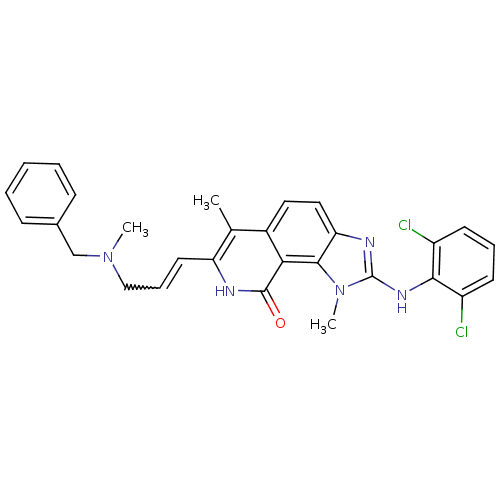
(7-[3-(Benzyl-methyl-amino)-propenyl]-2-(2,6-dichlo...)Show SMILES CN(CC=Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1C)Cc1ccccc1 |w:3.2| Show InChI InChI=1S/C29H27Cl2N5O/c1-18-20-14-15-24-27(36(3)29(33-24)34-26-21(30)11-7-12-22(26)31)25(20)28(37)32-23(18)13-8-16-35(2)17-19-9-5-4-6-10-19/h4-15H,16-17H2,1-3H3,(H,32,37)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-2
(Homo sapiens (Human)) | BDBM28404

((2E)-3-{3-[6-(1,4-diazepan-1-yl)pyrazin-2-yl]pheny...)Show InChI InChI=1S/C18H20N4O2/c23-18(24)6-5-14-3-1-4-15(11-14)16-12-20-13-17(21-16)22-9-2-7-19-8-10-22/h1,3-6,11-13,19H,2,7-10H2,(H,23,24)/b6-5+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Boehringer Ingelheim Pharmaceuticals Inc.
| Assay Description
The activity of Pim kinase was measured in a homogeneous luciferase assay using GST-Pim, biotinylated peptide substrate and a luciferin-luciferase de... |
J Med Chem 52: 1814-27 (2009)
Article DOI: 10.1021/jm801242y
BindingDB Entry DOI: 10.7270/Q25T3HS3 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196959

(CHEMBL396929 | N2-(2-nitrobenzyl)-N4-((4-(aminomet...)Show SMILES NCC1CCC(CNc2nc(NCc3ccccc3[N+]([O-])=O)ncc2[N+]([O-])=O)CC1 |(36.8,-26.77,;36.8,-25.23,;35.47,-24.46,;34.13,-25.22,;32.8,-24.45,;32.81,-22.92,;31.48,-22.15,;31.47,-20.61,;30.14,-19.84,;28.8,-20.62,;27.47,-19.85,;26.13,-20.61,;24.8,-19.84,;23.46,-20.61,;22.13,-19.84,;20.8,-20.6,;20.8,-22.15,;22.14,-22.92,;23.47,-22.15,;24.8,-22.91,;24.81,-24.46,;26.14,-22.14,;27.47,-18.3,;28.8,-17.53,;30.13,-18.29,;31.47,-17.52,;32.8,-18.28,;31.46,-15.98,;34.13,-22.14,;35.47,-22.91,)| Show InChI InChI=1S/C19H25N7O4/c20-9-13-5-7-14(8-6-13)10-21-18-17(26(29)30)12-23-19(24-18)22-11-15-3-1-2-4-16(15)25(27)28/h1-4,12-14H,5-11,20H2,(H2,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50325431

((S)-2-amino-N-(7-chloro-1-oxo-1,2-dihydroisoquinol...)Show SMILES CC(C)[C@H](N)C(=O)Nc1cc2cc[nH]c(=O)c2cc1Cl |r| Show InChI InChI=1S/C14H16ClN3O2/c1-7(2)12(16)14(20)18-11-5-8-3-4-17-13(19)9(8)6-10(11)15/h3-7,12H,16H2,1-2H3,(H,17,19)(H,18,20)/t12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 |
Bioorg Med Chem Lett 20: 5153-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.014
BindingDB Entry DOI: 10.7270/Q2RR1ZDC |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50319634
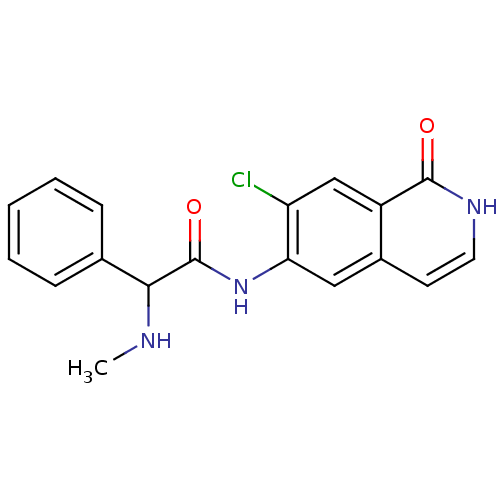
