

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [600-1027,P835L]/[600-1155,P835L] (Human immunodeficiency virus type 1) | BDBM2019 (2-(6-Azaindol-3-yl)-5,11-dihydro-11-ethyl-5-methyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 40: 2430-3 (1997) Article DOI: 10.1021/jm960837y BindingDB Entry DOI: 10.7270/Q2P26W9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121562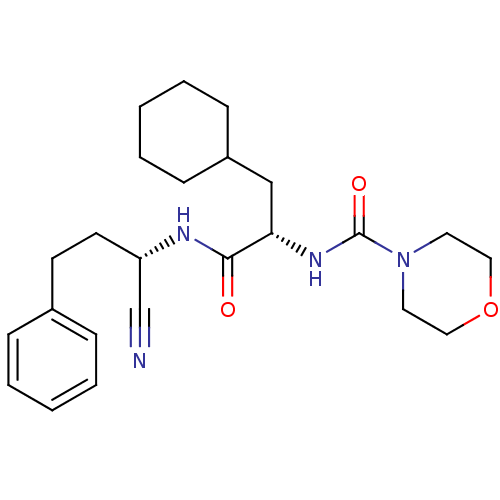 (CHEMBL153239 | Morpholine-4-carboxylic acid [1-(1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121542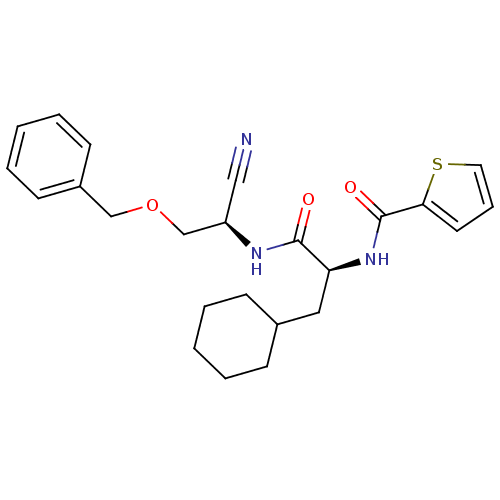 (CHEMBL155560 | Thiophene-2-carboxylic acid {1-[(be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121549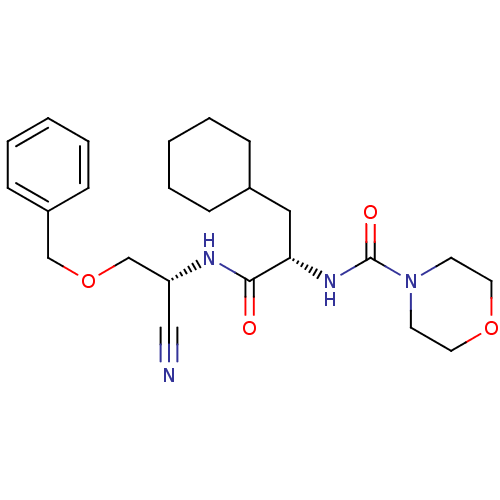 (CHEMBL347111 | Morpholine-4-carboxylic acid {1-[(b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121554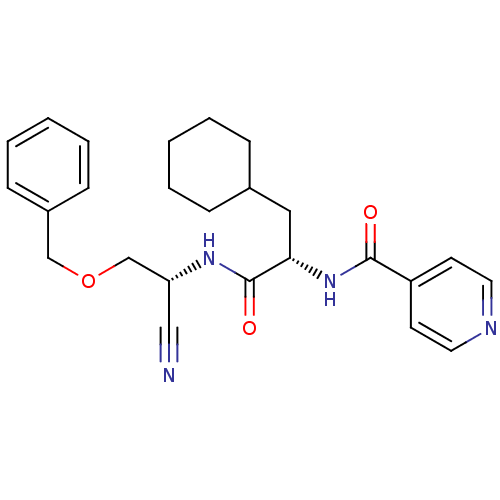 (CHEMBL356155 | N-{1-[(Benzyloxymethyl-cyano-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121575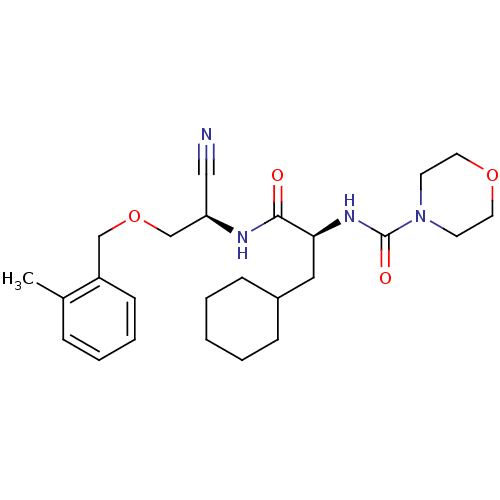 (CHEMBL356442 | Morpholine-4-carboxylic acid (1-{[c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50057000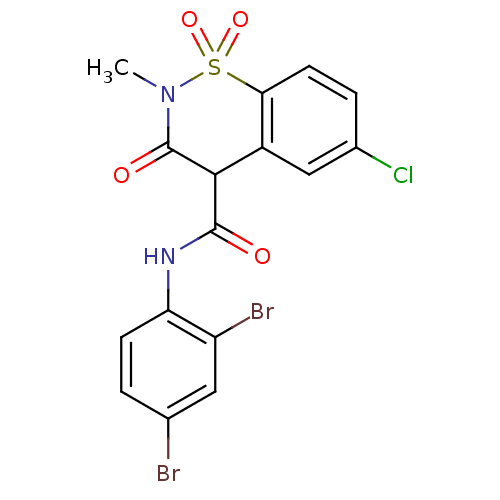 (6-Chloro-3-hydroxy-2-methyl-1,1-dioxo-1,2-dihydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration of drug that causes a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 2 activity as measured by PGE2 production('+... | J Med Chem 40: 980-9 (1997) Article DOI: 10.1021/jm9607010 BindingDB Entry DOI: 10.7270/Q2WH2P37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121569 (CHEMBL153783 | Morpholine-4-carboxylic acid {1-[(b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50126413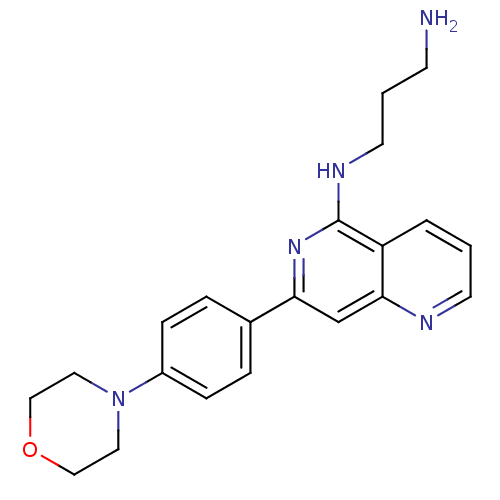 (CHEMBL287478 | N*1*-[7-(4-Morpholin-4-yl-phenyl)-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against spleen tyrosine kinase (SYK) | Bioorg Med Chem Lett 13: 1415-8 (2003) BindingDB Entry DOI: 10.7270/Q2MC8ZCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50057004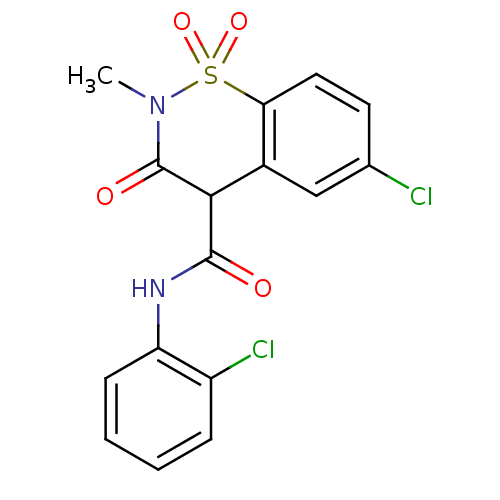 (6-Chloro-3-hydroxy-2-methyl-1,1-dioxo-1,2-dihydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration of drug that causes a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 2 activity as measured by PGE2 production('+... | J Med Chem 40: 980-9 (1997) Article DOI: 10.1021/jm9607010 BindingDB Entry DOI: 10.7270/Q2WH2P37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121581 (CHEMBL150253 | Morpholine-4-carboxylic acid (1-{[(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121561 (CHEMBL356167 | Morpholine-4-carboxylic acid (1-{[(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121545 (CHEMBL149523 | Morpholine-4-carboxylic acid (1-{[(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121544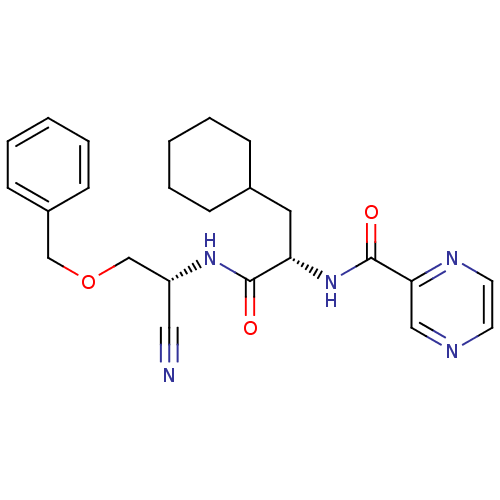 (CHEMBL153248 | Pyrazine-2-carboxylic acid {1-[(ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121543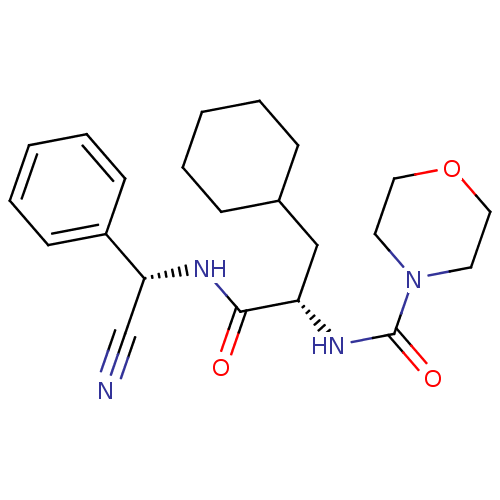 (CHEMBL435440 | Morpholine-4-carboxylic acid {1-[(c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50056994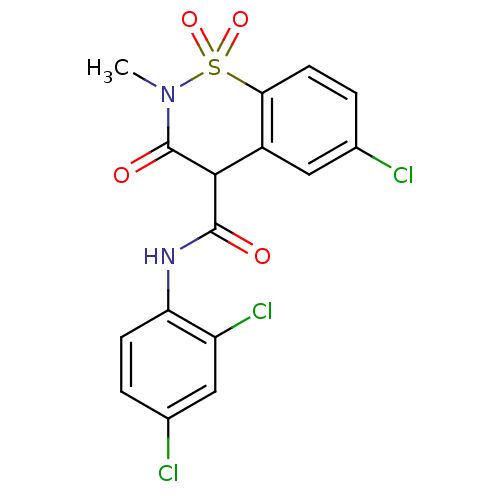 (6-Chloro-3-hydroxy-2-methyl-1,1-dioxo-1,2-dihydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Concentration of drug that causes a 50% decrease in the maximal inhibition of Prostaglandin G/H synthase 2 activity as measured by PGE-2 production | J Med Chem 40: 980-9 (1997) Article DOI: 10.1021/jm9607010 BindingDB Entry DOI: 10.7270/Q2WH2P37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1539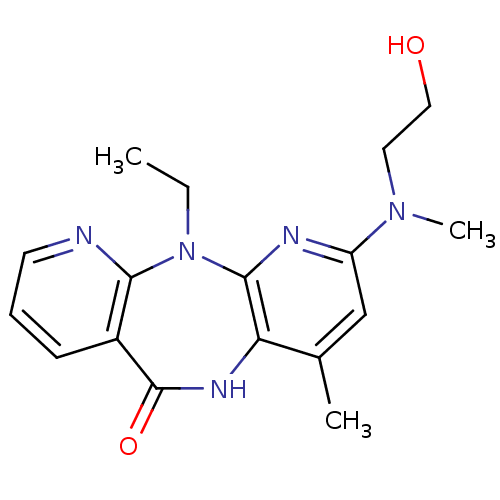 (2-ethyl-5-[(2-hydroxyethyl)(methyl)amino]-7-methyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 38: 4830-8 (1995) Article DOI: 10.1021/jm00024a010 BindingDB Entry DOI: 10.7270/Q23B5XBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1528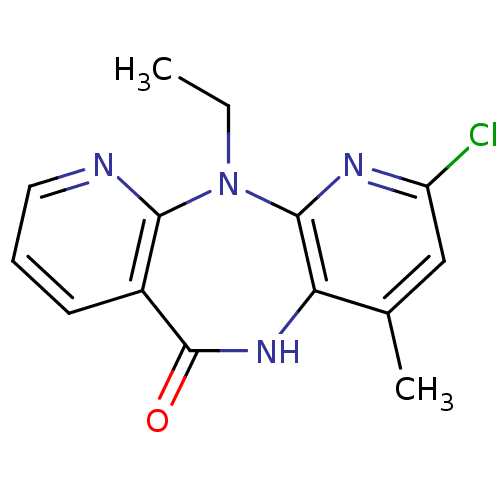 (5-chloro-2-ethyl-7-methyl-2,4,9,15-tetraazatricycl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 38: 4830-8 (1995) Article DOI: 10.1021/jm00024a010 BindingDB Entry DOI: 10.7270/Q23B5XBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,G789A]/[600-1155,G789A] (Human immunodeficiency virus type 1) | BDBM2014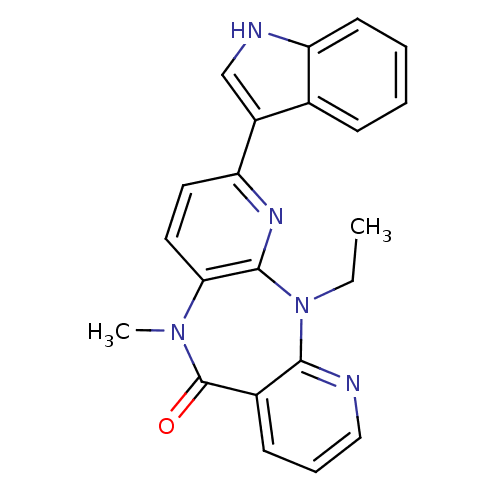 (2-ethyl-5-(1H-indol-3-yl)-9-methyl-2,4,9,15-tetraa...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 40: 2430-3 (1997) Article DOI: 10.1021/jm960837y BindingDB Entry DOI: 10.7270/Q2P26W9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121558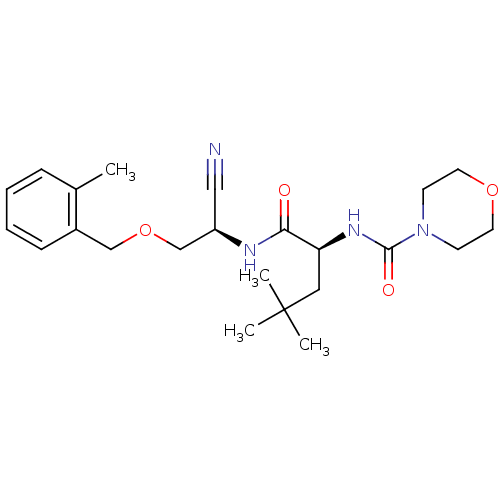 (CHEMBL152940 | Morpholine-4-carboxylic acid (1-{[c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121572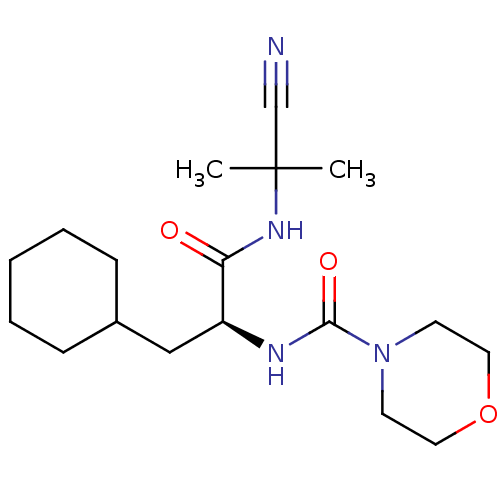 (CHEMBL150358 | Morpholine-4-carboxylic acid {1-[(c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50126419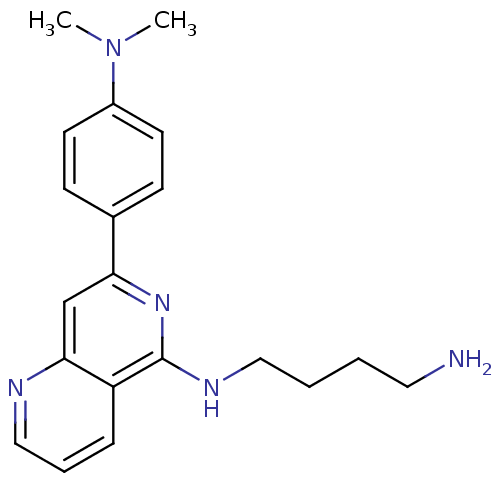 (CHEMBL282342 | N*1*-[7-(4-Dimethylamino-phenyl)-[1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against spleen tyrosine kinase (SYK) | Bioorg Med Chem Lett 13: 1415-8 (2003) BindingDB Entry DOI: 10.7270/Q2MC8ZCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,G789A]/[600-1155,G789A] (Human immunodeficiency virus type 1) | BDBM2019 (2-(6-Azaindol-3-yl)-5,11-dihydro-11-ethyl-5-methyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 40: 2430-3 (1997) Article DOI: 10.1021/jm960837y BindingDB Entry DOI: 10.7270/Q2P26W9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121548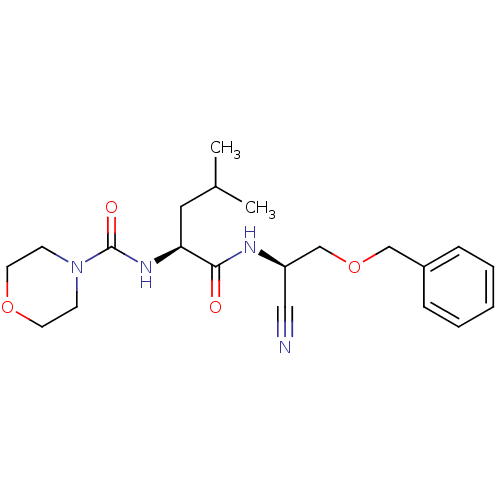 (CHEMBL153813 | MORPHOLINE-4-CARBOXYLIC ACID [1S-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121541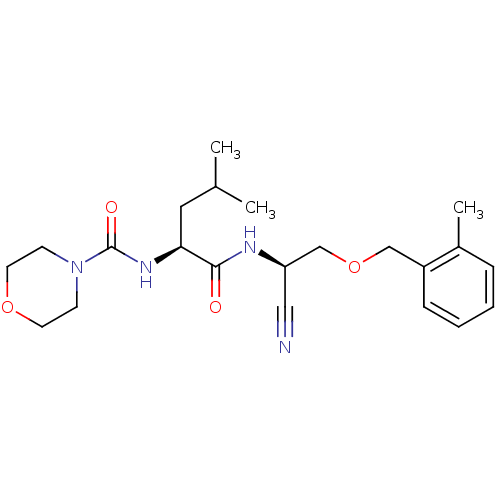 (CHEMBL346448 | Morpholine-4-carboxylic acid (1-{[c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM2008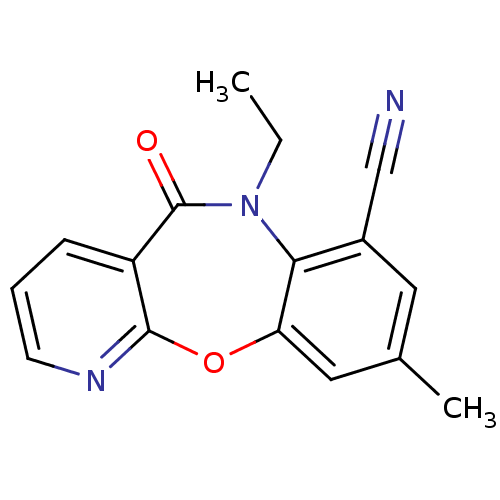 (10-ethyl-14-methyl-9-oxo-2-oxa-4,10-diazatricyclo[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 35: 1887-97 (1992) Article DOI: 10.1021/jm00088a027 BindingDB Entry DOI: 10.7270/Q2ST7N0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50126408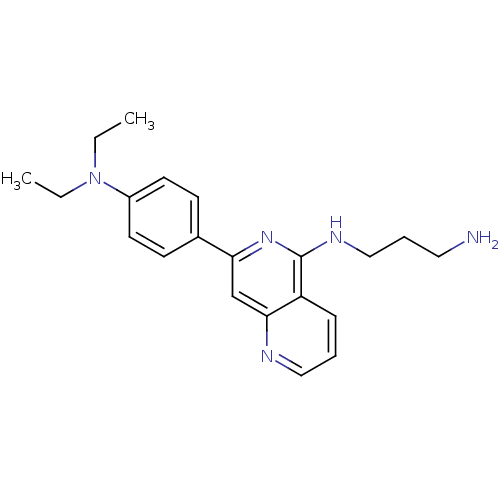 (CHEMBL30381 | N*1*-[7-(4-Diethylamino-phenyl)-[1,6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against spleen tyrosine kinase (SYK) | Bioorg Med Chem Lett 13: 1415-8 (2003) BindingDB Entry DOI: 10.7270/Q2MC8ZCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1520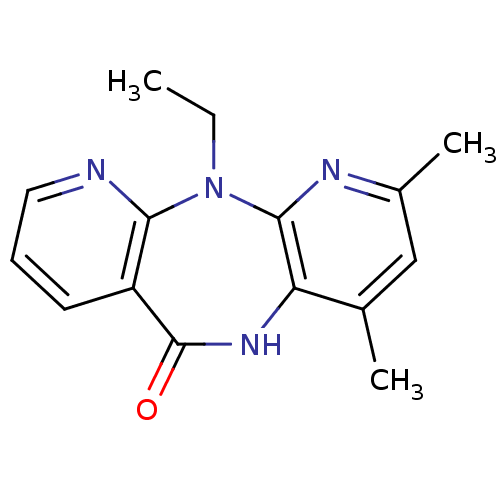 (2-ethyl-5,7-dimethyl-2,4,9,15-tetraazatricyclo[9.4...