 Found 5076 hits with Last Name = 'berry' and Initial = 'a'
Found 5076 hits with Last Name = 'berry' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prothrombin
(Homo sapiens (Human)) | BDBM50072528
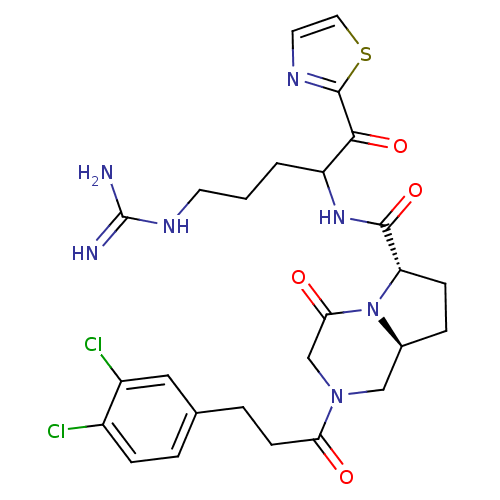
((6S,8aS)-2-[3-(3,4-Dichloro-phenyl)-propionyl]-4-o...)Show SMILES NC(=N)NCCCC(NC(=O)[C@@H]1CC[C@H]2CN(CC(=O)N12)C(=O)CCc1ccc(Cl)c(Cl)c1)C(=O)c1nccs1 Show InChI InChI=1S/C26H31Cl2N7O4S/c27-17-6-3-15(12-18(17)28)4-8-21(36)34-13-16-5-7-20(35(16)22(37)14-34)24(39)33-19(2-1-9-32-26(29)30)23(38)25-31-10-11-40-25/h3,6,10-12,16,19-20H,1-2,4-5,7-9,13-14H2,(H,33,39)(H4,29,30,32)/t16-,19?,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM10246
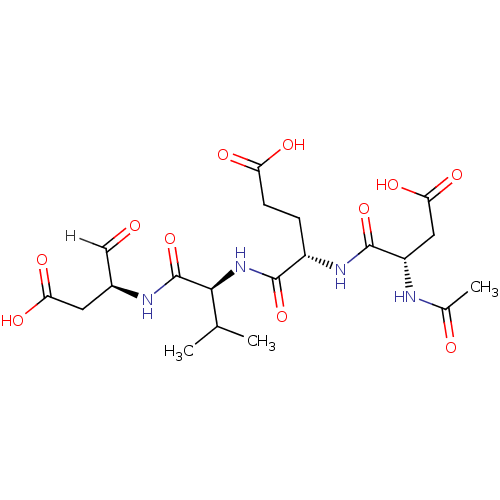
((4S)-4-{[(1S)-1-{[(2S)-1-carboxy-3-oxopropan-2-yl]...)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(=O)N[C@@H](CC(O)=O)C=O |r| Show InChI InChI=1S/C20H30N4O11/c1-9(2)17(20(35)22-11(8-25)6-15(29)30)24-18(33)12(4-5-14(27)28)23-19(34)13(7-16(31)32)21-10(3)26/h8-9,11-13,17H,4-7H2,1-3H3,(H,21,26)(H,22,35)(H,23,34)(H,24,33)(H,27,28)(H,29,30)(H,31,32)/t11-,12-,13-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.230 | -55.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Research Laboratories
| Assay Description
The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ... |
J Biol Chem 273: 32608-13 (1998)
Article DOI: 10.1074/jbc.273.49.32608
BindingDB Entry DOI: 10.7270/Q26D5R5V |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072536
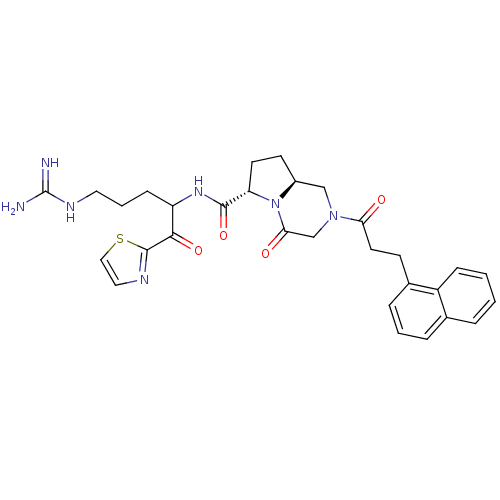
((6S,8aS)-2-(3-Naphthalen-1-yl-propionyl)-4-oxo-oct...)Show SMILES NC(=N)NCCCC(NC(=O)[C@@H]1CC[C@H]2CN(CC(=O)N12)C(=O)CCc1cccc2ccccc12)C(=O)c1nccs1 Show InChI InChI=1S/C30H35N7O4S/c31-30(32)34-14-4-9-23(27(40)29-33-15-16-42-29)35-28(41)24-12-11-21-17-36(18-26(39)37(21)24)25(38)13-10-20-7-3-6-19-5-1-2-8-22(19)20/h1-3,5-8,15-16,21,23-24H,4,9-14,17-18H2,(H,35,41)(H4,31,32,34)/t21-,23?,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072537
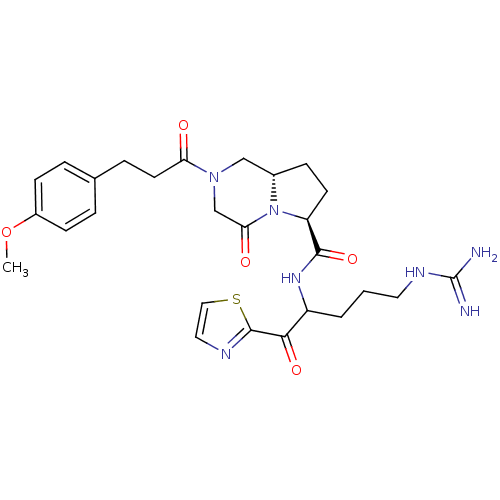
((6S,8aS)-2-[3-(4-Methoxy-phenyl)-propionyl]-4-oxo-...)Show SMILES COc1ccc(CCC(=O)N2C[C@@H]3CC[C@H](N3C(=O)C2)C(=O)NC(CCCNC(N)=N)C(=O)c2nccs2)cc1 Show InChI InChI=1S/C27H35N7O5S/c1-39-19-8-4-17(5-9-19)6-11-22(35)33-15-18-7-10-21(34(18)23(36)16-33)25(38)32-20(3-2-12-31-27(28)29)24(37)26-30-13-14-40-26/h4-5,8-9,13-14,18,20-21H,2-3,6-7,10-12,15-16H2,1H3,(H,32,38)(H4,28,29,31)/t18-,20?,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072524
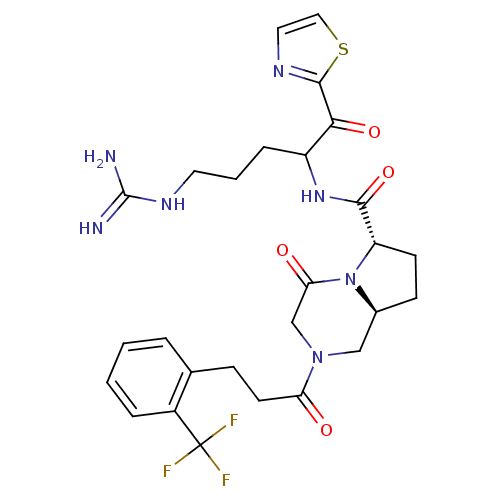
((6S,8aS)-4-Oxo-2-[3-(2-trifluoromethyl-phenyl)-pro...)Show SMILES NC(=N)NCCCC(NC(=O)[C@@H]1CC[C@H]2CN(CC(=O)N12)C(=O)CCc1ccccc1C(F)(F)F)C(=O)c1nccs1 Show InChI InChI=1S/C27H32F3N7O4S/c28-27(29,30)18-5-2-1-4-16(18)7-10-21(38)36-14-17-8-9-20(37(17)22(39)15-36)24(41)35-19(6-3-11-34-26(31)32)23(40)25-33-12-13-42-25/h1-2,4-5,12-13,17,19-20H,3,6-11,14-15H2,(H,35,41)(H4,31,32,34)/t17-,19?,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072527
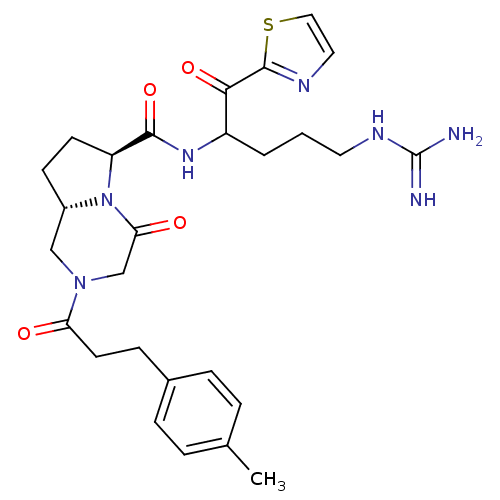
((6S,8aS)-4-Oxo-2-(3-p-tolyl-propionyl)-octahydro-p...)Show SMILES Cc1ccc(CCC(=O)N2C[C@@H]3CC[C@H](N3C(=O)C2)C(=O)NC(CCCNC(N)=N)C(=O)c2nccs2)cc1 Show InChI InChI=1S/C27H35N7O4S/c1-17-4-6-18(7-5-17)8-11-22(35)33-15-19-9-10-21(34(19)23(36)16-33)25(38)32-20(3-2-12-31-27(28)29)24(37)26-30-13-14-39-26/h4-7,13-14,19-21H,2-3,8-12,15-16H2,1H3,(H,32,38)(H4,28,29,31)/t19-,20?,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072534
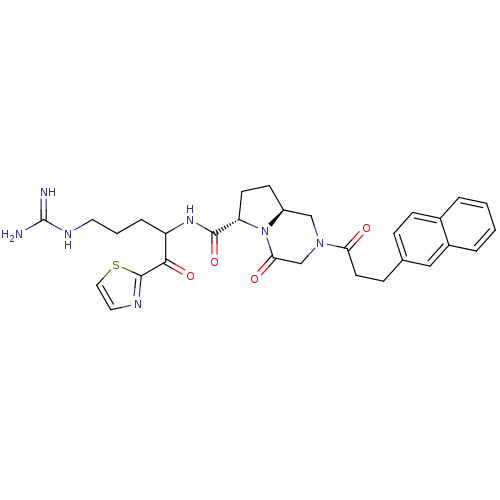
((6S,8aS)-2-(3-Naphthalen-2-yl-propionyl)-4-oxo-oct...)Show SMILES NC(=N)NCCCC(NC(=O)[C@@H]1CC[C@H]2CN(CC(=O)N12)C(=O)CCc1ccc2ccccc2c1)C(=O)c1nccs1 Show InChI InChI=1S/C30H35N7O4S/c31-30(32)34-13-3-6-23(27(40)29-33-14-15-42-29)35-28(41)24-11-10-22-17-36(18-26(39)37(22)24)25(38)12-8-19-7-9-20-4-1-2-5-21(20)16-19/h1-2,4-5,7,9,14-16,22-24H,3,6,8,10-13,17-18H2,(H,35,41)(H4,31,32,34)/t22-,23?,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072532