(CHEMBL1084892 | N-(7-chloro-1-oxo-1,2-dihydroisoqu...)Show InChI InChI=1S/C18H16ClN3O2/c1-20-16(11-5-3-2-4-6-11)18(24)22-15-9-12-7-8-21-17(23)13(12)10-14(15)19/h2-10,16,20H,1H3,(H,21,23)(H,22,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 |
Bioorg Med Chem Lett 20: 3235-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.070
BindingDB Entry DOI: 10.7270/Q21N819Z |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-2
(Homo sapiens (Human)) | BDBM28397
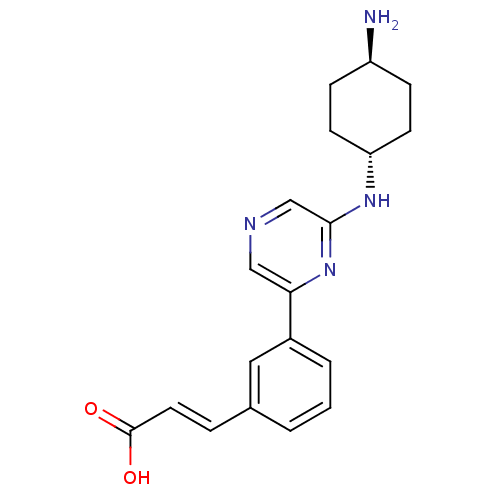
((2E)-3-(3-{6-[(4-aminocyclohexyl)amino]pyrazin-2-y...)Show SMILES N[C@H]1CC[C@@H](CC1)Nc1cncc(n1)-c1cccc(\C=C\C(O)=O)c1 |r,wU:4.7,wD:1.0,(-2.78,-3.68,;-2.78,-2.14,;-1.45,-1.37,;-1.45,.17,;-2.78,.94,;-4.12,.17,;-4.12,-1.37,;-2.78,2.48,;-1.45,3.25,;-1.45,4.8,;-.12,5.57,;1.22,4.8,;1.22,3.25,;-.12,2.48,;2.55,2.48,;3.88,3.26,;5.22,2.48,;5.22,.94,;3.88,.17,;3.88,-1.37,;5.22,-2.14,;5.22,-3.68,;6.55,-4.45,;3.88,-4.45,;2.55,.94,)| Show InChI InChI=1S/C19H22N4O2/c20-15-5-7-16(8-6-15)22-18-12-21-11-17(23-18)14-3-1-2-13(10-14)4-9-19(24)25/h1-4,9-12,15-16H,5-8,20H2,(H,22,23)(H,24,25)/b9-4+/t15-,16- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
| Assay Description
The activity of Pim kinase was measured in a homogeneous luciferase assay using GST-Pim, biotinylated peptide substrate and a luciferin-luciferase de... |
J Med Chem 52: 1814-27 (2009)
Article DOI: 10.1021/jm801242y
BindingDB Entry DOI: 10.7270/Q25T3HS3 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196951
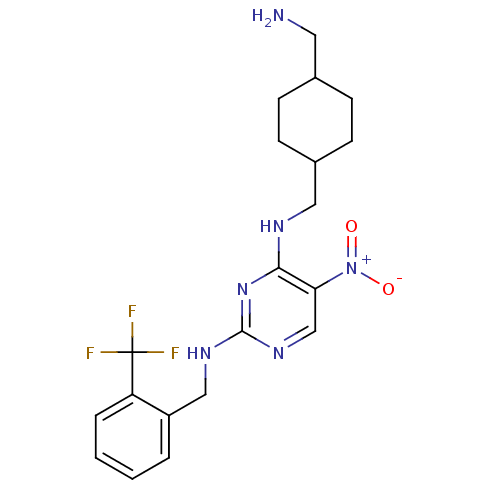
(CHEMBL247979 | N2-(2-(trifluoromethyl)benzyl)-N4-(...)Show SMILES NCC1CCC(CNc2nc(NCc3ccccc3C(F)(F)F)ncc2[N+]([O-])=O)CC1 |(20.15,-4.22,;20.15,-2.68,;18.82,-1.91,;17.48,-2.67,;16.15,-1.91,;16.16,-.37,;14.82,.4,;14.82,1.94,;13.49,2.7,;12.15,1.93,;10.81,2.7,;9.48,1.93,;8.15,2.7,;6.81,1.94,;5.48,2.71,;4.15,1.94,;4.15,.4,;5.49,-.37,;6.82,.4,;8.15,-.36,;9.48,-1.13,;8.92,.97,;7.39,-1.7,;10.82,4.25,;12.15,5.02,;13.48,4.25,;14.82,5.03,;16.15,4.27,;14.81,6.57,;17.48,.41,;18.82,-.37,)| Show InChI InChI=1S/C20H25F3N6O2/c21-20(22,23)16-4-2-1-3-15(16)11-26-19-27-12-17(29(30)31)18(28-19)25-10-14-7-5-13(9-24)6-8-14/h1-4,12-14H,5-11,24H2,(H2,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50116391
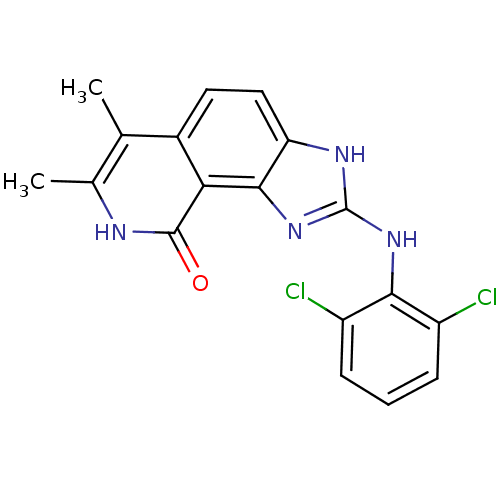
(2-(2,6-Dichloro-phenylamino)-6,7-dimethyl-1,8-dihy...)Show SMILES Cc1[nH]c(=O)c2c3nc(Nc4c(Cl)cccc4Cl)[nH]c3ccc2c1C Show InChI InChI=1S/C18H14Cl2N4O/c1-8-9(2)21-17(25)14-10(8)6-7-13-16(14)24-18(22-13)23-15-11(19)4-3-5-12(15)20/h3-7H,1-2H3,(H,21,25)(H2,22,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of c-SRC with 1 uM ATP |
