

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14028 ((S)-2-METHYL-1-[(4-METHYL-5-ISOQUINOLINE)SULFONYL]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | DrugBank PDB Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ROCK1 by homogenous luciferase assay | J Med Chem 53: 759-77 (2010) Article DOI: 10.1021/jm9014263 BindingDB Entry DOI: 10.7270/Q2V125RD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50027431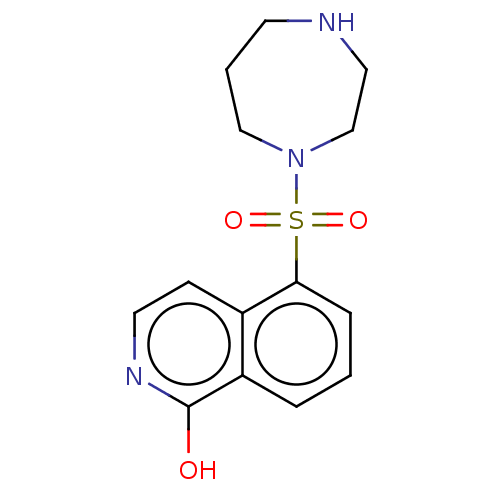 (HYDROXYFASUDIL | Hydroxy-Fasudil) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ROCK1 by homogenous luciferase assay | J Med Chem 53: 759-77 (2010) Article DOI: 10.1021/jm9014263 BindingDB Entry DOI: 10.7270/Q2V125RD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14029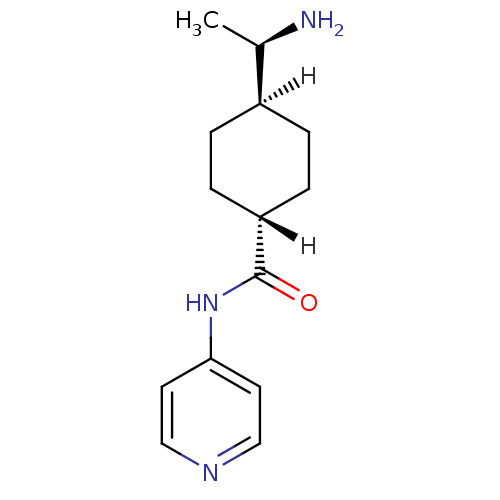 ((R)-TRANS-4-(1-AMINOETHYL)-N-(4-PYRIDYL) CYCLOHEXA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ROCK1 by homogenous luciferase assay | J Med Chem 53: 759-77 (2010) Article DOI: 10.1021/jm9014263 BindingDB Entry DOI: 10.7270/Q2V125RD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM14029 ((R)-TRANS-4-(1-AMINOETHYL)-N-(4-PYRIDYL) CYCLOHEXA...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human ROCK2 by homogenous luciferase assay | J Med Chem 53: 759-77 (2010) Article DOI: 10.1021/jm9014263 BindingDB Entry DOI: 10.7270/Q2V125RD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50126732 (2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Src protein tyrosine kinase | J Med Chem 46: 1337-49 (2003) Article DOI: 10.1021/jm020446l BindingDB Entry DOI: 10.7270/Q2DN44FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50126732 (2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Brutons tyrosine kinase | J Med Chem 46: 1337-49 (2003) Article DOI: 10.1021/jm020446l BindingDB Entry DOI: 10.7270/Q2DN44FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50126732 (2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | J Med Chem 46: 1337-49 (2003) Article DOI: 10.1021/jm020446l BindingDB Entry DOI: 10.7270/Q2DN44FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50126732 (2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | J Med Chem 46: 1337-49 (2003) Article DOI: 10.1021/jm020446l BindingDB Entry DOI: 10.7270/Q2DN44FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50078118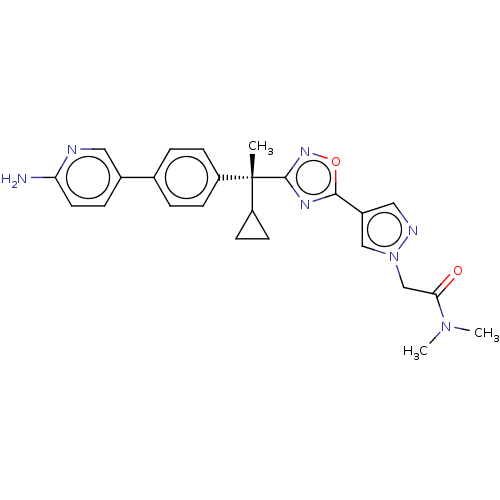 (CHEMBL3417524) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]-L-691831 from human FLAP expressed in insect SF9 cell membranes after 2 hrs by Topcount analysis | J Med Chem 58: 1669-90 (2015) Article DOI: 10.1021/jm501185j BindingDB Entry DOI: 10.7270/Q2SB47GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50078123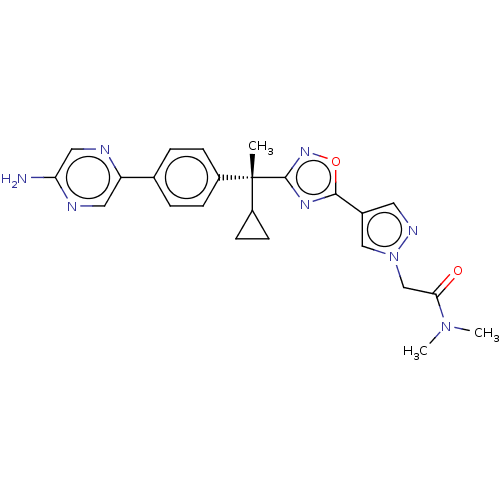 (CHEMBL3417526) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]-L-691831 from human FLAP expressed in insect SF9 cell membranes after 2 hrs by Topcount analysis | J Med Chem 58: 1669-90 (2015) Article DOI: 10.1021/jm501185j BindingDB Entry DOI: 10.7270/Q2SB47GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM104552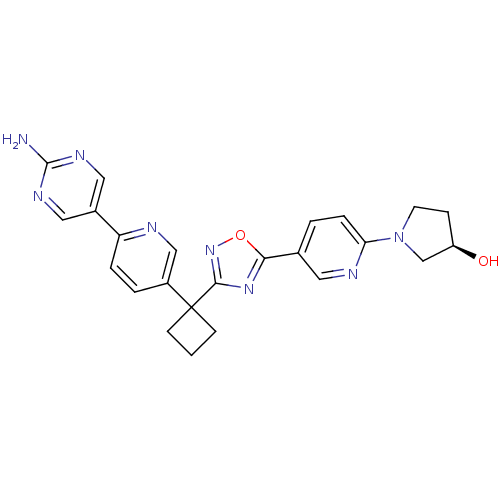 (US8575201, 43) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Compounds area assessed for the ability to bind to FLAP in a binding assay that measures compound-specific displacement of an iodinated (125I) FLAP i... | US