((6S,8aS)-4-Oxo-2-(3-phenyl-propionyl)-octahydro-py...)Show SMILES NC(=N)NCCCC(NC(=O)[C@@H]1CC[C@H]2CN(CC(=O)N12)C(=O)CCc1ccccc1)C(=O)c1nccs1 Show InChI InChI=1S/C26H33N7O4S/c27-26(28)30-12-4-7-19(23(36)25-29-13-14-38-25)31-24(37)20-10-9-18-15-32(16-22(35)33(18)20)21(34)11-8-17-5-2-1-3-6-17/h1-3,5-6,13-14,18-20H,4,7-12,15-16H2,(H,31,37)(H4,27,28,30)/t18-,19?,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50003020
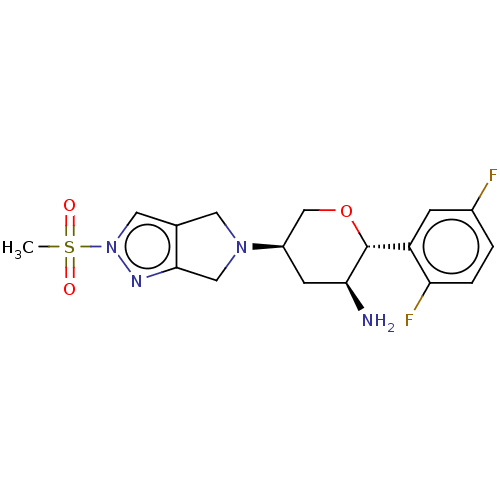
(MK-3102 | OMARIGLIPTIN | US10155775, Omarigliptin ...)Show SMILES CS(=O)(=O)n1cc2CN(Cc2n1)[C@H]1CO[C@@H]([C@@H](N)C1)c1cc(F)ccc1F |r| Show InChI InChI=1S/C17H20F2N4O3S/c1-27(24,25)23-7-10-6-22(8-16(10)21-23)12-5-15(20)17(26-9-12)13-4-11(18)2-3-14(13)19/h2-4,7,12,15,17H,5-6,8-9,20H2,1H3/t12-,15+,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Competitive reversible inhibition of DPP4 (unknown origin) |
J Med Chem 57: 3205-12 (2014)
Article DOI: 10.1021/jm401992e
BindingDB Entry DOI: 10.7270/Q2WD423H |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072520
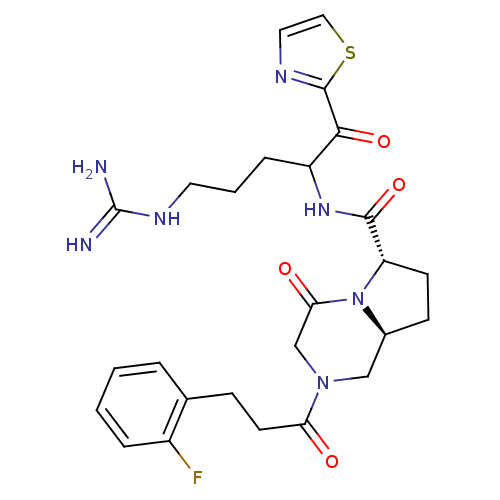
((6S,8aS)-2-[3-(2-Fluoro-phenyl)-propionyl]-4-oxo-o...)Show SMILES NC(=N)NCCCC(NC(=O)[C@@H]1CC[C@H]2CN(CC(=O)N12)C(=O)CCc1ccccc1F)C(=O)c1nccs1 Show InChI InChI=1S/C26H32FN7O4S/c27-18-5-2-1-4-16(18)7-10-21(35)33-14-17-8-9-20(34(17)22(36)15-33)24(38)32-19(6-3-11-31-26(28)29)23(37)25-30-12-13-39-25/h1-2,4-5,12-13,17,19-20H,3,6-11,14-15H2,(H,32,38)(H4,28,29,31)/t17-,19?,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50080924
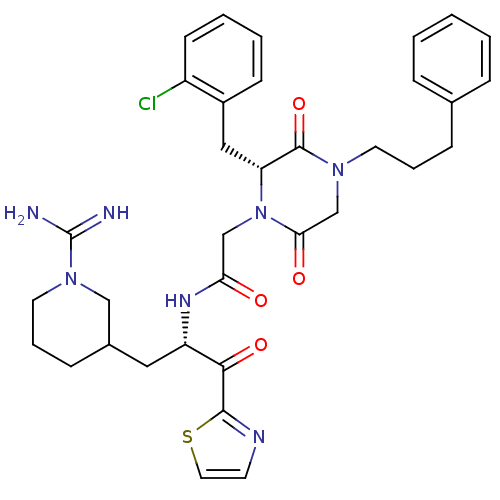
(CHEMBL83260 | N-[(S)-1-(1-Carbamimidoyl-piperidin-...)Show SMILES NC(=N)N1CCCC(C[C@H](NC(=O)CN2[C@H](Cc3ccccc3Cl)C(=O)N(CCCc3ccccc3)CC2=O)C(=O)c2nccs2)C1 Show InChI InChI=1S/C34H40ClN7O4S/c35-26-13-5-4-12-25(26)19-28-33(46)40(15-6-10-23-8-2-1-3-9-23)22-30(44)42(28)21-29(43)39-27(31(45)32-38-14-17-47-32)18-24-11-7-16-41(20-24)34(36)37/h1-5,8-9,12-14,17,24,27-28H,6-7,10-11,15-16,18-22H2,(H3,36,37)(H,39,43)/t24?,27-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 9: 2503-8 (1999)
BindingDB Entry DOI: 10.7270/Q2028QRW |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072533
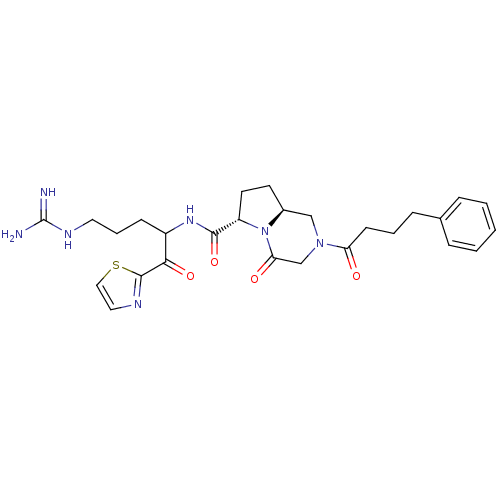
((6S,8aS)-4-Oxo-2-(4-phenyl-butyryl)-octahydro-pyrr...)Show SMILES NC(=N)NCCCC(NC(=O)[C@@H]1CC[C@H]2CN(CC(=O)N12)C(=O)CCCc1ccccc1)C(=O)c1nccs1 Show InChI InChI=1S/C27H35N7O4S/c28-27(29)31-13-5-9-20(24(37)26-30-14-15-39-26)32-25(38)21-12-11-19-16-33(17-23(36)34(19)21)22(35)10-4-8-18-6-2-1-3-7-18/h1-3,6-7,14-15,19-21H,4-5,8-13,16-17H2,(H,32,38)(H4,28,29,31)/t19-,20?,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072530
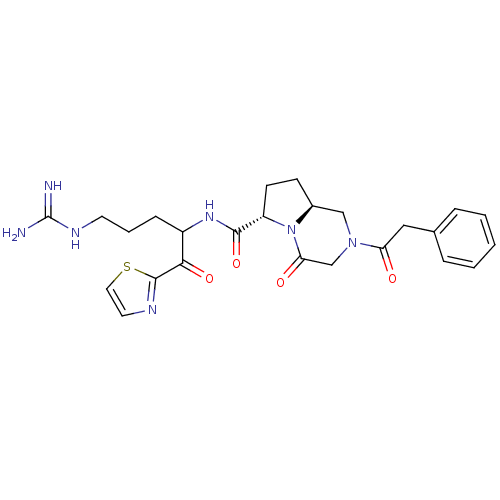
((6S,8aS)-4-Oxo-2-phenylacetyl-octahydro-pyrrolo[1,...)Show SMILES NC(=N)NCCCC(NC(=O)[C@@H]1CC[C@H]2CN(CC(=O)N12)C(=O)Cc1ccccc1)C(=O)c1nccs1 Show InChI InChI=1S/C25H31N7O4S/c26-25(27)29-10-4-7-18(22(35)24-28-11-12-37-24)30-23(36)19-9-8-17-14-31(15-21(34)32(17)19)20(33)13-16-5-2-1-3-6-16/h1-3,5-6,11-12,17-19H,4,7-10,13-15H2,(H,30,36)(H4,26,27,29)/t17-,18?,19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50282978
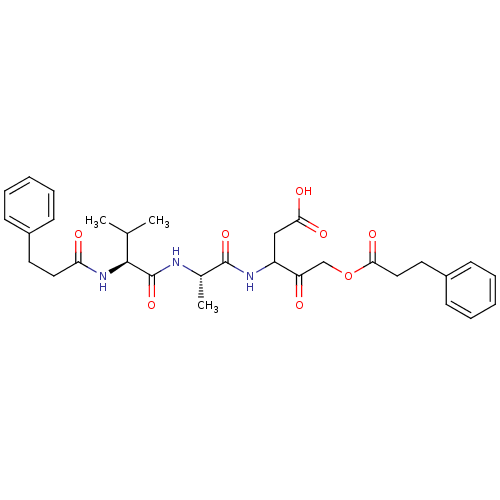
(3-{(S)-2-[(S)-3-Methyl-2-(3-phenyl-propionylamino)...)Show SMILES CC(C)[C@H](NC(=O)CCc1ccccc1)C(=O)N[C@@H](C)C(=O)NC(CC(O)=O)C(=O)COC(=O)CCc1ccccc1 Show InChI InChI=1S/C31H39N3O8/c1-20(2)29(34-26(36)16-14-22-10-6-4-7-11-22)31(41)32-21(3)30(40)33-24(18-27(37)38)25(35)19-42-28(39)17-15-23-12-8-5-9-13-23/h4-13,20-21,24,29H,14-19H2,1-3H3,(H,32,41)(H,33,40)(H,34,36)(H,37,38)/t21-,24?,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition of cysteine proteinase IL-1 beta converting enzyme (ICE) |
Bioorg Med Chem Lett 4: 1965-1968 (1994)
Article DOI: 10.1016/S0960-894X(01)80544-8
BindingDB Entry DOI: 10.7270/Q24M94GD |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072541