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 38: 4830-8 (1995) Article DOI: 10.1021/jm00024a010 BindingDB Entry DOI: 10.7270/Q23B5XBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1526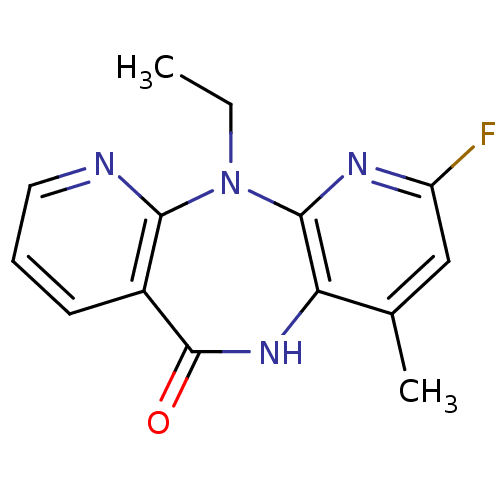 (2-ethyl-5-fluoro-7-methyl-2,4,9,15-tetraazatricycl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 38: 4830-8 (1995) Article DOI: 10.1021/jm00024a010 BindingDB Entry DOI: 10.7270/Q23B5XBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1527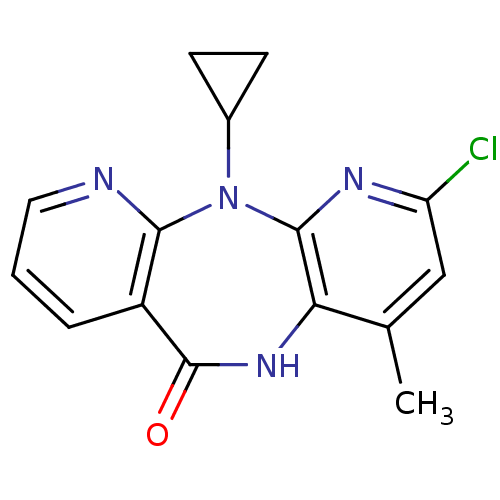 (5-chloro-2-cyclopropyl-7-methyl-2,4,9,15-tetraazat...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 38: 4830-8 (1995) Article DOI: 10.1021/jm00024a010 BindingDB Entry DOI: 10.7270/Q23B5XBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1540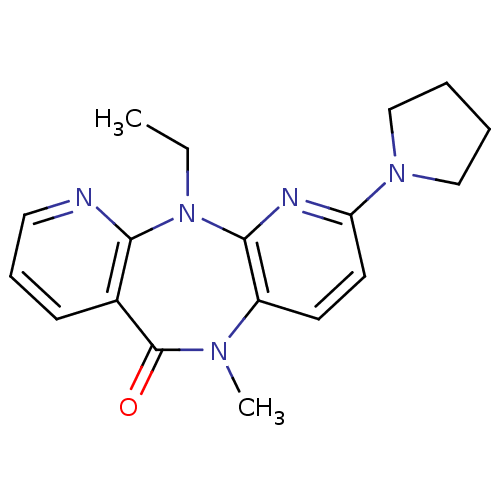 (2-ethyl-9-methyl-5-(pyrrolidin-1-yl)-2,4,9,15-tetr...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 38: 4830-8 (1995) Article DOI: 10.1021/jm00024a010 BindingDB Entry DOI: 10.7270/Q23B5XBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1549 (2-ethyl-7-methyl-5-(methylsulfanyl)-2,4,9,15-tetra...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 38: 4830-8 (1995) Article DOI: 10.1021/jm00024a010 BindingDB Entry DOI: 10.7270/Q23B5XBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1562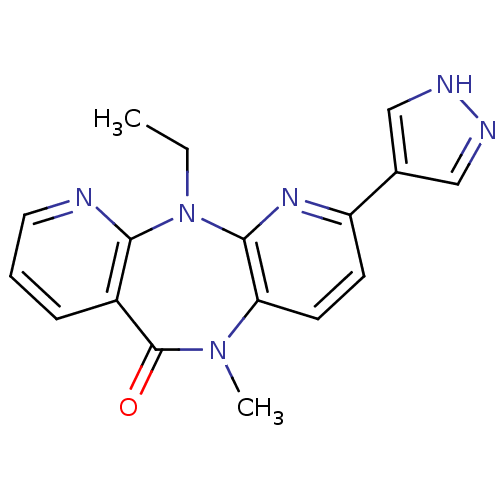 (2-ethyl-9-methyl-5-(1H-pyrazol-4-yl)-2,4,9,15-tetr...