

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50116391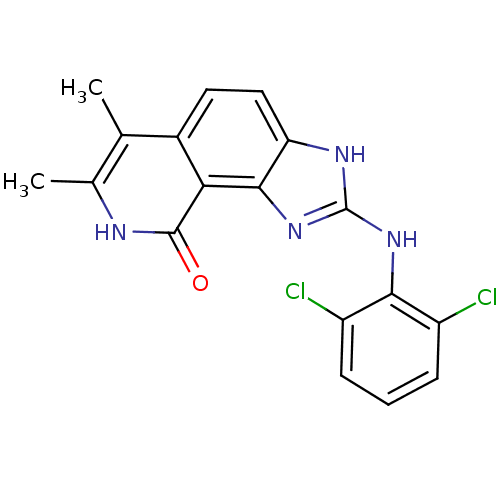 (2-(2,6-Dichloro-phenylamino)-6,7-dimethyl-1,8-dihy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of c-SRC with 1 uM ATP | J Med Chem 45: 3394-405 (2002) BindingDB Entry DOI: 10.7270/Q2CJ8CTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50116401 (6,7-Dimethyl-2-(2,4,6-trichloro-phenylamino)-1,8-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human p56 Lck tyrosine kinase | J Med Chem 45: 3394-405 (2002) BindingDB Entry DOI: 10.7270/Q2CJ8CTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50116405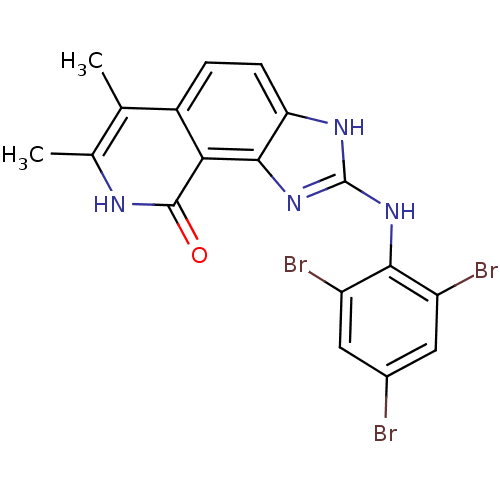 (6,7-Dimethyl-2-(2,4,6-tribromo-phenylamino)-1,8-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human p56 Lck tyrosine kinase | J Med Chem 45: 3394-405 (2002) BindingDB Entry DOI: 10.7270/Q2CJ8CTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50116391 (2-(2,6-Dichloro-phenylamino)-6,7-dimethyl-1,8-dihy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human p56 Lck tyrosine kinase | J Med Chem 45: 3394-405 (2002) BindingDB Entry DOI: 10.7270/Q2CJ8CTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50116399 (2-(2-Chloro-6-methyl-phenylamino)-6,7-dimethyl-1,8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human p56 Lck tyrosine kinase | J Med Chem 45: 3394-405 (2002) BindingDB Entry DOI: 