J Med Chem 45: 3394-405 (2002)
BindingDB Entry DOI: 10.7270/Q2CJ8CTW |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196987
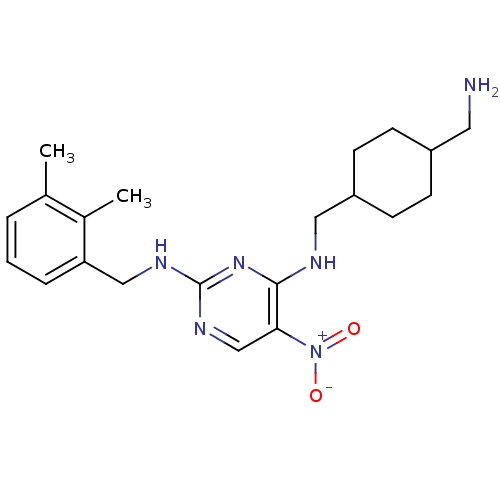
(CHEMBL245912 | N2-(2,3-dimethylbenzyl)-N4-((4-(ami...)Show SMILES Cc1cccc(CNc2ncc(c(NCC3CCC(CN)CC3)n2)[N+]([O-])=O)c1C |(20.87,-.91,;20.86,.63,;19.52,1.4,;19.52,2.95,;20.86,3.71,;22.19,2.94,;23.52,3.71,;24.86,2.94,;26.19,3.71,;26.19,5.25,;27.52,6.02,;28.86,5.26,;28.86,3.71,;30.2,2.94,;30.2,1.4,;31.53,.63,;31.53,-.9,;32.86,-1.67,;34.19,-.91,;35.53,-1.68,;35.52,-3.22,;34.2,.64,;32.86,1.41,;27.53,2.93,;30.19,6.03,;31.53,5.27,;30.19,7.58,;22.19,1.41,;23.53,.64,)| Show InChI InChI=1S/C21H30N6O2/c1-14-4-3-5-18(15(14)2)12-24-21-25-13-19(27(28)29)20(26-21)23-11-17-8-6-16(10-22)7-9-17/h3-5,13,16-17H,6-12,22H2,1-2H3,(H2,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM28397

((2E)-3-(3-{6-[(4-aminocyclohexyl)amino]pyrazin-2-y...)Show SMILES N[C@H]1CC[C@@H](CC1)Nc1cncc(n1)-c1cccc(\C=C\C(O)=O)c1 |r,wU:4.7,wD:1.0,(-2.78,-3.68,;-2.78,-2.14,;-1.45,-1.37,;-1.45,.17,;-2.78,.94,;-4.12,.17,;-4.12,-1.37,;-2.78,2.48,;-1.45,3.25,;-1.45,4.8,;-.12,5.57,;1.22,4.8,;1.22,3.25,;-.12,2.48,;2.55,2.48,;3.88,3.26,;5.22,2.48,;5.22,.94,;3.88,.17,;3.88,-1.37,;5.22,-2.14,;5.22,-3.68,;6.55,-4.45,;3.88,-4.45,;2.55,.94,)| Show InChI InChI=1S/C19H22N4O2/c20-15-5-7-16(8-6-15)22-18-12-21-11-17(23-18)14-3-1-2-13(10-14)4-9-19(24)25/h1-4,9-12,15-16H,5-8,20H2,(H,22,23)(H,24,25)/b9-4+/t15-,16- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Boehringer Ingelheim Pharmaceuticals Inc.
| Assay Description
The activity of Pim kinase was measured in a homogeneous luciferase assay using GST-Pim, biotinylated peptide substrate and a luciferin-luciferase de... |
J Med Chem 52: 1814-27 (2009)
Article DOI: 10.1021/jm801242y
BindingDB Entry DOI: 10.7270/Q25T3HS3 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50319634

(CHEMBL1084892 | N-(7-chloro-1-oxo-1,2-dihydroisoqu...)Show InChI InChI=1S/C18H16ClN3O2/c1-20-16(11-5-3-2-4-6-11)18(24)22-15-9-12-7-8-21-17(23)13(12)10-14(15)19/h2-10,16,20H,1H3,(H,21,23)(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 |
Bioorg Med Chem Lett 20: 3235-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.070
BindingDB Entry DOI: 10.7270/Q21N819Z |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196991
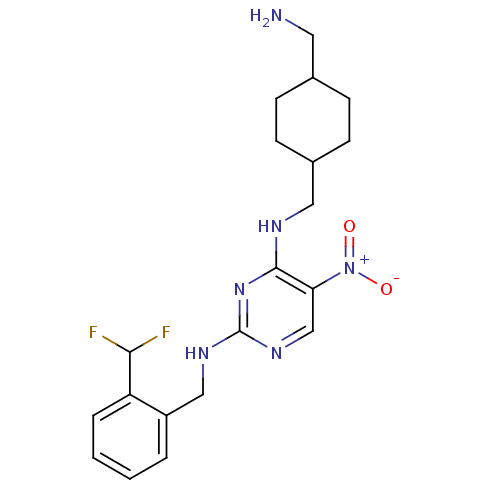