((6S,8aS)-4-Oxo-2-(3-pyridin-2-yl-propionyl)-octahy...)Show SMILES NC(=N)NCCCC(NC(=O)[C@@H]1CC[C@H]2CN(CC(=O)N12)C(=O)CCc1ccccn1)C(=O)c1nccs1 Show InChI InChI=1S/C25H32N8O4S/c26-25(27)30-11-3-5-18(22(36)24-29-12-13-38-24)31-23(37)19-8-7-17-14-32(15-21(35)33(17)19)20(34)9-6-16-4-1-2-10-28-16/h1-2,4,10,12-13,17-19H,3,5-9,11,14-15H2,(H,31,37)(H4,26,27,30)/t17-,18?,19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072519

((6S,8aS)-2-[(S)-2-Amino-3-(4-fluoro-phenyl)-propio...)Show SMILES N[C@@H](Cc1ccc(F)cc1)C(=O)N1C[C@@H]2CC[C@H](N2C(=O)C1)C(=O)NC(CCCNC(N)=N)C(=O)c1nccs1 Show InChI InChI=1S/C26H33FN8O4S/c27-16-5-3-15(4-6-16)12-18(28)25(39)34-13-17-7-8-20(35(17)21(36)14-34)23(38)33-19(2-1-9-32-26(29)30)22(37)24-31-10-11-40-24/h3-6,10-11,17-20H,1-2,7-9,12-14,28H2,(H,33,38)(H4,29,30,32)/t17-,18-,19?,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50080911