10.7270/Q2CJ8CTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005159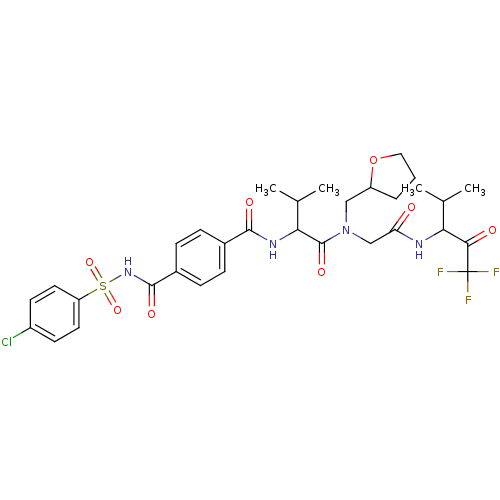 (4-(4-Chloro-benzenesulfonylaminocarbonyl)-N-(2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005147 ((S)-1-{(S)-2-[4-(4-Bromo-benzenesulfonylaminocarbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005166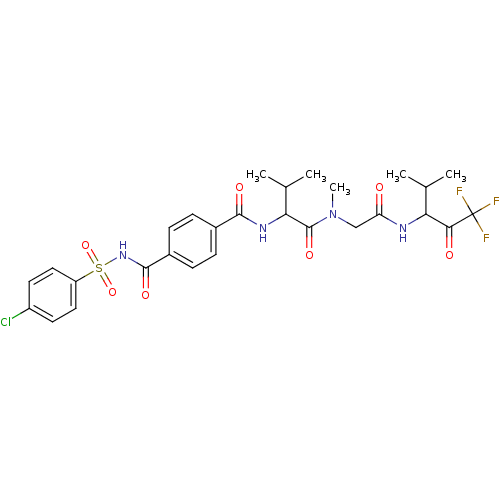 (4-(4-Chloro-benzenesulfonylaminocarbonyl)-N-(2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005176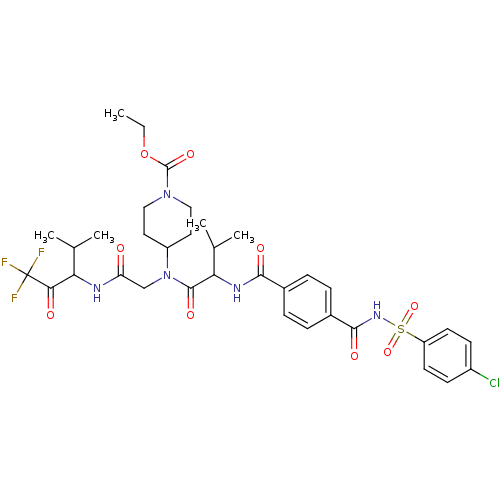 (4-{{2-[4-(4-Chloro-benzenesulfonylaminocarbonyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004182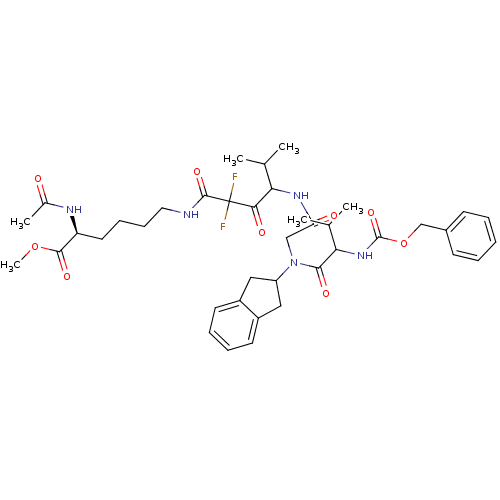 (2-Acetylamino-6-(4-{2-[(2-benzyloxycarbonylamino-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 | J Med Chem 35: 4795-808 (1993) BindingDB Entry DOI: 10.7270/Q2HX1BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005174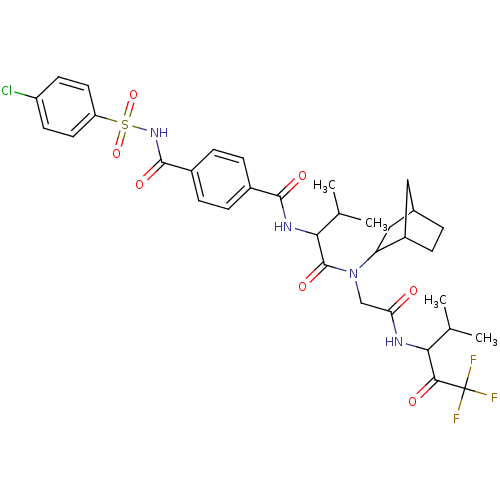 (CHEMBL435649 | N-(1-{Bicyclo[2.2.1]hept-2-yl-[(3,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005154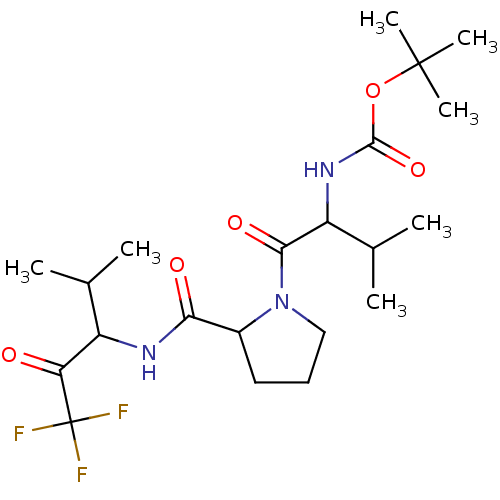 (CHEMBL423067 | {2-Methyl-1-[2-(3,3,3-trifluoro-1-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005156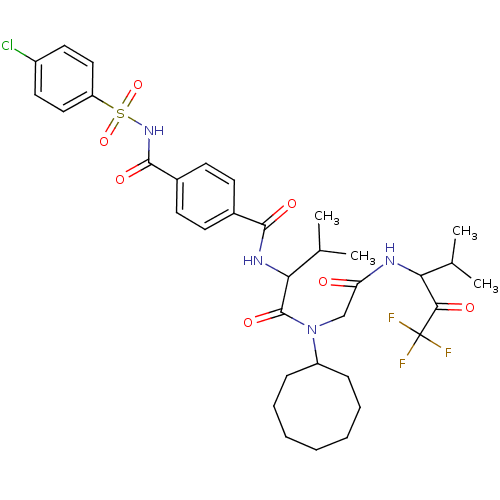 (4-(4-Chloro-benzenesulfonylaminocarbonyl)-N-(1-{cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004192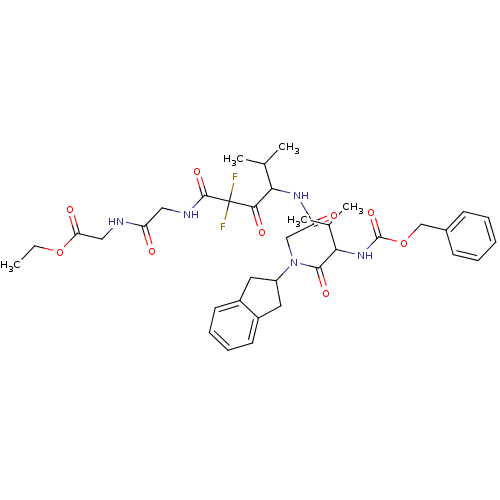 (CHEMBL344981 | [2-(4-{2-[(2-Benzyloxycarbonylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 | J Med Chem 35: 4795-808 (1993) BindingDB Entry DOI: 10.7270/Q2HX1BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005152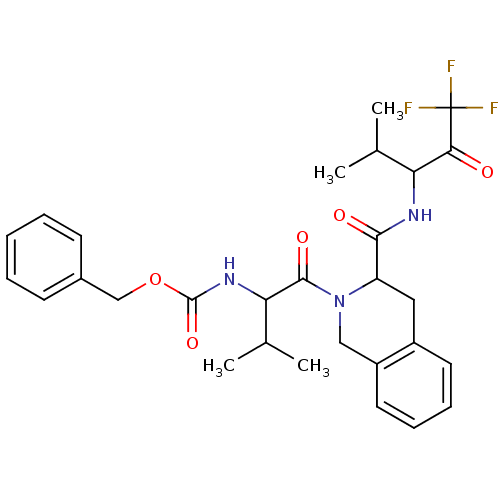 (CHEMBL160365 | {2-Methyl-1-[3-(3,3,3-trifluoro-1-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005178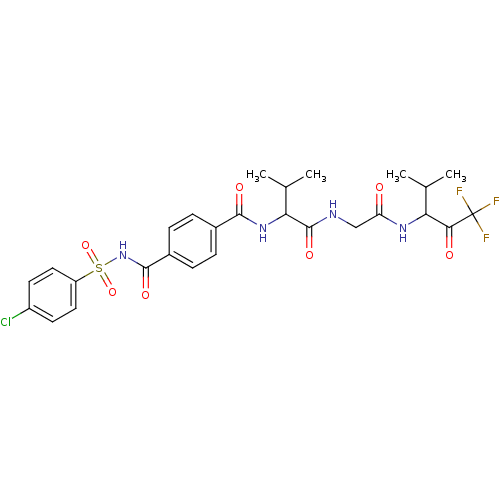 (4-(4-Chloro-benzenesulfonylaminocarbonyl)-N-(2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005152 (CHEMBL160365 | {2-Methyl-1-[3-(3,3,3-trifluoro-1-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004184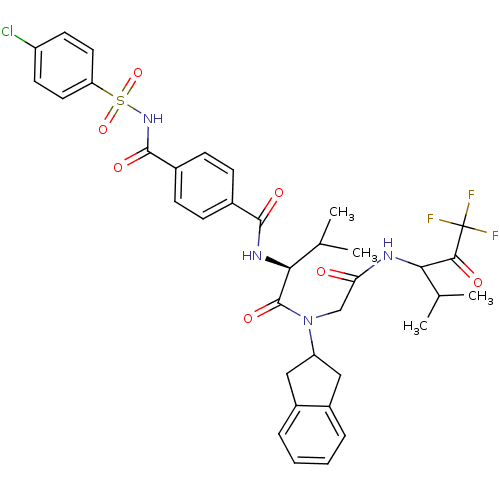 (4-(4-Chloro-benzenesulfonylaminocarbonyl)-N-((S)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | Bioorg Med Chem Lett 3: 773-778 (1993) Article DOI: 10.1016/S0960-894X(01)81273-7 BindingDB Entry DOI: 10.7270/Q2FJ2GQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004184 (4-(4-Chloro-benzenesulfonylaminocarbonyl)-N-((S)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004184 (4-(4-Chloro-benzenesulfonylaminocarbonyl)-N-((S)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 | J Med Chem 35: 4795-808 (1993) BindingDB Entry DOI: 10.7270/Q2HX1BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005164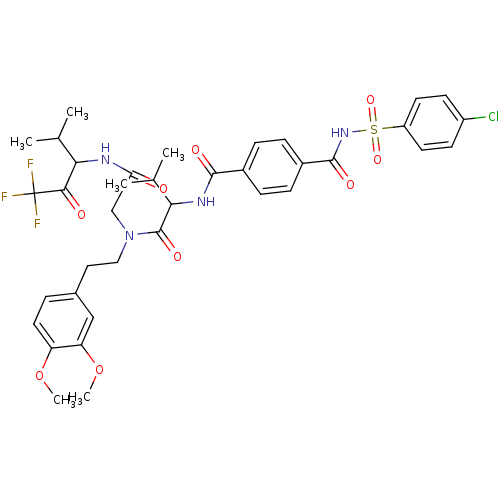 (4-(4-Chloro-benzenesulfonylaminocarbonyl)-N-(1-{[2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005153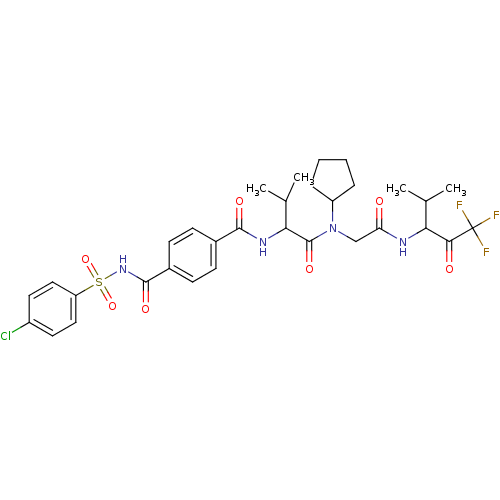 (4-(4-Chloro-benzenesulfonylaminocarbonyl)-N-(1-{cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004190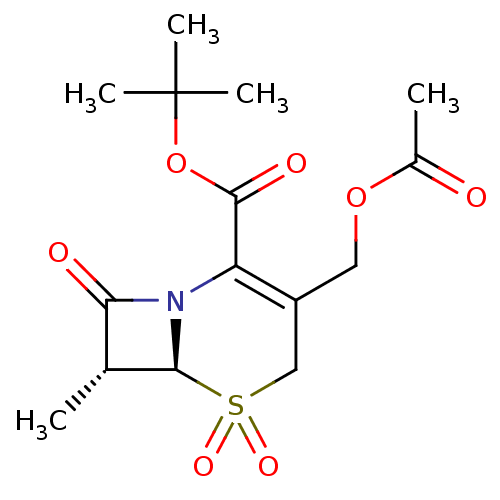 (3-Acetoxymethyl-7-methyl-5,5,8-trioxo-5lambda*6*-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 107 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 | J Med Chem 35: 4795-808 (1993) BindingDB Entry DOI: 10.7270/Q2HX1BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005169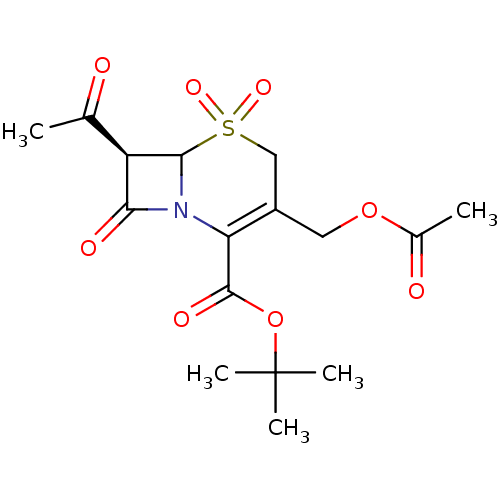 (3-Acetoxymethyl-7-acetyl-5,5-dihydroxy-8-oxo-5lamb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005149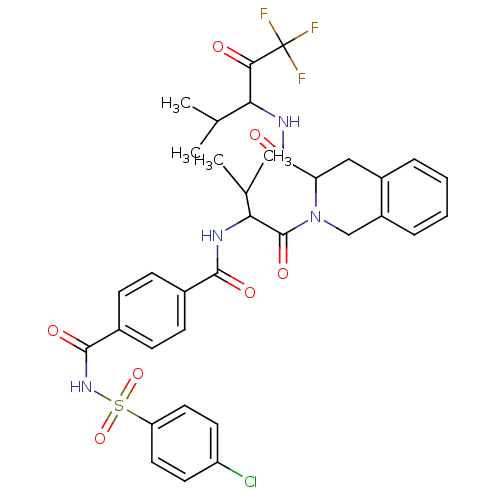 (2-{2-[4-(4-Chloro-benzenesulfonylaminocarbonyl)-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005167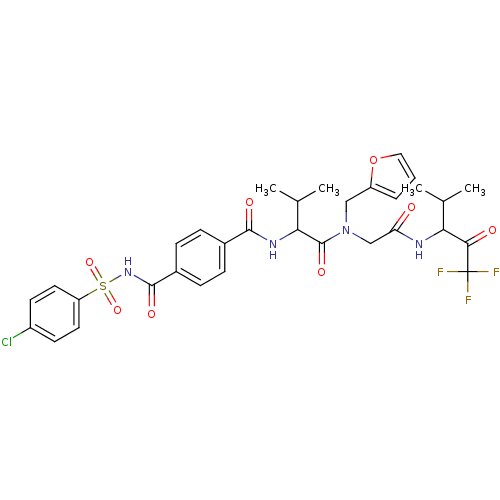 (4-(4-Chloro-benzenesulfonylaminocarbonyl)-N-(1-{fu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005168 (4-(4-Bromo-benzenesulfonylaminocarbonyl)-N-(1-{ind...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 149 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50116408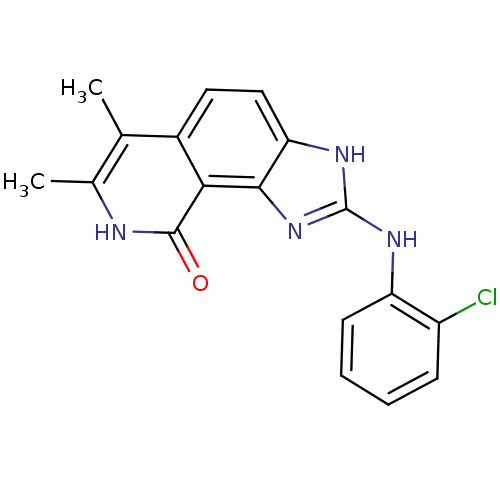 (2-(2-Chloro-phenylamino)-6,7-dimethyl-1,8-dihydro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human p56 Lck tyrosine kinase | J Med Chem 45: 3394-405 (2002) BindingDB Entry DOI: 10.7270/Q2CJ8CTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005148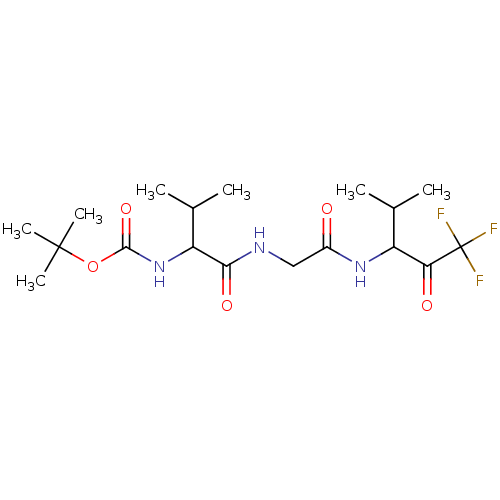 ((2-Methyl-1-{[(3,3,3-trifluoro-1-isopropyl-2-oxo-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 