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 38: 4830-8 (1995) Article DOI: 10.1021/jm00024a010 BindingDB Entry DOI: 10.7270/Q23B5XBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1987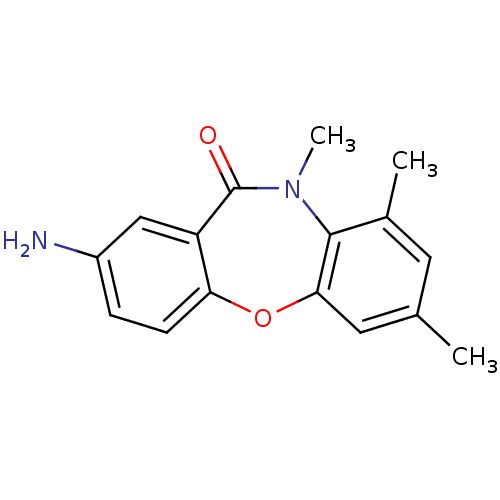 (13-amino-5,7,9-trimethyl-2-oxa-9-azatricyclo[9.4.0...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 35: 1887-97 (1992) Article DOI: 10.1021/jm00088a027 BindingDB Entry DOI: 10.7270/Q2ST7N0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121576 (CHEMBL153018 | Furan-2-carboxylic acid {1-[(benzyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM2012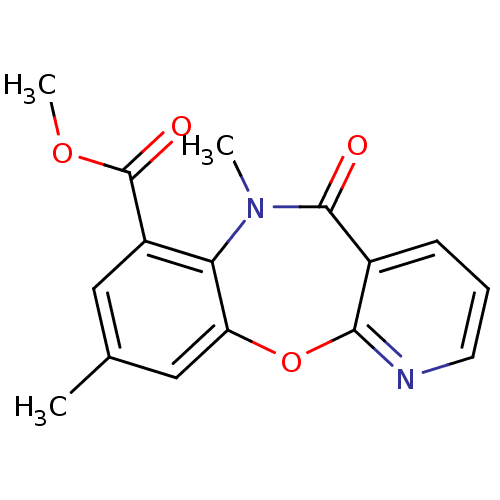 (Pyridobenzoxazepinone 80 | methyl 10,14-dimethyl-9...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 35: 1887-97 (1992) Article DOI: 10.1021/jm00088a027 BindingDB Entry DOI: 10.7270/Q2ST7N0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM2010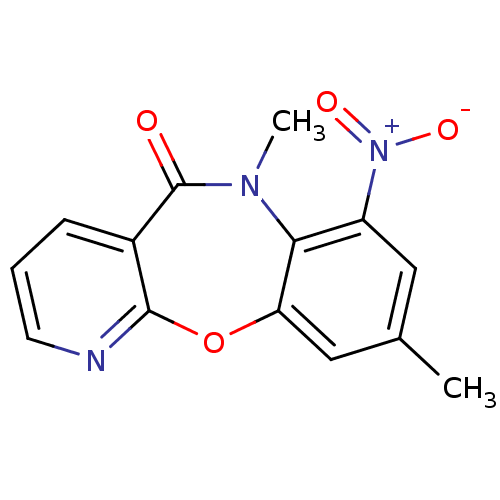 (10,14-dimethyl-12-nitro-2-oxa-4,10-diazatricyclo[9...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 35: 1887-97 (1992) Article DOI: 10.1021/jm00088a027 BindingDB Entry DOI: 10.7270/Q2ST7N0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121570 (CHEMBL348679 | Morpholine-4-carboxylic acid [1-(1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM2003 (6-amino-10-ethyl-12,14-dimethyl-2-oxa-4,10-diazatr...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 35: 1887-97 (1992) Article DOI: 10.1021/jm00088a027 BindingDB Entry DOI: 10.7270/Q2ST7N0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50121557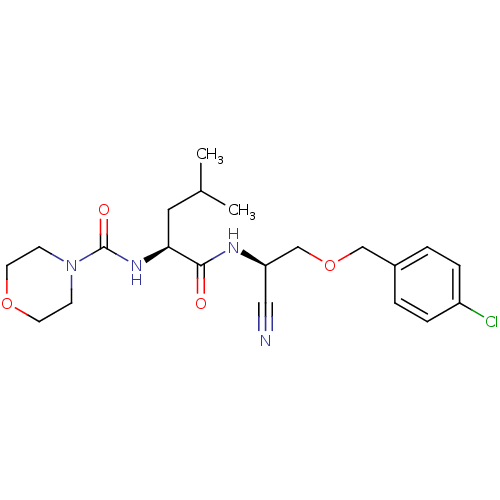 (CHEMBL151642 | Morpholine-4-carboxylic acid (1-{[(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration against human recombinant Cathepsin S expressed in baculovirus | J Med Chem 45: 5471-82 (2002) BindingDB Entry DOI: 10.7270/Q2P26XG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,Y780C]/[600-1155,Y780C] (Human immunodeficiency virus type 1) | BDBM2014 (2-ethyl-5-(1H-indol-3-yl)-9-methyl-2,4,9,15-tetraa...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 40: 2430-3 (1997) Article DOI: 10.1021/jm960837y BindingDB Entry DOI: 10.7270/Q2P26W9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM2014 (2-ethyl-5-(1H-indol-3-yl)-9-methyl-2,4,9,15-tetraa...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 40: 2430-3 (1997) Article DOI: 10.1021/jm960837y BindingDB Entry DOI: 10.7270/Q2P26W9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM2004 (10-ethyl-12,14-dimethyl-2-oxa-4,10-diazatricyclo[9...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 35: 1887-97 (1992) Article DOI: 10.1021/jm00088a027 BindingDB Entry DOI: 10.7270/Q2ST7N0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1531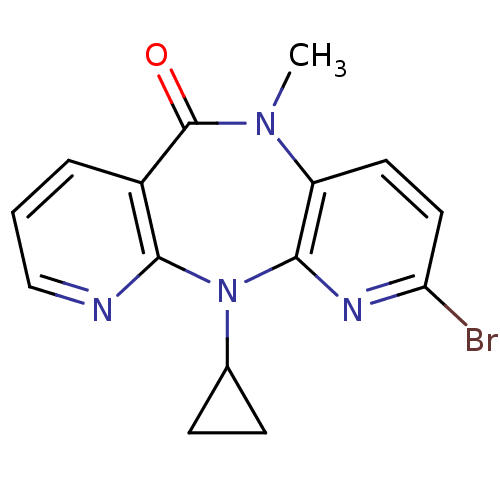 (2-Bromo-11-cyclopropyl-5-methyl-5,11-dihydro-6H-di...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 38: 4830-8 (1995) Article DOI: 10.1021/jm00024a010 BindingDB Entry DOI: 10.7270/Q23B5XBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1979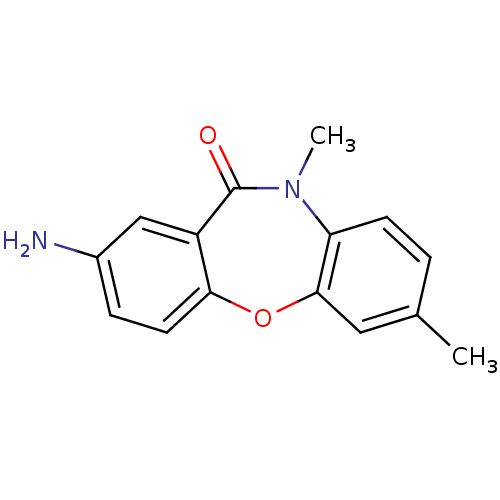 (13-amino-5,9-dimethyl-2-oxa-9-azatricyclo[9.4.0.0^...