(2-((S)-4-Benzyl-2-naphthalen-2-ylmethyl-3,6-dioxo-...)Show SMILES NC(=N)N1CCCC(C[C@H](NC(=O)CN2[C@@H](Cc3ccc4ccccc4c3)C(=O)N(Cc3ccccc3)CC2=O)C(=O)c2nccs2)C1 Show InChI InChI=1S/C36H39N7O4S/c37-36(38)41-15-6-9-26(21-41)18-29(33(46)34-39-14-16-48-34)40-31(44)22-43-30(19-25-12-13-27-10-4-5-11-28(27)17-25)35(47)42(23-32(43)45)20-24-7-2-1-3-8-24/h1-5,7-8,10-14,16-17,26,29-30H,6,9,15,18-23H2,(H3,37,38)(H,40,44)/t26?,29-,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound required against thrombin was determined |
Bioorg Med Chem Lett 9: 2503-8 (1999)
BindingDB Entry DOI: 10.7270/Q2028QRW |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072529
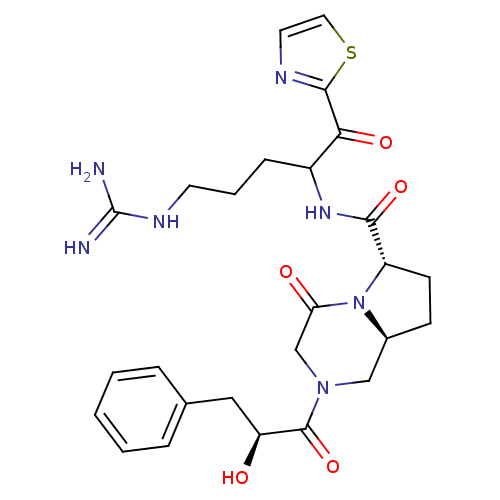
((6S,8aS)-2-((S)-2-Hydroxy-3-phenyl-propionyl)-4-ox...)Show SMILES NC(=N)NCCCC(NC(=O)[C@@H]1CC[C@H]2CN(CC(=O)N12)C(=O)[C@@H](O)Cc1ccccc1)C(=O)c1nccs1 Show InChI InChI=1S/C26H33N7O5S/c27-26(28)30-10-4-7-18(22(36)24-29-11-12-39-24)31-23(37)19-9-8-17-14-32(15-21(35)33(17)19)25(38)20(34)13-16-5-2-1-3-6-16/h1-3,5-6,11-12,17-20,34H,4,7-10,13-15H2,(H,31,37)(H4,27,28,30)/t17-,18?,19-,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50282981
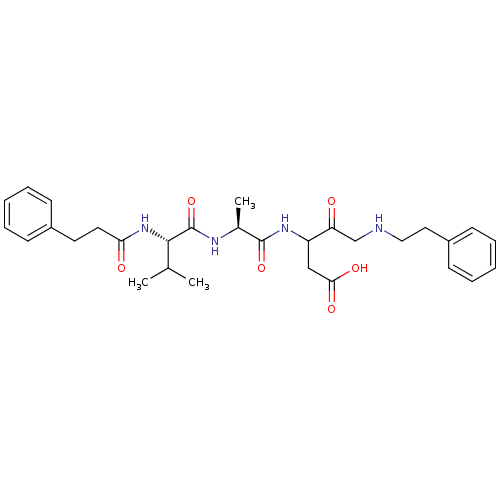
(3-{(S)-2-[(S)-3-Methyl-2-(3-phenyl-propionylamino)...)Show SMILES CC(C)[C@H](NC(=O)CCc1ccccc1)C(=O)N[C@@H](C)C(=O)NC(CC(O)=O)C(=O)CNCCc1ccccc1 Show InChI InChI=1S/C30H40N4O6/c1-20(2)28(34-26(36)15-14-22-10-6-4-7-11-22)30(40)32-21(3)29(39)33-24(18-27(37)38)25(35)19-31-17-16-23-12-8-5-9-13-23/h4-13,20-21,24,28,31H,14-19H2,1-3H3,(H,32,40)(H,33,39)(H,34,36)(H,37,38)/t21-,24?,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition of cysteine proteinase IL-1 beta converting enzyme (ICE) |
Bioorg Med Chem Lett 4: 1965-1968 (1994)
Article DOI: 10.1016/S0960-894X(01)80544-8
BindingDB Entry DOI: 10.7270/Q24M94GD |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072535
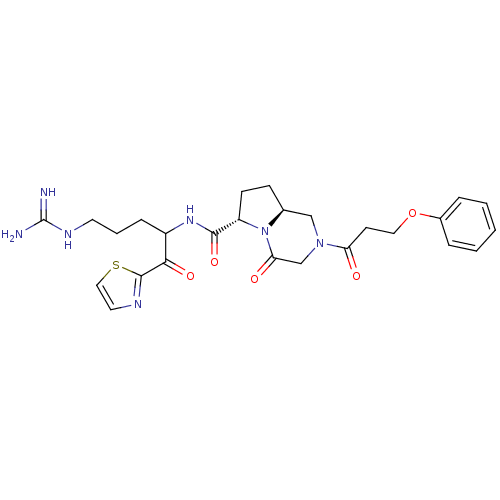
((6S,8aS)-4-Oxo-2-(3-phenoxy-propionyl)-octahydro-p...)Show SMILES NC(=N)NCCCC(NC(=O)[C@@H]1CC[C@H]2CN(CC(=O)N12)C(=O)CCOc1ccccc1)C(=O)c1nccs1 Show InChI InChI=1S/C26H33N7O5S/c27-26(28)30-11-4-7-19(23(36)25-29-12-14-39-25)31-24(37)20-9-8-17-15-32(16-22(35)33(17)20)21(34)10-13-38-18-5-2-1-3-6-18/h1-3,5-6,12,14,17,19-20H,4,7-11,13,15-16H2,(H,31,37)(H4,27,28,30)/t17-,19?,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116264

((S)-3-({(S)-(S)-5-[(S)-2-((S)-Acetyl-methyl-amino)...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)N[C@@H](CC(O)=O)C(=O)c1nnc(o1)-c1ccccc1 Show InChI InChI=1S/C34H38N6O8/c1-5-18(2)27(39(4)19(3)41)31(46)35-23-15-14-20-12-9-13-22-16-25(40(28(20)22)34(23)47)30(45)36-24(17-26(42)43)29(44)33-38-37-32(48-33)21-10-7-6-8-11-21/h6-13,18,23-25,27H,5,14-17H2,1-4H3,(H,35,46)(H,36,45)(H,42,43)/t18-,23-,24-,25-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116262
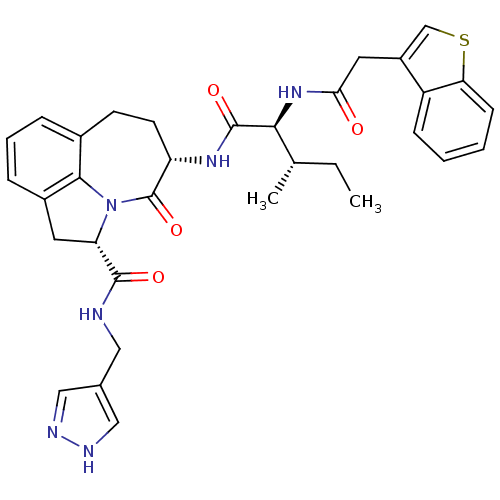
((2S,5S)-5-[(S)-2-(2-Benzo[b]thiophen-3-yl-acetylam...)Show SMILES CC[C@H](C)[C@H](NC(=O)Cc1csc2ccccc12)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)NCc1cn[nH]c1 Show InChI InChI=1S/C33H36N6O4S/c1-3-19(2)29(38-28(40)14-23-18-44-27-10-5-4-9-24(23)27)32(42)37-25-12-11-21-7-6-8-22-13-26(39(30(21)22)33(25)43)31(41)34-15-20-16-35-36-17-20/h4-10,16-19,25-26,29H,3,11-15H2,1-2H3,(H,34,41)(H,35,36)(H,37,42)(H,38,40)/t19-,25-,26-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50080920