153 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005144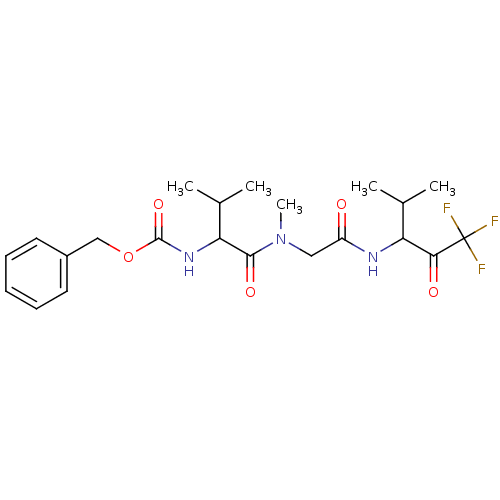 ((2-Methyl-1-{methyl-[(3,3,3-trifluoro-1-isopropyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 153 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005155 ((1-{Cyclopentyl-[(3,3,3-trifluoro-1-isopropyl-2-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 156 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005154 (CHEMBL423067 | {2-Methyl-1-[2-(3,3,3-trifluoro-1-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 172 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005145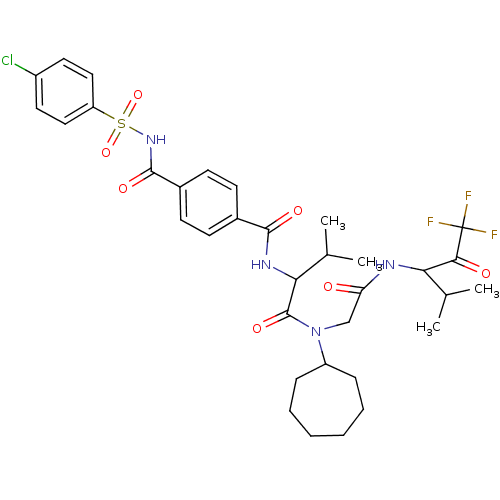 (4-(4-Chloro-benzenesulfonylaminocarbonyl)-N-(1-{cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 175 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005175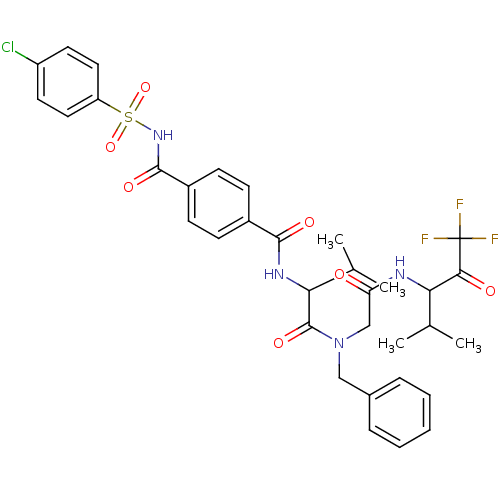 (CHEMBL159116 | N-(1-{Benzyl-[(3,3,3-trifluoro-1-is...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 217 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004187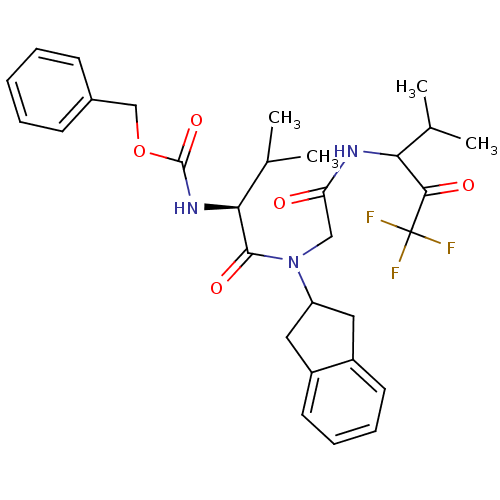 (((S)-1-{Indan-2-yl-[(3,3,3-trifluoro-1-isopropyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 365 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | Bioorg Med Chem Lett 3: 773-778 (1993) Article DOI: 10.1016/S0960-894X(01)81273-7 BindingDB Entry DOI: 10.7270/Q2FJ2GQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004187 (((S)-1-{Indan-2-yl-[(3,3,3-trifluoro-1-isopropyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 365 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 | J Med Chem 35: 4795-808 (1993) BindingDB Entry DOI: 10.7270/Q2HX1BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004187 (((S)-1-{Indan-2-yl-[(3,3,3-trifluoro-1-isopropyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 365 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50116398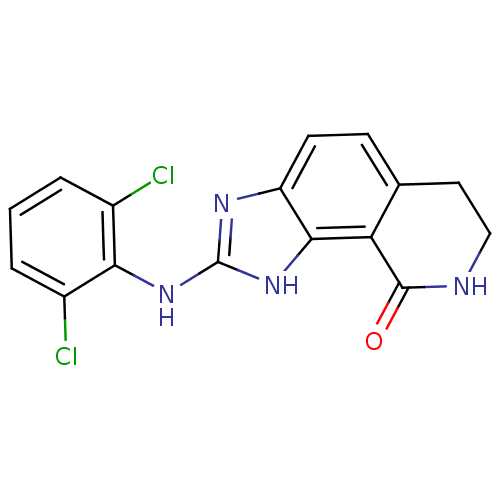 (2-(2,6-Dichloro-phenylamino)-1,6,7,8-tetrahydro-im...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human p56 Lck tyrosine kinase | J Med Chem 45: 3394-405 (2002) BindingDB Entry DOI: 10.7270/Q2CJ8CTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004185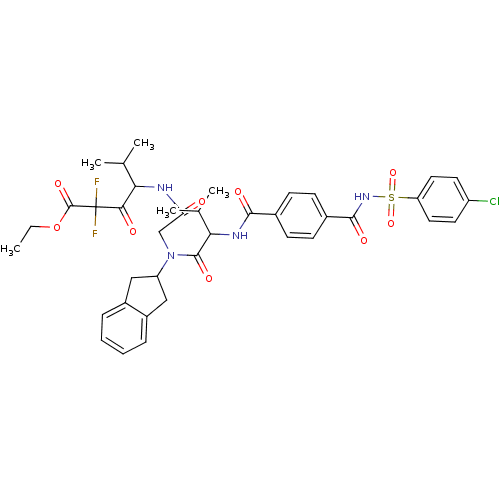 (4-[2-({2-[4-(4-Chloro-benzenesulfonylaminocarbonyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 404 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 | J Med Chem 35: 4795-808 (1993) BindingDB Entry DOI: 10.7270/Q2HX1BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005170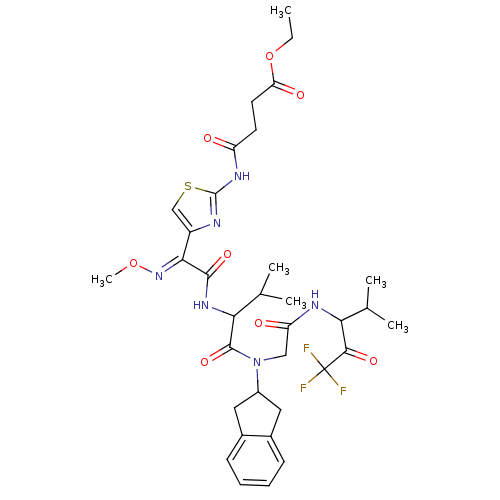 (CHEMBL158647 | N-{4-[(1-{Indan-2-yl-[(3,3,3-triflu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 452 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50116402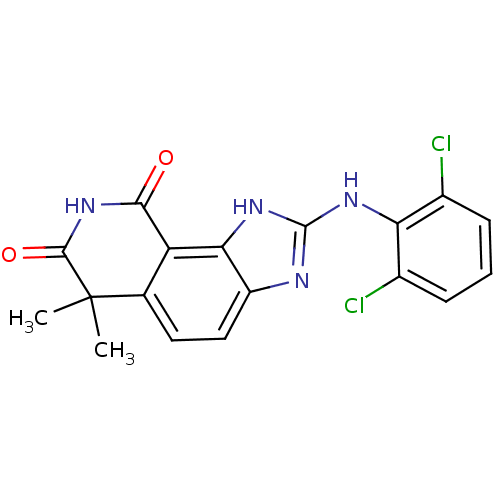 (2-(2,6-Dichloro-phenylamino)-6,6-dimethyl-1,6-dihy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human