(CHEMBL245977 | N2-(2-(difluoromethyl)benzyl)-N4-((...)Show SMILES NCC1CCC(CNc2nc(NCc3ccccc3C(F)F)ncc2[N+]([O-])=O)CC1 |(5.34,-13.55,;5.35,-12.01,;4.02,-11.23,;2.68,-12,;1.35,-11.23,;1.36,-9.7,;.02,-8.93,;.02,-7.39,;-1.32,-6.62,;-2.65,-7.39,;-3.99,-6.62,;-5.32,-7.39,;-6.65,-6.62,;-7.99,-7.39,;-9.32,-6.61,;-10.65,-7.38,;-10.66,-8.92,;-9.31,-9.69,;-7.99,-8.92,;-6.65,-9.69,;-6.65,-11.23,;-5.32,-8.92,;-3.98,-5.08,;-2.66,-4.31,;-1.32,-5.07,;.01,-4.29,;1.35,-5.06,;.01,-2.75,;2.68,-8.92,;4.02,-9.69,)| Show InChI InChI=1S/C20H26F2N6O2/c21-18(22)16-4-2-1-3-15(16)11-25-20-26-12-17(28(29)30)19(27-20)24-10-14-7-5-13(9-23)6-8-14/h1-4,12-14,18H,5-11,23H2,(H2,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50325444
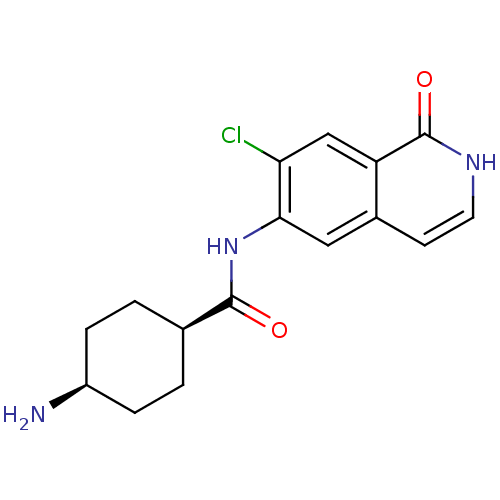
(CHEMBL1222571 | cis-4-amino-N-(7-chloro-1-oxo-1,2-...)Show SMILES N[C@H]1CC[C@H](CC1)C(=O)Nc1cc2cc[nH]c(=O)c2cc1Cl |r,wU:4.7,1.0,(1.58,6.82,;.25,6.05,;.24,4.51,;-1.08,3.74,;-2.42,4.51,;-2.43,6.05,;-1.09,6.82,;-3.75,3.74,;-5.08,4.5,;-3.74,2.2,;-5.07,1.42,;-6.4,2.18,;-7.73,1.42,;-9.05,2.19,;-10.38,1.42,;-10.38,-.12,;-9.05,-.89,;-9.05,-2.43,;-7.73,-.12,;-6.4,-.89,;-5.07,-.12,;-3.74,-.89,)| Show InChI InChI=1S/C16H18ClN3O2/c17-13-8-12-10(5-6-19-16(12)22)7-14(13)20-15(21)9-1-3-11(18)4-2-9/h5-9,11H,1-4,18H2,(H,19,22)(H,20,21)/t9-,11+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 |
Bioorg Med Chem Lett 20: 5153-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.014
BindingDB Entry DOI: 10.7270/Q2RR1ZDC |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196952
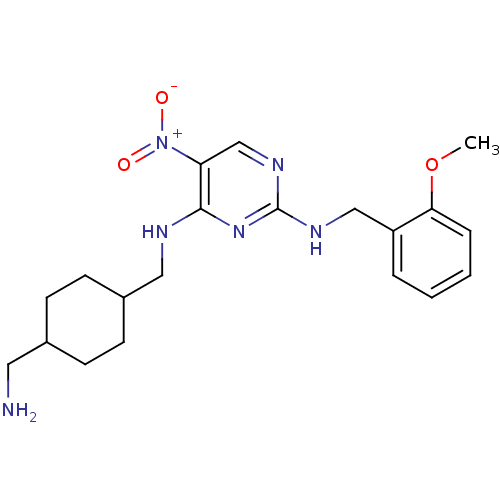
(CHEMBL395690 | N2-(2-methoxybenzyl)-N4-((4-(aminom...)Show SMILES COc1ccccc1CNc1ncc(c(NCC2CCC(CN)CC2)n1)[N+]([O-])=O |(25.24,-35.25,;25.23,-33.71,;23.9,-32.95,;22.57,-33.72,;21.23,-32.95,;21.23,-31.41,;22.56,-30.64,;23.89,-31.42,;25.23,-30.65,;26.56,-31.42,;27.89,-30.65,;27.9,-29.1,;29.23,-28.33,;30.56,-29.1,;30.57,-30.65,;31.9,-31.42,;31.9,-32.96,;33.24,-33.72,;33.23,-35.26,;34.56,-36.03,;35.9,-35.26,;37.23,-36.03,;37.23,-37.57,;35.9,-33.72,;34.56,-32.94,;29.23,-31.42,;31.9,-28.32,;33.23,-29.08,;31.89,-26.78,)| Show InChI InChI=1S/C20H28N6O3/c1-29-18-5-3-2-4-16(18)12-23-20-24-13-17(26(27)28)19(25-20)22-11-15-8-6-14(10-21)7-9-15/h2-5,13-15H,6-12,21H2,1H3,(H2,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-2
(Homo sapiens (Human)) | BDBM28417

((2E)-3-(3-{6-[4-(aminomethyl)piperidin-1-yl]pyrazi...)Show SMILES NCC1CCN(CC1)c1cncc(n1)-c1cccc(\C=C\C(O)=O)c1 Show InChI InChI=1S/C19H22N4O2/c20-11-15-6-8-23(9-7-15)18-13-21-12-17(22-18)16-3-1-2-14(10-16)4-5-19(24)25/h1-5,10,12-13,15H,6-9,11,20H2,(H,24,25)/b5-4+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