(CHEMBL313769 | N-[(S)-1-(1-Carbamimidoyl-piperidin...)Show SMILES NC(=N)N1CCCC(C[C@H](NC(=O)CN2[C@H](Cc3ccc(Cl)c(Cl)c3)C(=O)N(CCCc3ccccc3)CC2=O)C(=O)c2nccs2)C1 Show InChI InChI=1S/C34H39Cl2N7O4S/c35-25-11-10-23(16-26(25)36)18-28-33(47)41(13-4-8-22-6-2-1-3-7-22)21-30(45)43(28)20-29(44)40-27(31(46)32-39-12-15-48-32)17-24-9-5-14-42(19-24)34(37)38/h1-3,6-7,10-12,15-16,24,27-28H,4-5,8-9,13-14,17-21H2,(H3,37,38)(H,40,44)/t24?,27-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 9: 2503-8 (1999)
BindingDB Entry DOI: 10.7270/Q2028QRW |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116260
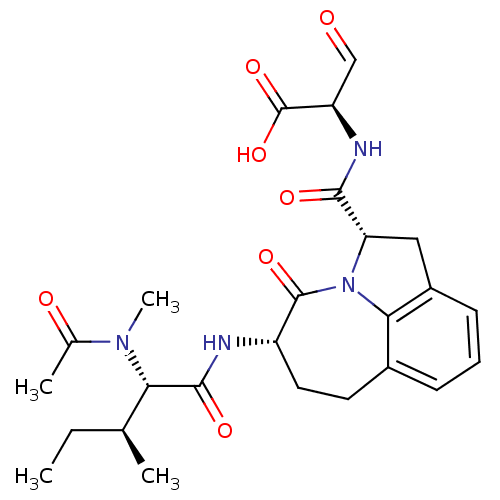
((R)-2-({(2S,5S)-5-[(S)-2-(Acetyl-methyl-amino)-3-(...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)N[C@H](C=O)C(O)=O Show InChI InChI=1S/C25H32N4O7/c1-5-13(2)20(28(4)14(3)31)23(33)26-17-10-9-15-7-6-8-16-11-19(29(21(15)16)24(17)34)22(32)27-18(12-30)25(35)36/h6-8,12-13,17-20H,5,9-11H2,1-4H3,(H,26,33)(H,27,32)(H,35,36)/t13-,17-,18+,19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50282984
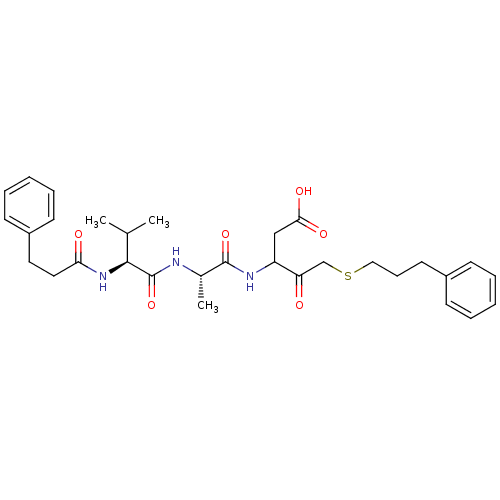
(3-{(S)-2-[(S)-3-Methyl-2-(3-phenyl-propionylamino)...)Show SMILES CC(C)[C@H](NC(=O)CCc1ccccc1)C(=O)N[C@@H](C)C(=O)NC(CC(O)=O)C(=O)CSCCCc1ccccc1 Show InChI InChI=1S/C31H41N3O6S/c1-21(2)29(34-27(36)17-16-24-13-8-5-9-14-24)31(40)32-22(3)30(39)33-25(19-28(37)38)26(35)20-41-18-10-15-23-11-6-4-7-12-23/h4-9,11-14,21-22,25,29H,10,15-20H2,1-3H3,(H,32,40)(H,33,39)(H,34,36)(H,37,38)/t22-,25?,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition of cysteine proteinase IL-1 beta converting enzyme (ICE) |
Bioorg Med Chem Lett 4: 1965-1968 (1994)
Article DOI: 10.1016/S0960-894X(01)80544-8
BindingDB Entry DOI: 10.7270/Q24M94GD |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116256
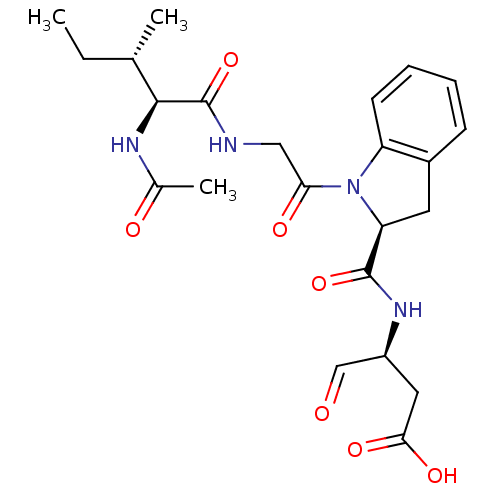
((S)-3-({1-[(S)-2-((3S,4S)-2-Acetylamino-3-methyl-p...)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)NCC(=O)N1[C@@H](Cc2ccccc12)C(=O)N[C@@H](CC(O)=O)C=O Show InChI InChI=1S/C23H30N4O7/c1-4-13(2)21(25-14(3)29)23(34)24-11-19(30)27-17-8-6-5-7-15(17)9-18(27)22(33)26-16(12-28)10-20(31)32/h5-8,12-13,16,18,21H,4,9-11H2,1-3H3,(H,24,34)(H,25,29)(H,26,33)(H,31,32)/t13-,16-,18-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116255
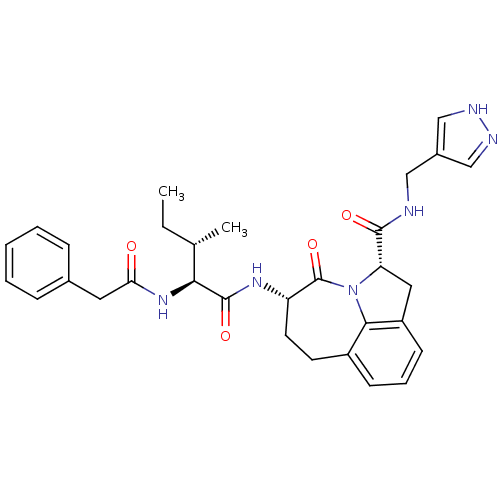
((2S,5S)-5-((S)-3-(S)-Methyl-2-phenylacetylamino-pe...)Show SMILES CC[C@H](C)[C@H](NC(=O)Cc1ccccc1)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)NCc1cn[nH]c1 Show InChI InChI=1S/C31H36N6O4/c1-3-19(2)27(36-26(38)14-20-8-5-4-6-9-20)30(40)35-24-13-12-22-10-7-11-23-15-25(37(28(22)23)31(24)41)29(39)32-16-21-17-33-34-18-21/h4-11,17-19,24-25,27H,3,12-16H2,1-2H3,(H,32,39)(H,33,34)(H,35,40)(H,36,38)/t19-,24-,25-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072521
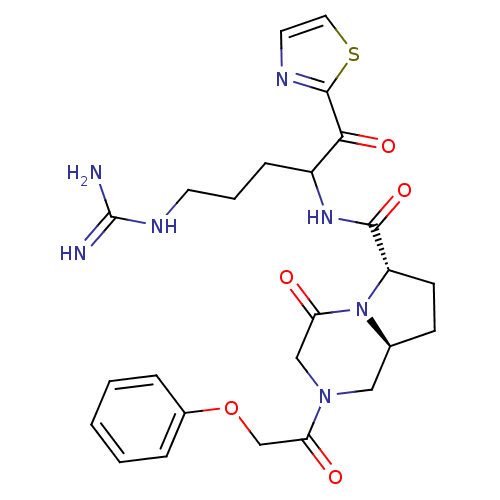
((6S,8aS)-4-Oxo-2-(2-phenoxy-acetyl)-octahydro-pyrr...)Show SMILES NC(=N)NCCCC(NC(=O)[C@@H]1CC[C@H]2CN(CC(=O)N12)C(=O)COc1ccccc1)C(=O)c1nccs1 Show InChI InChI=1S/C25H31N7O5S/c26-25(27)29-10-4-7-18(22(35)24-28-11-12-38-24)30-23(36)19-9-8-16-13-31(14-20(33)32(16)19)21(34)15-37-17-5-2-1-3-6-17/h1-3,5-6,11-12,16,18-19H,4,7-10,13-15H2,(H,30,36)(H4,26,27,29)/t16-,18?,19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116267
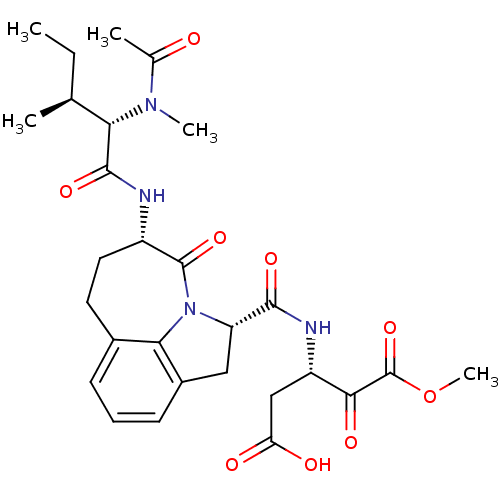
((S)-3-({(S)-(S)-5-[(S)-2-((S)-Acetyl-methyl-amino)...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)N[C@@H](CC(O)=O)C(=O)C(=O)OC Show InChI InChI=1S/C28H36N4O9/c1-6-14(2)22(31(4)15(3)33)26(38)29-18-11-10-16-8-7-9-17-12-20(32(23(16)17)27(18)39)25(37)30-19(13-21(34)35)24(36)28(40)41-5/h7-9,14,18-20,22H,6,10-13H2,1-5H3,(H,29,38)(H,30,37)(H,34,35)/t14-,18-,19-,20-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072538
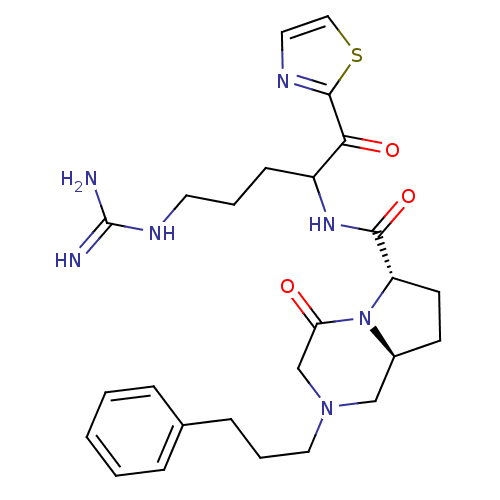
((6S,8aS)-4-Oxo-2-(3-phenyl-propyl)-octahydro-pyrro...)Show SMILES NC(=N)NCCCC(NC(=O)[C@@H]1CC[C@H]2CN(CCCc3ccccc3)CC(=O)N12)C(=O)c1nccs1 Show InChI InChI=1S/C26H35N7O3S/c27-26(28)30-12-4-9-20(23(35)25-29-13-15-37-25)31-24(36)21-11-10-19-16-32(17-22(34)33(19)21)14-5-8-18-6-2-1-3-7-18/h1-3,6-7,13,15,19-21H,4-5,8-12,14,16-17H2,(H,31,36)(H4,27,28,30)/t19-,20?,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM10246