p56 Lck tyrosine kinase | J Med Chem 45: 3394-405 (2002) BindingDB Entry DOI: 10.7270/Q2CJ8CTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50116410 (2-(2,6-Dimethyl-phenylamino)-6,6-dimethyl-1,6-dihy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human p56 Lck tyrosine kinase | J Med Chem 45: 3394-405 (2002) BindingDB Entry DOI: 10.7270/Q2CJ8CTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005158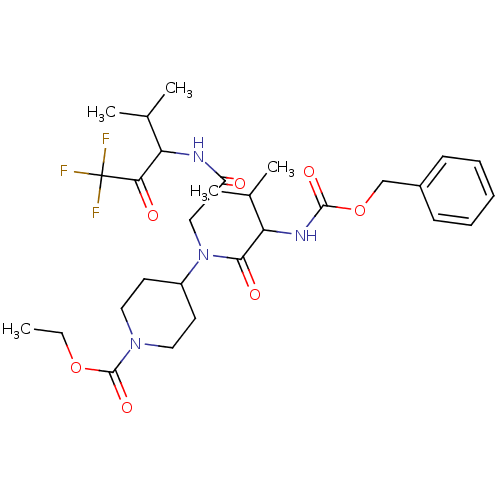 (4-{(2-Benzyloxycarbonylamino-3-methyl-butyryl)-[(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 507 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50116397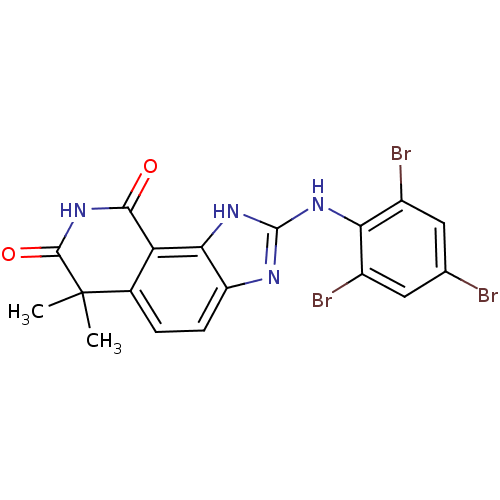 (6,6-Dimethyl-2-(2,4,6-tribromo-phenylamino)-1,6-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human p56 Lck tyrosine kinase | J Med Chem 45: 3394-405 (2002) BindingDB Entry DOI: 10.7270/Q2CJ8CTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50004183 (4-{2-[(2-Benzyloxycarbonylamino-3-methyl-butyryl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 635 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase (HLE) at pH 7.5 | J Med Chem 35: 4795-808 (1993) BindingDB Entry DOI: 10.7270/Q2HX1BM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005177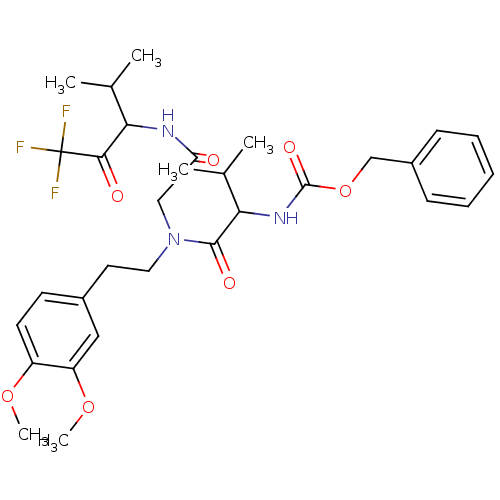 ((1-{[2-(3,4-Dimethoxy-phenyl)-ethyl]-[(3,3,3-trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 693 