| Assay Description
The activity of Pim kinase was measured in a homogeneous luciferase assay using GST-Pim, biotinylated peptide substrate and a luciferin-luciferase de... |
J Med Chem 52: 1814-27 (2009)
Article DOI: 10.1021/jm801242y
BindingDB Entry DOI: 10.7270/Q25T3HS3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50116401

(6,7-Dimethyl-2-(2,4,6-trichloro-phenylamino)-1,8-d...)Show SMILES Cc1[nH]c(=O)c2c3nc(Nc4c(Cl)cc(Cl)cc4Cl)[nH]c3ccc2c1C Show InChI InChI=1S/C18H13Cl3N4O/c1-7-8(2)22-17(26)14-10(7)3-4-13-16(14)25-18(23-13)24-15-11(20)5-9(19)6-12(15)21/h3-6H,1-2H3,(H,22,26)(H2,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human p56 Lck tyrosine kinase |
J Med Chem 45: 3394-405 (2002)
BindingDB Entry DOI: 10.7270/Q2CJ8CTW |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
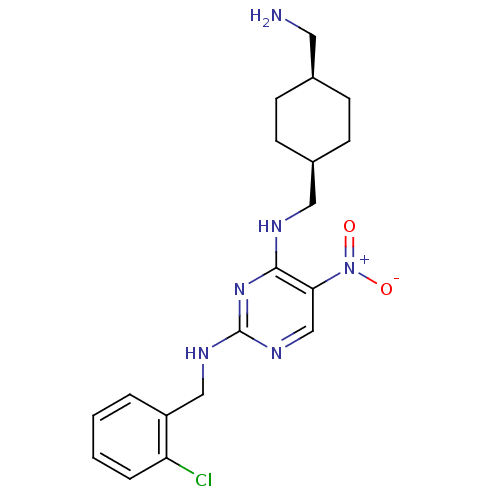
(Homo sapiens (Human)) | BDBM50196958
(CHEMBL246159 | N2-(2-chlorobenzyl)-N4-(((1s,4s)-4-...)Show SMILES NC[C@H]1CC[C@@H](CNc2nc(NCc3ccccc3Cl)ncc2[N+]([O-])=O)CC1 |wU:5.5,2.1,(4.42,-27.37,;4.42,-25.83,;3.09,-25.06,;1.75,-25.82,;.43,-25.05,;.43,-23.52,;-.9,-22.75,;-.91,-21.21,;-2.24,-20.44,;-3.58,-21.22,;-4.91,-20.44,;-6.25,-21.21,;-7.58,-20.44,;-8.91,-21.21,;-10.25,-20.44,;-11.58,-21.2,;-11.6,-22.75,;-10.24,-23.52,;-8.91,-22.74,;-7.58,-23.51,;-4.91,-18.9,;-3.58,-18.13,;-2.25,-18.89,;-.91,-18.12,;.43,-18.88,;-.92,-16.58,;1.76,-22.74,;3.09,-23.51,)| Show InChI InChI=1S/C19H25ClN6O2/c20-16-4-2-1-3-15(16)11-23-19-24-12-17(26(27)28)18(25-19)22-10-14-7-5-13(9-21)6-8-14/h1-4,12-14H,5-11,21H2,(H2,22,23,24,25)/t13-,14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196972

(CHEMBL248183 | N2-(2-(trifluoromethoxy)benzyl)-N4-...)Show SMILES NCC1CCC(CNc2nc(NCc3ccccc3OC(F)(F)F)ncc2[N+]([O-])=O)CC1 |(34.71,-3.87,;34.71,-2.33,;33.38,-1.56,;32.04,-2.32,;30.71,-1.56,;30.72,-.02,;29.38,.75,;29.38,2.29,;28.05,3.05,;26.71,2.28,;25.37,3.05,;24.04,2.28,;22.71,3.05,;21.37,2.29,;20.04,3.06,;18.71,2.29,;18.71,.75,;20.05,-.02,;21.38,.75,;22.71,-.01,;22.72,-1.55,;22.7,-3.09,;24.26,-1.56,;21.18,-1.56,;25.38,4.6,;26.7,5.37,;28.04,4.6,;29.38,5.38,;30.71,4.62,;29.37,6.92,;32.04,.76,;33.38,-.02,)| Show InChI InChI=1S/C20H25F3N6O3/c21-20(22,23)32-17-4-2-1-3-15(17)11-26-19-27-12-16(29(30)31)18(28-19)25-10-14-7-5-13(9-24)6-8-14/h1-4,12-14H,5-11,24H2,(H2,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50325434
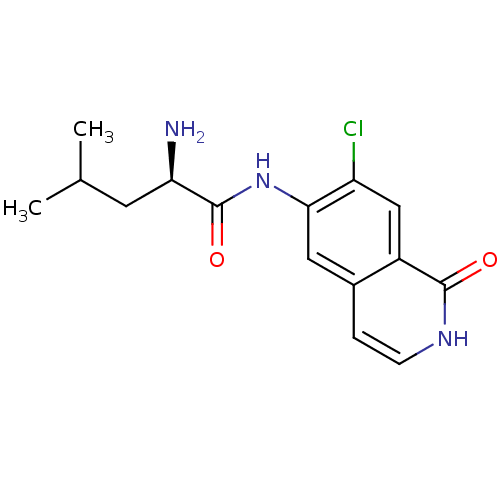