((4S)-4-{[(1S)-1-{[(2S)-1-carboxy-3-oxopropan-2-yl]...)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(=O)N[C@@H](CC(O)=O)C=O |r| Show InChI InChI=1S/C20H30N4O11/c1-9(2)17(20(35)22-11(8-25)6-15(29)30)24-18(33)12(4-5-14(27)28)23-19(34)13(7-16(31)32)21-10(3)26/h8-9,11-13,17H,4-7H2,1-3H3,(H,21,26)(H,22,35)(H,23,34)(H,24,33)(H,27,28)(H,29,30)(H,31,32)/t11-,12-,13-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 18 | -44.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Research Laboratories
| Assay Description
The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ... |
J Biol Chem 273: 32608-13 (1998)
Article DOI: 10.1074/jbc.273.49.32608
BindingDB Entry DOI: 10.7270/Q26D5R5V |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072526
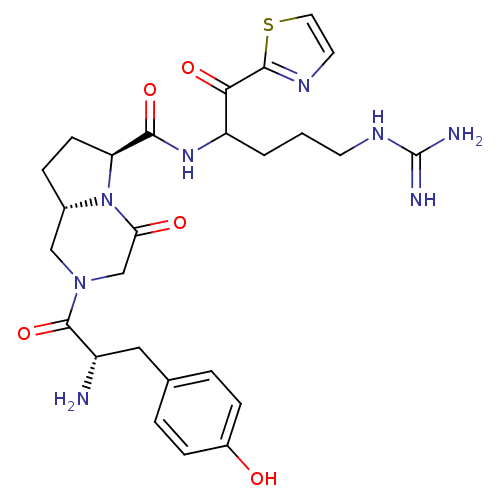
((6S,8aS)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propi...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1C[C@@H]2CC[C@H](N2C(=O)C1)C(=O)NC(CCCNC(N)=N)C(=O)c1nccs1 Show InChI InChI=1S/C26H34N8O5S/c27-18(12-15-3-6-17(35)7-4-15)25(39)33-13-16-5-8-20(34(16)21(36)14-33)23(38)32-19(2-1-9-31-26(28)29)22(37)24-30-10-11-40-24/h3-4,6-7,10-11,16,18-20,35H,1-2,5,8-9,12-14,27H2,(H,32,38)(H4,28,29,31)/t16-,18-,19?,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072526

((6S,8aS)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propi...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1C[C@@H]2CC[C@H](N2C(=O)C1)C(=O)NC(CCCNC(N)=N)C(=O)c1nccs1 Show InChI InChI=1S/C26H34N8O5S/c27-18(12-15-3-6-17(35)7-4-15)25(39)33-13-16-5-8-20(34(16)21(36)14-33)23(38)32-19(2-1-9-31-26(28)29)22(37)24-30-10-11-40-24/h3-4,6-7,10-11,16,18-20,35H,1-2,5,8-9,12-14,27H2,(H,32,38)(H4,28,29,31)/t16-,18-,19?,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM12197
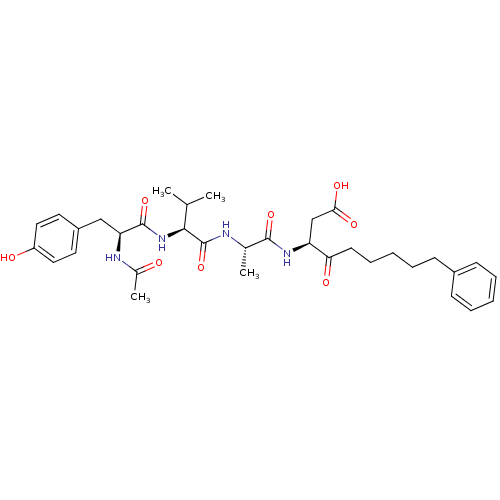
((3S)-3-[(2S)-2-[(2S)-2-[(2S)-2-acetamido-3-(4-hydr...)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)CCCCCc1ccccc1 |r| Show InChI InChI=1S/C34H46N4O8/c1-21(2)31(38-33(45)28(36-23(4)39)19-25-15-17-26(40)18-16-25)34(46)35-22(3)32(44)37-27(20-30(42)43)29(41)14-10-6-9-13-24-11-7-5-8-12-24/h5,7-8,11-12,15-18,21-22,27-28,31,40H,6,9-10,13-14,19-20H2,1-4H3,(H,35,46)(H,36,39)(H,37,44)(H,38,45)(H,42,43)/t22-,27-,28-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was tested for its inhibitory activity against IL-1 beta converting enzyme |
Bioorg Med Chem Lett 3: 2689-2692 (1993)
Article DOI: 10.1016/S0960-894X(01)80743-5
BindingDB Entry DOI: 10.7270/Q2BV7GJZ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50080916

(CHEMBL84084 | N-[(S)-1-(1-Carbamimidoyl-piperidin-...)Show SMILES NC(=N)N1CCCC(C[C@H](NC(=O)CN2[C@H](Cc3cccc(Cl)c3)C(=O)N(CCCc3ccccc3)CC2=O)C(=O)c2nccs2)C1 Show InChI InChI=1S/C34H40ClN7O4S/c35-26-12-4-9-24(17-26)19-28-33(46)40(14-5-10-23-7-2-1-3-8-23)22-30(44)42(28)21-29(43)39-27(31(45)32-38-13-16-47-32)18-25-11-6-15-41(20-25)34(36)37/h1-4,7-9,12-13,16-17,25,27-28H,5-6,10-11,14-15,18-22H2,(H3,36,37)(H,39,43)/t25?,27-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 9: 2503-8 (1999)
BindingDB Entry DOI: 10.7270/Q2028QRW |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072539
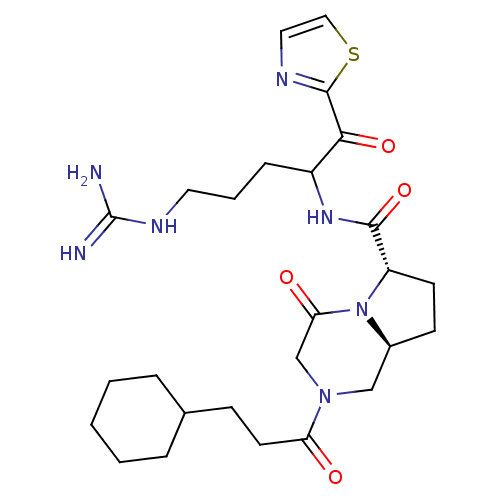
((6S,8aS)-2-(3-Cyclohexyl-propionyl)-4-oxo-octahydr...)Show SMILES NC(=N)NCCCC(NC(=O)[C@@H]1CC[C@H]2CN(CC(=O)N12)C(=O)CCC1CCCCC1)C(=O)c1nccs1 Show InChI InChI=1S/C26H39N7O4S/c27-26(28)30-12-4-7-19(23(36)25-29-13-14-38-25)31-24(37)20-10-9-18-15-32(16-22(35)33(18)20)21(34)11-8-17-5-2-1-3-6-17/h13-14,17-20H,1-12,15-16H2,(H,31,37)(H4,27,28,30)/t18-,19?,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072540

((6S,8aS)-2-Benzoyl-4-oxo-octahydro-pyrrolo[1,2-a]p...)Show SMILES NC(=N)NCCCC(NC(=O)[C@@H]1CC[C@H]2CN(CC(=O)N12)C(=O)c1ccccc1)C(=O)c1nccs1 Show InChI InChI=1S/C24H29N7O4S/c25-24(26)28-10-4-7-17(20(33)22-27-11-12-36-22)29-21(34)18-9-8-16-13-30(14-19(32)31(16)18)23(35)15-5-2-1-3-6-15/h1-3,5-6,11-12,16-18H,4,7-10,13-14H2,(H,29,34)(H4,25,26,28)/t16-,17?,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50116264