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50116406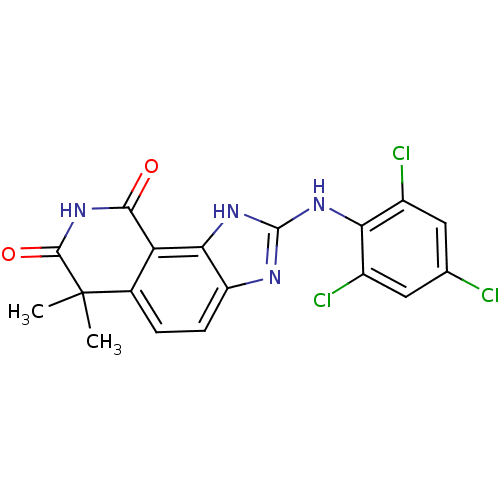 (6,6-Dimethyl-2-(2,4,6-trichloro-phenylamino)-1,6-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human p56 Lck tyrosine kinase | J Med Chem 45: 3394-405 (2002) BindingDB Entry DOI: 10.7270/Q2CJ8CTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50116409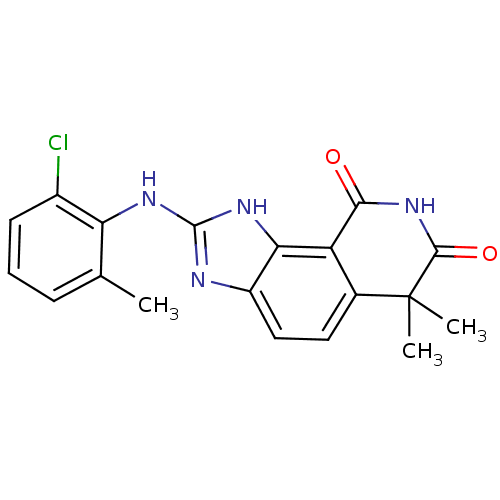 (2-(2-Chloro-6-methyl-phenylamino)-6,6-dimethyl-1,6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human p56 Lck tyrosine kinase | J Med Chem 45: 3394-405 (2002) BindingDB Entry DOI: 10.7270/Q2CJ8CTW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50005151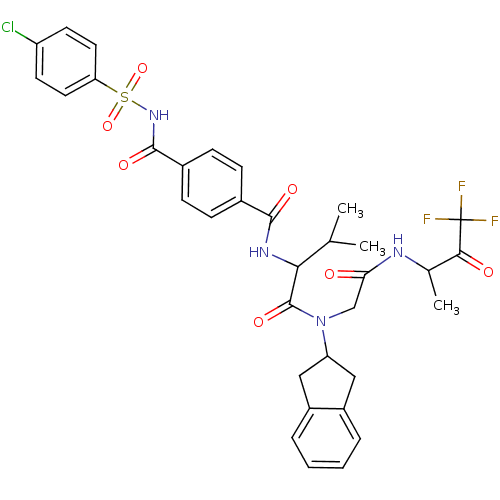 (4-(4-Chloro-benzenesulfonylaminocarbonyl)-N-(1-{in...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 817 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibitory activity against human leukocyte elastase | J Med Chem 35: 641-62 (1992) BindingDB Entry DOI: 10.7270/Q2K074X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit beta (Homo sapiens (Human)) | BDBM50137196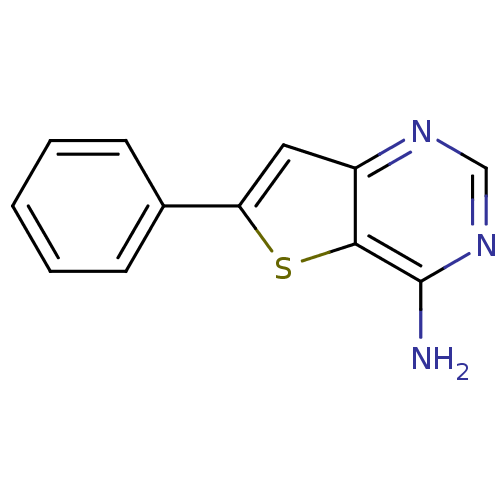 (6-Phenyl-thieno[3,2-d]pyrimidin-4-ylamine | CHEMBL...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of IKK beta | J Med Chem 49: 2898-908 (2006) Article DOI: 10.1021/jm0510979 BindingDB Entry DOI: 10.7270/Q2TQ614S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 171 total ) | Next | Last >> |