((R)-2-amino-N-(7-chloro-1-oxo-1,2-dihydroisoquinol...)Show SMILES CC(C)C[C@@H](N)C(=O)Nc1cc2cc[nH]c(=O)c2cc1Cl |r| Show InChI InChI=1S/C15H18ClN3O2/c1-8(2)5-12(17)15(21)19-13-6-9-3-4-18-14(20)10(9)7-11(13)16/h3-4,6-8,12H,5,17H2,1-2H3,(H,18,20)(H,19,21)/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 |
Bioorg Med Chem Lett 20: 5153-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.014
BindingDB Entry DOI: 10.7270/Q2RR1ZDC |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50319636
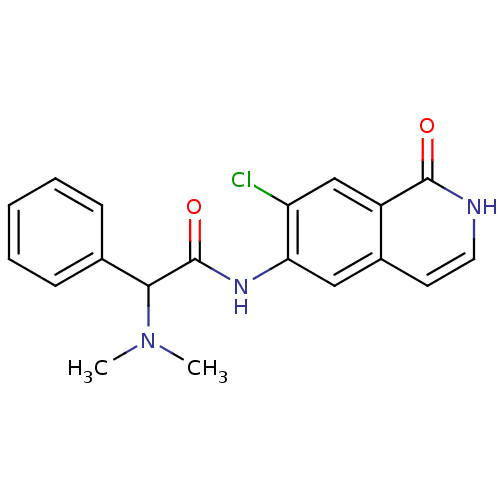
(CHEMBL1084107 | N-(7-chloro-1-oxo-1,2-dihydroisoqu...)Show SMILES CN(C)C(C(=O)Nc1cc2cc[nH]c(=O)c2cc1Cl)c1ccccc1 Show InChI InChI=1S/C19H18ClN3O2/c1-23(2)17(12-6-4-3-5-7-12)19(25)22-16-10-13-8-9-21-18(24)14(13)11-15(16)20/h3-11,17H,1-2H3,(H,21,24)(H,22,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 |
Bioorg Med Chem Lett 20: 5153-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.014
BindingDB Entry DOI: 10.7270/Q2RR1ZDC |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50319635
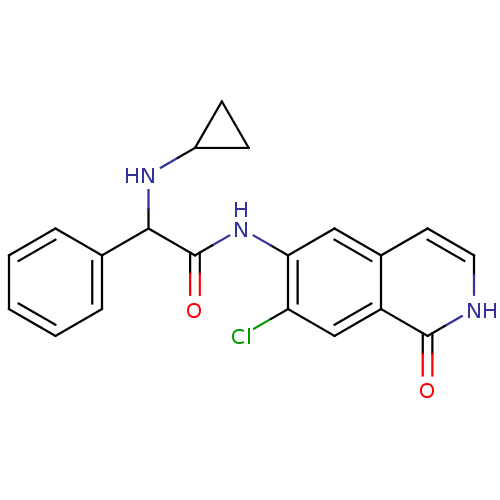
(CHEMBL1084106 | N-(7-chloro-1-oxo-1,2-dihydroisoqu...)Show SMILES Clc1cc2c(cc[nH]c2=O)cc1NC(=O)C(NC1CC1)c1ccccc1 Show InChI InChI=1S/C20H18ClN3O2/c21-16-11-15-13(8-9-22-19(15)25)10-17(16)24-20(26)18(23-14-6-7-14)12-4-2-1-3-5-12/h1-5,8-11,14,18,23H,6-7H2,(H,22,25)(H,24,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 |
Bioorg Med Chem Lett 20: 3235-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.070
BindingDB Entry DOI: 10.7270/Q21N819Z |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-2
(Homo sapiens (Human)) | BDBM28418
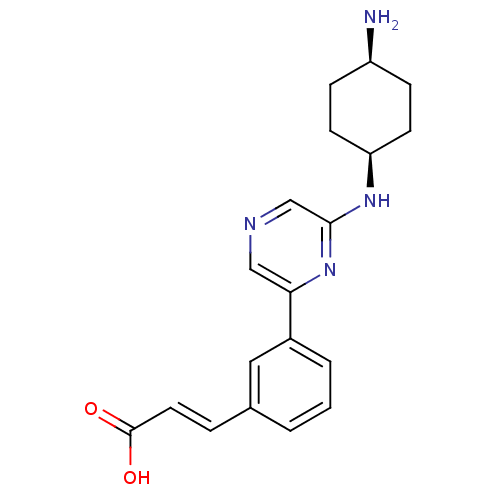
((2E)-3-(3-{6-[(4-aminocyclohexyl)amino]pyrazin-2-y...)Show SMILES N[C@H]1CC[C@H](CC1)Nc1cncc(n1)-c1cccc(\C=C\C(O)=O)c1 |r,wD:4.7,1.0,(-2.78,-3.68,;-2.78,-2.14,;-1.45,-1.37,;-1.45,.17,;-2.78,.94,;-4.12,.17,;-4.12,-1.37,;-2.78,2.48,;-1.45,3.25,;-1.45,4.8,;-.12,5.57,;1.22,4.8,;1.22,3.25,;-.12,2.48,;2.55,2.48,;3.88,3.26,;5.22,2.48,;5.22,.94,;3.88,.17,;3.88,-1.37,;5.22,-2.14,;5.22,-3.68,;6.55,-4.45,;3.88,-4.45,;2.55,.94,)| Show InChI InChI=1S/C19H22N4O2/c20-15-5-7-16(8-6-15)22-18-12-21-11-17(23-18)14-3-1-2-13(10-14)4-9-19(24)25/h1-4,9-12,15-16H,5-8,20H2,(H,22,23)(H,24,25)/b9-4+/t15-,16+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
| Assay Description
The activity of Pim kinase was measured in a homogeneous luciferase assay using GST-Pim, biotinylated peptide substrate and a luciferin-luciferase de... |
J Med Chem 52: 1814-27 (2009)