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 35: 1887-97 (1992) Article DOI: 10.1021/jm00088a027 BindingDB Entry DOI: 10.7270/Q2ST7N0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1553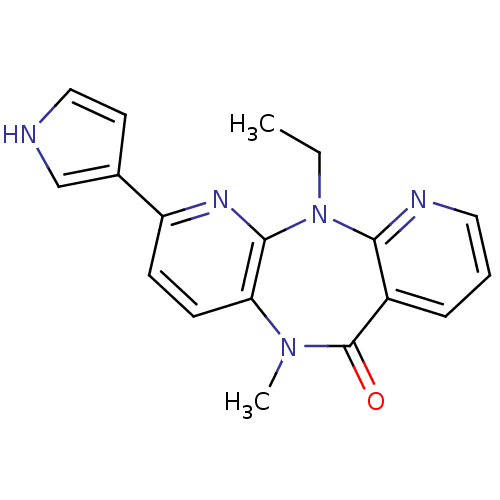 (2-ethyl-9-methyl-5-(1H-pyrrol-3-yl)-2,4,9,15-tetra...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 38: 4830-8 (1995) Article DOI: 10.1021/jm00024a010 BindingDB Entry DOI: 10.7270/Q23B5XBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM2006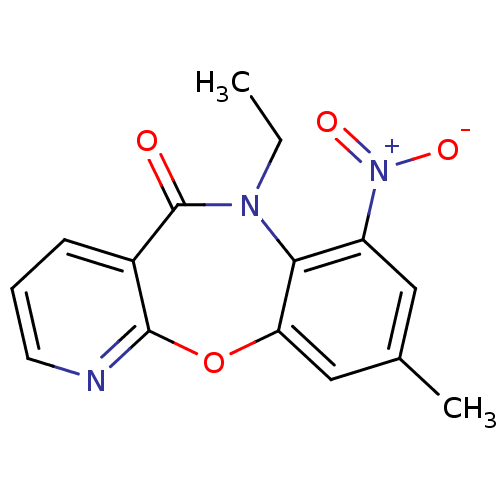 (10-ethyl-14-methyl-12-nitro-2-oxa-4,10-diazatricyc...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 35: 1887-97 (1992) Article DOI: 10.1021/jm00088a027 BindingDB Entry DOI: 10.7270/Q2ST7N0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,K702N]/[600-1155,K702N] (Human immunodeficiency virus type 1) | BDBM2019 (2-(6-Azaindol-3-yl)-5,11-dihydro-11-ethyl-5-methyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 40: 2430-3 (1997) Article DOI: 10.1021/jm960837y BindingDB Entry DOI: 10.7270/Q2P26W9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1553 (2-ethyl-9-methyl-5-(1H-pyrrol-3-yl)-2,4,9,15-tetra...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 40: 2430-3 (1997) Article DOI: 10.1021/jm960837y BindingDB Entry DOI: 10.7270/Q2P26W9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50126443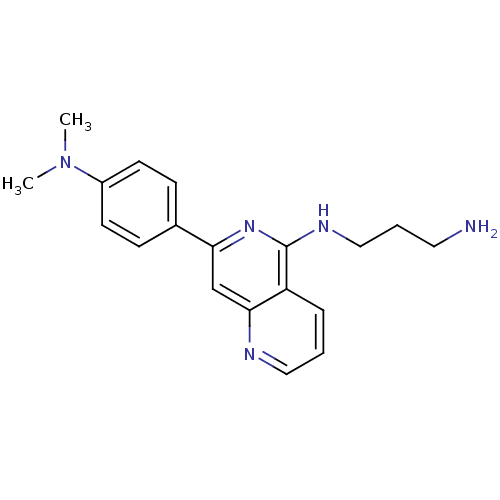 (CHEMBL30678 | N*1*-[7-(4-Dimethylamino-phenyl)-[1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against spleen tyrosine kinase (SYK) | Bioorg Med Chem Lett 13: 1415-8 (2003) BindingDB Entry DOI: 10.7270/Q2MC8ZCJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 508 total ) | Next | Last >> |