Patent US8575201 (2013) BindingDB Entry DOI: 10.7270/Q2ZK5FBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50078080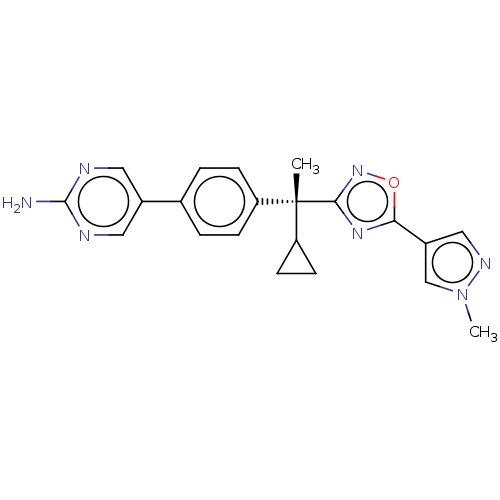 (CHEMBL3417520) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]-L-691831 from human FLAP expressed in insect SF9 cell membranes after 2 hrs by Topcount analysis | J Med Chem 58: 1669-90 (2015) Article DOI: 10.1021/jm501185j BindingDB Entry DOI: 10.7270/Q2SB47GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50078053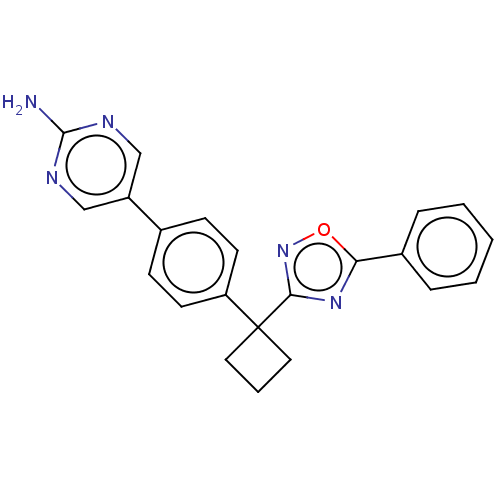 (CHEMBL3417414) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]-L-691831 from human FLAP expressed in insect SF9 cell membranes after 2 hrs by Topcount analysis | J Med Chem 58: 1669-90 (2015) Article DOI: 10.1021/jm501185j BindingDB Entry DOI: 10.7270/Q2SB47GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM104623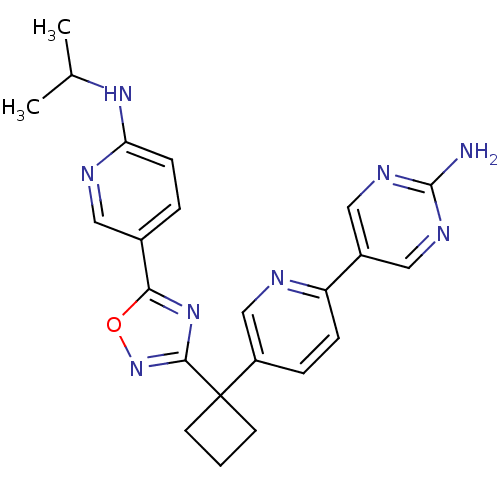 (US8575201, 114) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Compounds area assessed for the ability to bind to FLAP in a binding assay that measures compound-specific displacement of an iodinated (125I) FLAP i... | US Patent US8575201 (2013) BindingDB Entry DOI: 10.7270/Q2ZK5FBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50078078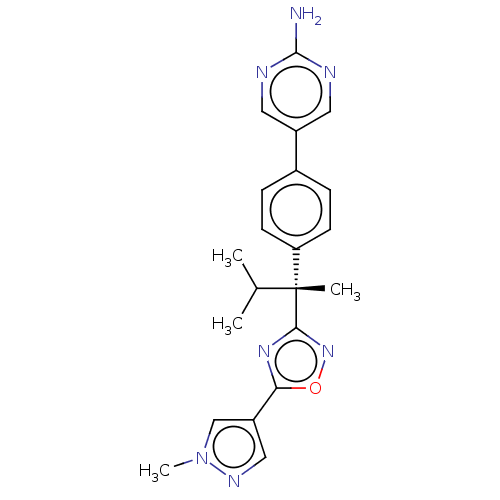 (CHEMBL3417518) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]-L-691831 from human FLAP expressed in insect SF9 cell membranes after 2 hrs by Topcount analysis | J Med Chem 58: 1669-90 (2015) Article DOI: 10.1021/jm501185j BindingDB Entry DOI: 10.7270/Q2SB47GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50078119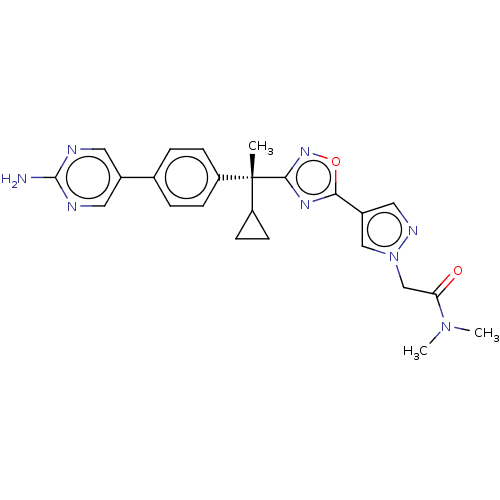 (CHEMBL3417525) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]-L-691831 from human FLAP expressed in insect SF9 cell membranes after 2 hrs by Topcount analysis | J Med Chem 58: 1669-90 (2015) Article DOI: 10.1021/jm501185j BindingDB Entry DOI: 10.7270/Q2SB47GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM104634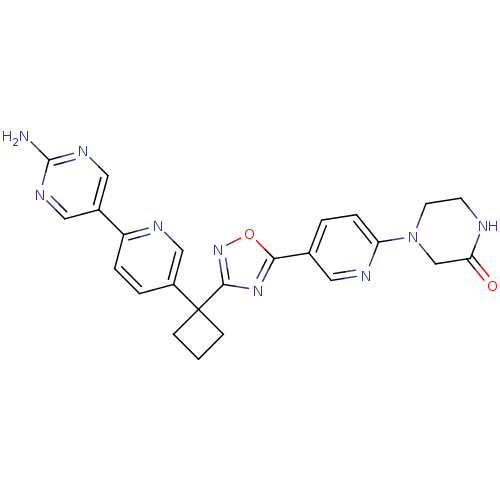 (US8575201, 125) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Compounds area assessed for the ability to bind to FLAP in a binding assay that measures compound-specific displacement of an iodinated (125I) FLAP i... | US Patent US8575201 (2013) BindingDB Entry DOI: 10.7270/Q2ZK5FBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM104570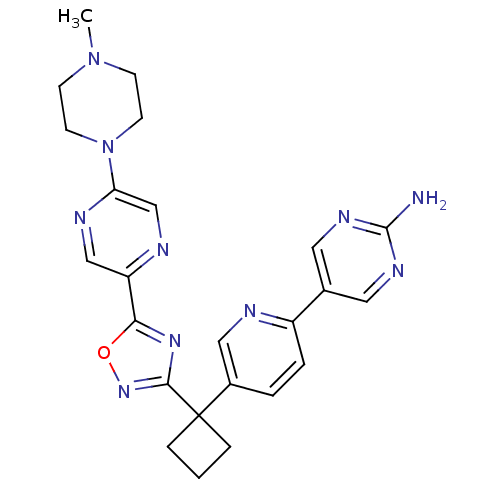 (US8575201, 61) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Compounds area assessed for the ability to bind to FLAP in a binding assay that measures compound-specific displacement of an iodinated (125I) FLAP i... | US Patent US8575201 (2013) BindingDB Entry DOI: 10.7270/Q2ZK5FBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM104527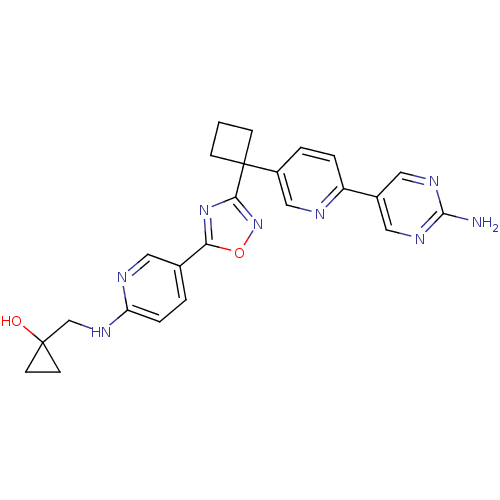 (US8575201, 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Compounds area assessed for the ability to bind to FLAP in a binding assay that measures compound-specific displacement of an iodinated (125I) FLAP i... | US Patent US8575201 (2013) BindingDB Entry DOI: 10.7270/Q2ZK5FBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50078035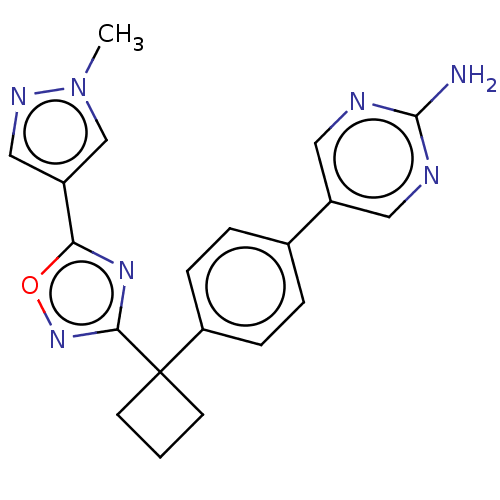 (CHEMBL3417429) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]-L-691831 from human FLAP expressed in insect SF9 cell membranes