Article DOI: 10.1021/jm801242y
BindingDB Entry DOI: 10.7270/Q25T3HS3 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50319636

(CHEMBL1084107 | N-(7-chloro-1-oxo-1,2-dihydroisoqu...)Show SMILES CN(C)C(C(=O)Nc1cc2cc[nH]c(=O)c2cc1Cl)c1ccccc1 Show InChI InChI=1S/C19H18ClN3O2/c1-23(2)17(12-6-4-3-5-7-12)19(25)22-16-10-13-8-9-21-18(24)14(13)11-15(16)20/h3-11,17H,1-2H3,(H,21,24)(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 |
Bioorg Med Chem Lett 20: 3235-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.070
BindingDB Entry DOI: 10.7270/Q21N819Z |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50126419
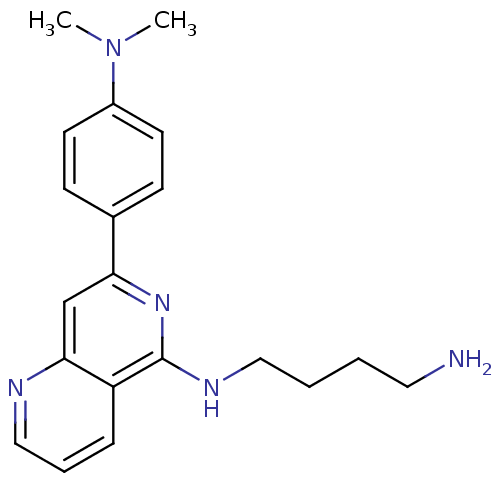
(CHEMBL282342 | N*1*-[7-(4-Dimethylamino-phenyl)-[1...)Show InChI InChI=1S/C20H25N5/c1-25(2)16-9-7-15(8-10-16)18-14-19-17(6-5-13-22-19)20(24-18)23-12-4-3-11-21/h5-10,13-14H,3-4,11-12,21H2,1-2H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against spleen tyrosine kinase (SYK) |
Bioorg Med Chem Lett 13: 1415-8 (2003)
BindingDB Entry DOI: 10.7270/Q2MC8ZCJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50126408
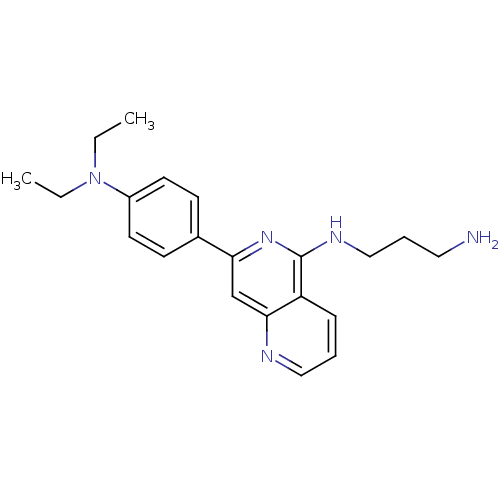
(CHEMBL30381 | N*1*-[7-(4-Diethylamino-phenyl)-[1,6...)Show InChI InChI=1S/C21H27N5/c1-3-26(4-2)17-10-8-16(9-11-17)19-15-20-18(7-5-13-23-20)21(25-19)24-14-6-12-22/h5,7-11,13,15H,3-4,6,12,14,22H2,1-2H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against spleen tyrosine kinase (SYK) |
Bioorg Med Chem Lett 13: 1415-8 (2003)
BindingDB Entry DOI: 10.7270/Q2MC8ZCJ |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50325437
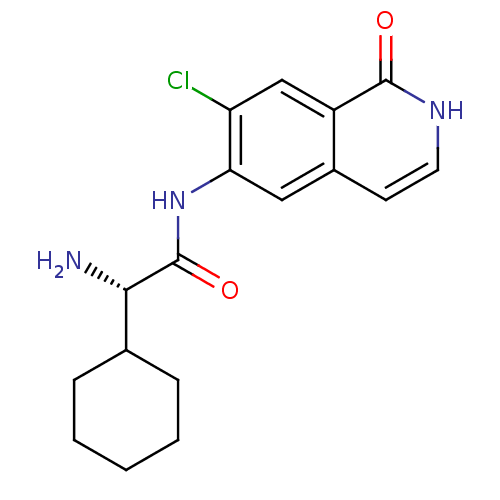
((S)-2-amino-N-(7-chloro-1-oxo-1,2-dihydroisoquinol...)Show SMILES N[C@@H](C1CCCCC1)C(=O)Nc1cc2cc[nH]c(=O)c2cc1Cl |r| Show InChI InChI=1S/C17H20ClN3O2/c18-13-9-12-11(6-7-20-16(12)22)8-14(13)21-17(23)15(19)10-4-2-1-3-5-10/h6-10,15H,1-5,19H2,(H,20,22)(H,21,23)/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 |
Bioorg Med Chem Lett 20: 5153-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.014
BindingDB Entry DOI: 10.7270/Q2RR1ZDC |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-2
(Homo sapiens (Human)) | BDBM28395
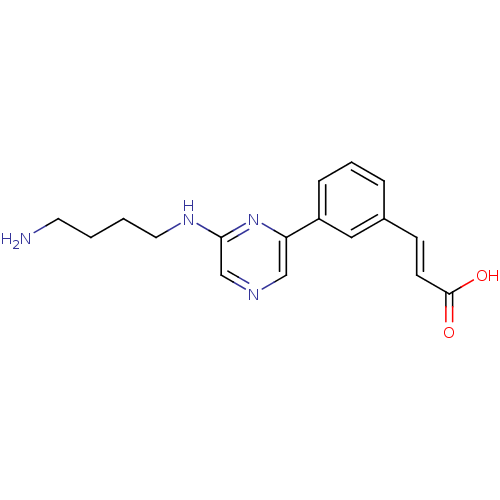
((2E)-3-(3-{6-[(4-aminobutyl)amino]pyrazin-2-yl}phe...)Show InChI InChI=1S/C17H20N4O2/c18-8-1-2-9-20-16-12-19-11-15(21-16)14-5-3-4-13(10-14)6-7-17(22)23/h3-7,10-12H,1-2,8-9,18H2,(H,20,21)(H,22,23)/b7-6+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Boehringer Ingelheim Pharmaceuticals Inc.