((S)-3-({(S)-(S)-5-[(S)-2-((S)-Acetyl-methyl-amino)...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)N[C@@H](CC(O)=O)C(=O)c1nnc(o1)-c1ccccc1 Show InChI InChI=1S/C34H38N6O8/c1-5-18(2)27(39(4)19(3)41)31(46)35-23-15-14-20-12-9-13-22-16-25(40(28(20)22)34(23)47)30(45)36-24(17-26(42)43)29(44)33-38-37-32(48-33)21-10-7-6-8-11-21/h6-13,18,23-25,27H,5,14-17H2,1-4H3,(H,35,46)(H,36,45)(H,42,43)/t18-,23-,24-,25-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibition against Caspase-3 |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50282979
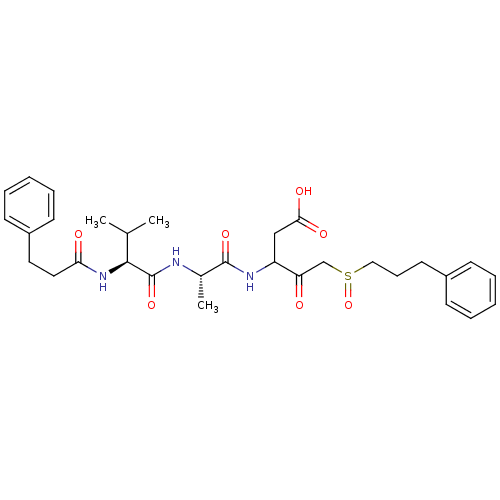
(3-{(S)-2-[(S)-3-Methyl-2-(3-phenyl-propionylamino)...)Show SMILES CC(C)[C@H](NC(=O)CCc1ccccc1)C(=O)N[C@@H](C)C(=O)NC(CC(O)=O)C(=O)CS(=O)CCCc1ccccc1 Show InChI InChI=1S/C31H41N3O7S/c1-21(2)29(34-27(36)17-16-24-13-8-5-9-14-24)31(40)32-22(3)30(39)33-25(19-28(37)38)26(35)20-42(41)18-10-15-23-11-6-4-7-12-23/h4-9,11-14,21-22,25,29H,10,15-20H2,1-3H3,(H,32,40)(H,33,39)(H,34,36)(H,37,38)/t22-,25?,29-,42?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition of cysteine proteinase IL-1 beta converting enzyme (ICE) |
Bioorg Med Chem Lett 4: 1965-1968 (1994)
Article DOI: 10.1016/S0960-894X(01)80544-8
BindingDB Entry DOI: 10.7270/Q24M94GD |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072525
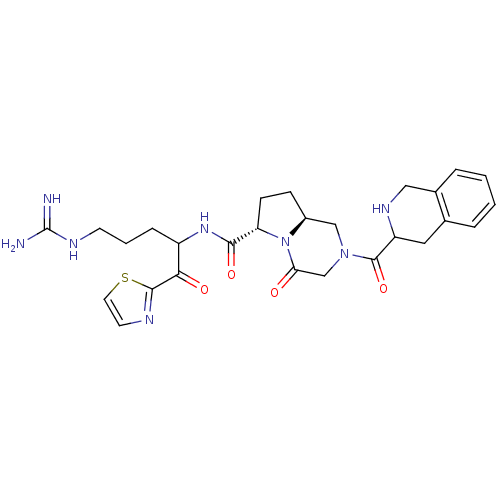
((6S,8aS)-4-Oxo-2-(1,2,3,4-tetrahydro-isoquinoline-...)Show SMILES NC(=N)NCCCC(NC(=O)[C@@H]1CC[C@H]2CN(CC(=O)N12)C(=O)C1Cc2ccccc2CN1)C(=O)c1nccs1 Show InChI InChI=1S/C27H34N8O4S/c28-27(29)31-9-3-6-19(23(37)25-30-10-11-40-25)33-24(38)21-8-7-18-14-34(15-22(36)35(18)21)26(39)20-12-16-4-1-2-5-17(16)13-32-20/h1-2,4-5,10-11,18-21,32H,3,6-9,12-15H2,(H,33,38)(H4,28,29,31)/t18-,19?,20?,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50080915
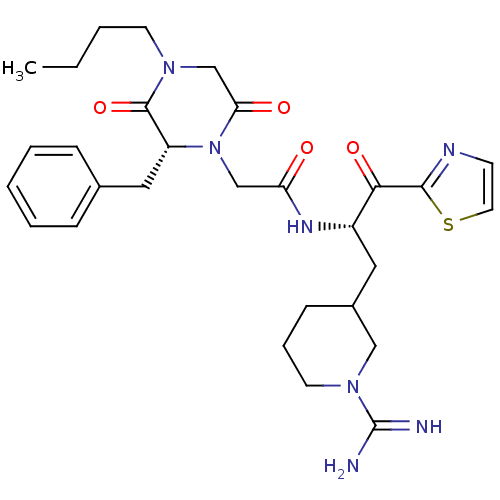
(2-((R)-2-Benzyl-4-butyl-3,6-dioxo-piperazin-1-yl)-...)Show SMILES CCCCN1CC(=O)N(CC(=O)N[C@@H](CC2CCCN(C2)C(N)=N)C(=O)c2nccs2)[C@H](Cc2ccccc2)C1=O Show InChI InChI=1S/C29H39N7O4S/c1-2-3-12-34-19-25(38)36(23(28(34)40)16-20-8-5-4-6-9-20)18-24(37)33-22(26(39)27-32-11-14-41-27)15-21-10-7-13-35(17-21)29(30)31/h4-6,8-9,11,14,21-23H,2-3,7,10,12-13,15-19H2,1H3,(H3,30,31)(H,33,37)/t21?,22-,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 9: 2503-8 (1999)
BindingDB Entry DOI: 10.7270/Q2028QRW |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116268
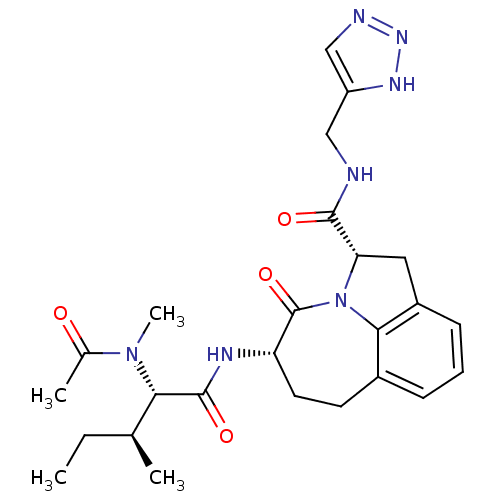
((2S,5S)-5-[(S)-2-(Acetyl-methyl-amino)-3-(S)-methy...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)NCc1cnn[nH]1 Show InChI InChI=1S/C25H33N7O4/c1-5-14(2)21(31(4)15(3)33)24(35)28-19-10-9-16-7-6-8-17-11-20(32(22(16)17)25(19)36)23(34)26-12-18-13-27-30-29-18/h6-8,13-14,19-21H,5,9-12H2,1-4H3,(H,26,34)(H,28,35)(H,27,29,30)/t14-,19-,20-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50281182
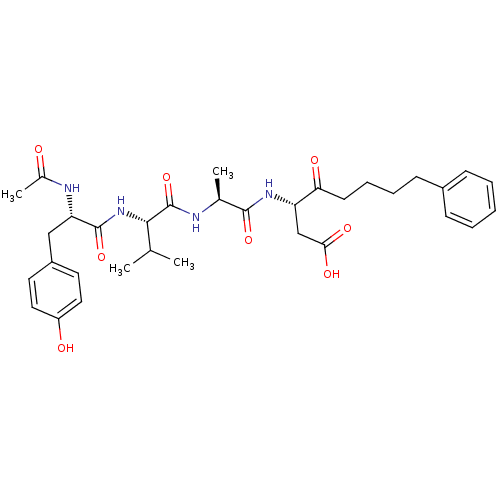
(3-(2-{2-[2-Acetylamino-3-(4-hydroxy-phenyl)-propio...)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)CCCCc1ccccc1 Show InChI InChI=1S/C33H44N4O8/c1-20(2)30(37-32(44)27(35-22(4)38)18-24-14-16-25(39)17-15-24)33(45)34-21(3)31(43)36-26(19-29(41)42)28(40)13-9-8-12-23-10-6-5-7-11-23/h5-7,10-11,14-17,20-21,26-27,30,39H,8-9,12-13,18-19H2,1-4H3,(H,34,45)(H,35,38)(H,36,43)(H,37,44)(H,41,42)/t21-,26-,27-,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was tested for its inhibitory activity against IL-1 beta converting enzyme |
Bioorg Med Chem Lett 3: 2689-2692 (1993)
Article DOI: 10.1016/S0960-894X(01)80743-5
BindingDB Entry DOI: 10.7270/Q2BV7GJZ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072518
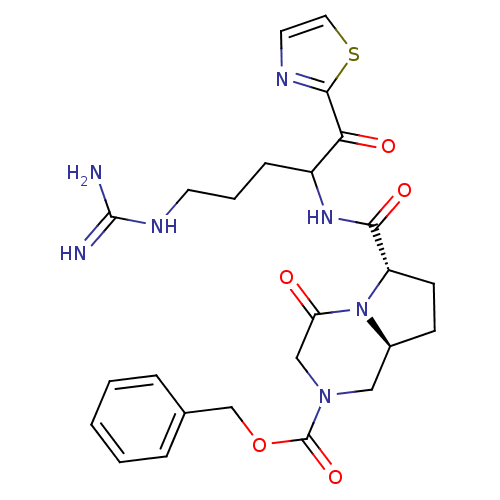
((6S,8aS)-6-[4-Guanidino-1-(thiazole-2-carbonyl)-bu...)Show SMILES NC(=N)NCCCC(NC(=O)[C@@H]1CC[C@H]2CN(CC(=O)N12)C(=O)OCc1ccccc1)C(=O)c1nccs1 Show InChI InChI=1S/C25H31N7O5S/c26-24(27)29-10-4-7-18(21(34)23-28-11-12-38-23)30-22(35)19-9-8-17-13-31(14-20(33)32(17)19)25(36)37-15-16-5-2-1-3-6-16/h1-3,5-6,11-12,17-19H,4,7-10,13-15H2,(H,30,35)(H4,26,27,29)/t17-,18?,19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50080918

(CHEMBL408553 | N-[(S)-1-(1-Carbamimidoyl-piperidin...)Show SMILES NC(=N)N1CCCC(C[C@H](NC(=O)CN2[C@H](Cc3ccc4ccccc4c3)C(=O)N(CCCc3ccccc3)CC2=O)C(=O)c2nccs2)C1 Show InChI InChI=1S/C38H43N7O4S/c39-38(40)44-18-7-11-28(23-44)21-31(35(48)36-41-16-19-50-36)42-33(46)24-45-32(22-27-14-15-29-12-4-5-13-30(29)20-27)37(49)43(25-34(45)47)17-6-10-26-8-2-1-3-9-26/h1-5,8-9,12-16,19-20,28,31-32H,6-7,10-11,17-18,21-25H2,(H3,39,40)(H,42,46)/t28?,31-,32+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 9: 2503-8 (1999)
BindingDB Entry DOI: 10.7270/Q2028QRW |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50080913
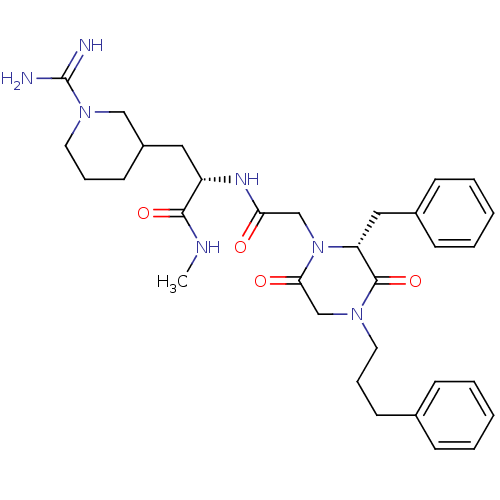
((S)-2-{2-[(R)-2-Benzyl-3,6-dioxo-4-(3-phenyl-propy...)Show SMILES CNC(=O)[C@H](CC1CCCN(C1)C(N)=N)NC(=O)CN1[C@H](Cc2ccccc2)C(=O)N(CCCc2ccccc2)CC1=O Show InChI InChI=1S/C32H43N7O4/c1-35-30(42)26(18-25-15-9-17-38(20-25)32(33)34)36-28(40)21-39-27(19-24-12-6-3-7-13-24)31(43)37(22-29(39)41)16-8-14-23-10-4-2-5-11-23/h2-7,10-13,25-27H,8-9,14-22H2,1H3,(H3,33,34)(H,35,42)(H,36,40)/t25?,26-,27+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 9: 2503-8 (1999)
BindingDB Entry DOI: 10.7270/Q2028QRW |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50080930

(2-[(R)-2-Benzyl-3,6-dioxo-4-(3-phenyl-propyl)-pipe...)Show SMILES [H][C@@]1(C[C@H](NC(=O)CN2[C@H](Cc3ccccc3)C(=O)N(CCCc3ccccc3)CC2=O)C(=O)c2nccs2)CCCN(C1)C(N)=N Show InChI InChI=1S/C34H41N7O4S/c35-34(36)40-17-8-14-26(21-40)19-27(31(44)32-37-15-18-46-32)38-29(42)22-41-28(20-25-11-5-2-6-12-25)33(45)39(23-30(41)43)16-7-13-24-9-3-1-4-10-24/h1-6,9-12,15,18,26-28H,7-8,13-14,16-17,19-23H2,(H3,35,36)(H,38,42)/t26?,27-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 9: 2503-8 (1999)
BindingDB Entry DOI: 10.7270/Q2028QRW |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072523

((6S,8aS)-4-Oxo-hexahydro-pyrrolo[1,2-a]pyrazine-2,...)Show SMILES NC(=N)NCCCC(NC(=O)[C@@H]1CC[C@H]2CN(CC(=O)N12)C(=O)NCc1ccccc1)C(=O)c1nccs1 Show InChI InChI=1S/C25H32N8O4S/c26-24(27)29-10-4-7-18(21(35)23-28-11-12-38-23)31-22(36)19-9-8-17-14-32(15-20(34)33(17)19)25(37)30-13-16-5-2-1-3-6-16/h1-3,5-6,11-12,17-19H,4,7-10,13-15H2,(H,30,37)(H,31,36)(H4,26,27,29)/t17-,18?,19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50282980
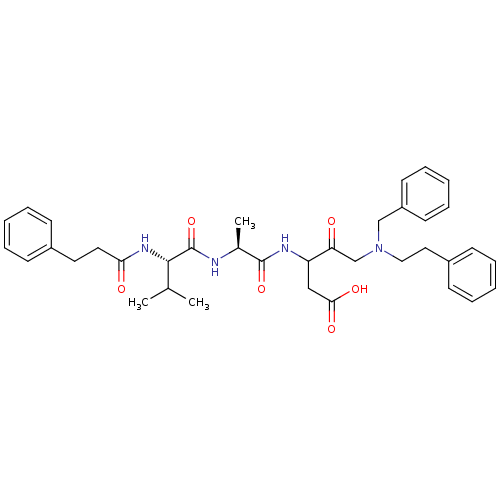
(5-(Benzyl-phenethyl-amino)-3-{(S)-2-[(S)-3-methyl-...)Show SMILES CC(C)[C@H](NC(=O)CCc1ccccc1)C(=O)N[C@@H](C)C(=O)NC(CC(O)=O)C(=O)CN(CCc1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C37H46N4O6/c1-26(2)35(40-33(43)20-19-28-13-7-4-8-14-28)37(47)38-27(3)36(46)39-31(23-34(44)45)32(42)25-41(24-30-17-11-6-12-18-30)22-21-29-15-9-5-10-16-29/h4-18,26-27,31,35H,19-25H2,1-3H3,(H,38,47)(H,39,46)(H,40,43)(H,44,45)/t27-,31?,35-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition of cysteine proteinase IL-1 beta converting enzyme (ICE) |
Bioorg Med Chem Lett 4: 1965-1968 (1994)
Article DOI: 10.1016/S0960-894X(01)80544-8
BindingDB Entry DOI: 10.7270/Q24M94GD |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50116260

((R)-2-({(2S,5S)-5-[(S)-2-(Acetyl-methyl-amino)-3-(...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)N[C@H](C=O)C(O)=O Show InChI InChI=1S/C25H32N4O7/c1-5-13(2)20(28(4)14(3)31)23(33)26-17-10-9-15-7-6-8-16-11-19(29(21(15)16)24(17)34)22(32)27-18(12-30)25(35)36/h6-8,12-13,17-20H,5,9-11H2,1-4H3,(H,26,33)(H,27,32)(H,35,36)/t13-,17-,18+,19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibition against Caspase-3 |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data