| Assay Description
The activity of Pim kinase was measured in a homogeneous luciferase assay using GST-Pim, biotinylated peptide substrate and a luciferin-luciferase de... |
J Med Chem 52: 1814-27 (2009)
Article DOI: 10.1021/jm801242y
BindingDB Entry DOI: 10.7270/Q25T3HS3 |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50196975

(CHEMBL245783 | N2-(2,3-difluorobenzyl)-N4-((4-(ami...)Show SMILES NCC1CCC(CNc2nc(NCc3cccc(F)c3F)ncc2[N+]([O-])=O)CC1 |(5.69,-35.63,;5.7,-34.09,;4.37,-33.32,;3.03,-34.08,;1.7,-33.31,;1.71,-31.78,;.37,-31.01,;.37,-29.47,;-.97,-28.7,;-2.3,-29.48,;-3.64,-28.7,;-4.97,-29.47,;-6.3,-28.7,;-7.64,-29.47,;-8.97,-28.7,;-10.3,-29.46,;-10.31,-31.01,;-8.96,-31.78,;-8.96,-33.32,;-7.64,-31,;-6.3,-31.77,;-3.63,-27.16,;-2.31,-26.39,;-.97,-27.15,;.36,-26.38,;1.7,-27.14,;.36,-24.84,;3.03,-31,;4.37,-31.77,)| Show InChI InChI=1S/C19H24F2N6O2/c20-15-3-1-2-14(17(15)21)10-24-19-25-11-16(27(28)29)18(26-19)23-9-13-6-4-12(8-22)5-7-13/h1-3,11-13H,4-10,22H2,(H2,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta by FP assay |
Bioorg Med Chem Lett 17: 225-30 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.056
BindingDB Entry DOI: 10.7270/Q2PC3211 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50126734
(2-(2,6-Dichloro-phenylamino)-1,6,7-trimethyl-1,8-d...)Show SMILES Cc1[nH]c(=O)c2c3n(C)c(Nc4c(Cl)cccc4Cl)nc3ccc2c1C Show InChI InChI=1S/C19H16Cl2N4O/c1-9-10(2)22-18(26)15-11(9)7-8-14-17(15)25(3)19(23-14)24-16-12(20)5-4-6-13(16)21/h4-8H,1-3H3,(H,22,26)(H,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
J Med Chem 46: 1337-49 (2003)
Article DOI: 10.1021/jm020446l
BindingDB Entry DOI: 10.7270/Q2DN44FW |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50325439
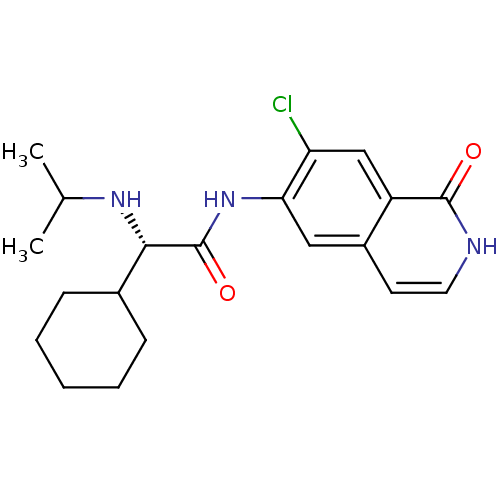
((S)-N-(7-chloro-1-oxo-1,2-dihydroisoquinolin-6-yl)...)Show SMILES CC(C)N[C@@H](C1CCCCC1)C(=O)Nc1cc2cc[nH]c(=O)c2cc1Cl |r| Show InChI InChI=1S/C20H26ClN3O2/c1-12(2)23-18(13-6-4-3-5-7-13)20(26)24-17-10-14-8-9-22-19(25)15(14)11-16(17)21/h8-13,18,23H,3-7H2,1-2H3,(H,22,25)(H,24,26)/t18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 |
Bioorg Med Chem Lett 20: 5153-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.014
BindingDB Entry DOI: 10.7270/Q2RR1ZDC |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-2
(Homo sapiens (Human)) | BDBM28416
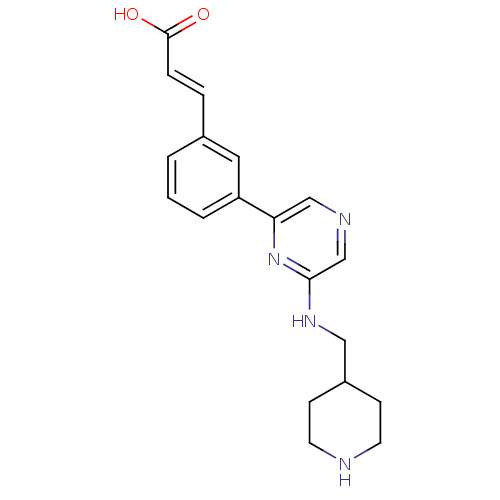
((2E)-3-(3-{6-[(piperidin-4-ylmethyl)amino]pyrazin-...)Show InChI InChI=1S/C19H22N4O2/c24-19(25)5-4-14-2-1-3-16(10-14)17-12-21-13-18(23-17)22-11-15-6-8-20-9-7-15/h1-5,10,12-13,15,20H,6-9,11H2,(H,22,23)(H,24,25)/b5-4+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc.
| Assay Description
The activity of Pim kinase was measured in a homogeneous luciferase assay using GST-Pim, biotinylated peptide substrate and a luciferin-luciferase de... |
J Med Chem 52: 1814-27 (2009)
Article DOI: 10.1021/jm801242y
BindingDB Entry DOI: 10.7270/Q25T3HS3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data