after 2 hrs by Topcount analysis | J Med Chem 58: 1669-90 (2015) Article DOI: 10.1021/jm501185j BindingDB Entry DOI: 10.7270/Q2SB47GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM104540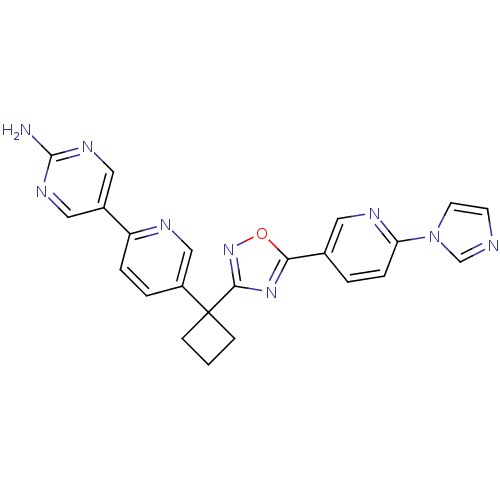 (US8575201, 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Compounds area assessed for the ability to bind to FLAP in a binding assay that measures compound-specific displacement of an iodinated (125I) FLAP i... | US Patent US8575201 (2013) BindingDB Entry DOI: 10.7270/Q2ZK5FBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM104624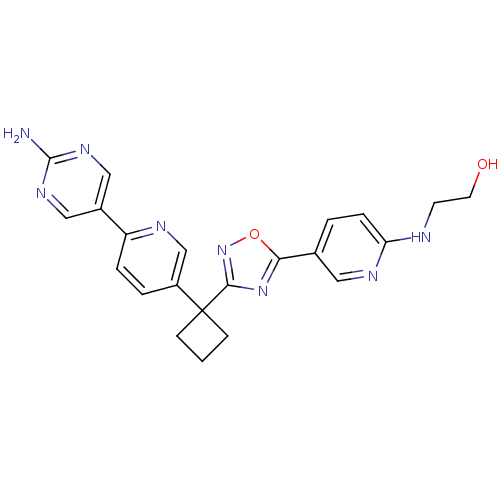 (US8575201, 115) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Compounds area assessed for the ability to bind to FLAP in a binding assay that measures compound-specific displacement of an iodinated (125I) FLAP i... | US Patent US8575201 (2013) BindingDB Entry DOI: 10.7270/Q2ZK5FBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM104517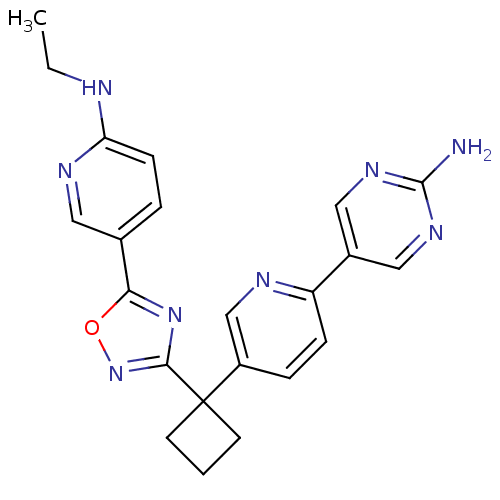 (US8575201, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Compounds area assessed for the ability to bind to FLAP in a binding assay that measures compound-specific displacement of an iodinated (125I) FLAP i... | US Patent US8575201 (2013) BindingDB Entry DOI: 10.7270/Q2ZK5FBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lyn (Homo sapiens (Human)) | BDBM50126732 (2-(2,6-Dichloro-phenylamino)-7-(3-diethylamino-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Protein tyrosine kinase Lyn | J Med Chem 46: 1337-49 (2003) Article DOI: 10.1021/jm020446l BindingDB Entry DOI: 10.7270/Q2DN44FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50126735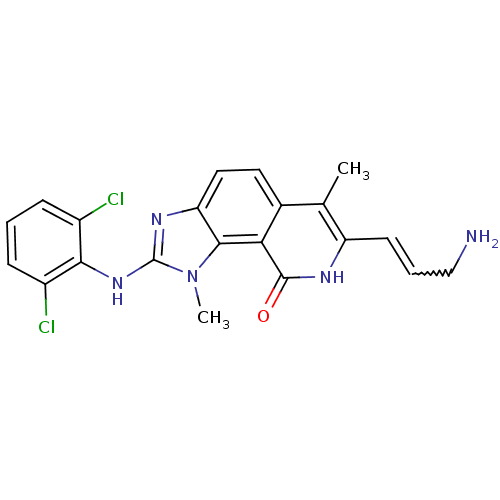 (7-(3-Amino-propenyl)-2-(2,6-dichloro-phenylamino)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | J Med Chem 46: 1337-49 (2003) Article DOI: 10.1021/jm020446l BindingDB Entry DOI: 10.7270/Q2DN44FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50078077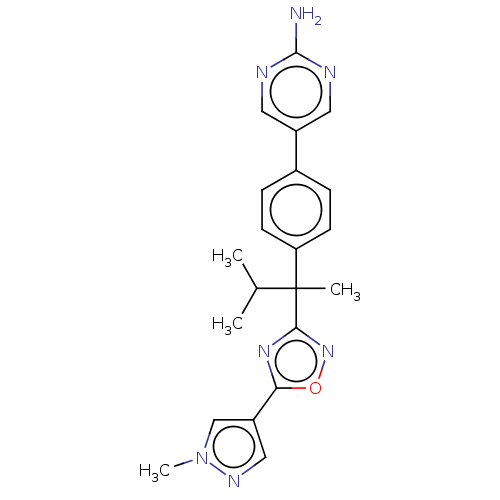 (CHEMBL3417517) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]-L-691831 from human FLAP expressed in insect SF9 cell membranes after 2 hrs by Topcount analysis | J Med Chem 58: 1669-90 (2015) Article DOI: 10.1021/jm501185j BindingDB Entry DOI: 10.7270/Q2SB47GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50126751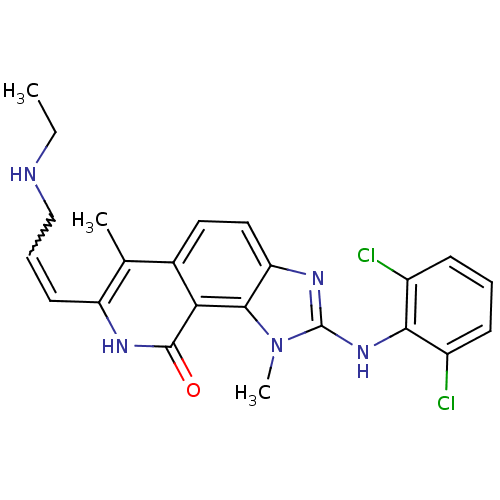 (2-(2,6-Dichloro-phenylamino)-7-(3-ethylamino-prope...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | J Med Chem 46: 1337-49 (2003) Article DOI: 10.1021/jm020446l BindingDB Entry DOI: 10.7270/Q2DN44FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50126739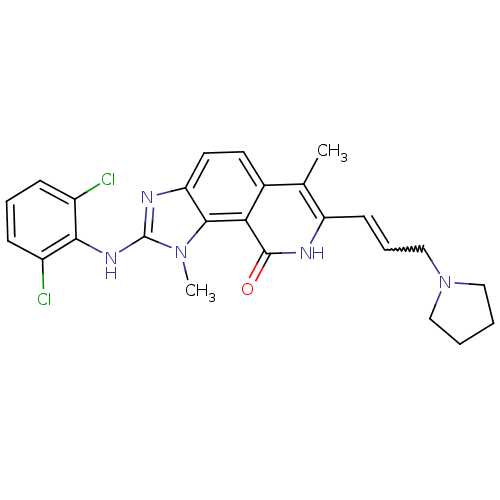 (2-(2,6-Dichloro-phenylamino)-1,6-dimethyl-7-(3-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | J Med Chem 46: 1337-49 (2003) Article DOI: 10.1021/jm020446l BindingDB Entry DOI: 10.7270/Q2DN44FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50078146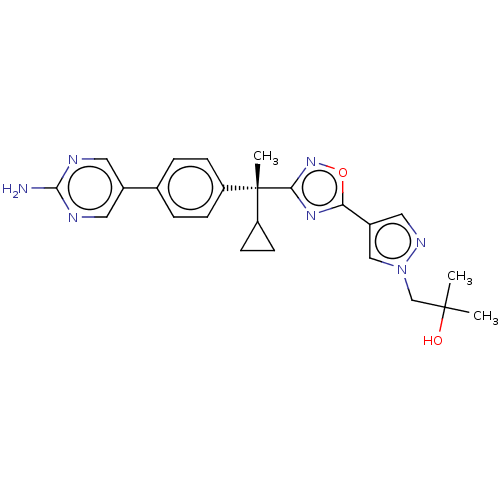 (CHEMBL3417531) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]-L-691831 from human FLAP expressed in insect SF9 cell membranes after 2 hrs by Topcount analysis | J Med Chem 58: 1669-90 (2015) Article DOI: 10.1021/jm501185j BindingDB Entry DOI: 10.7270/Q2SB47GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM104521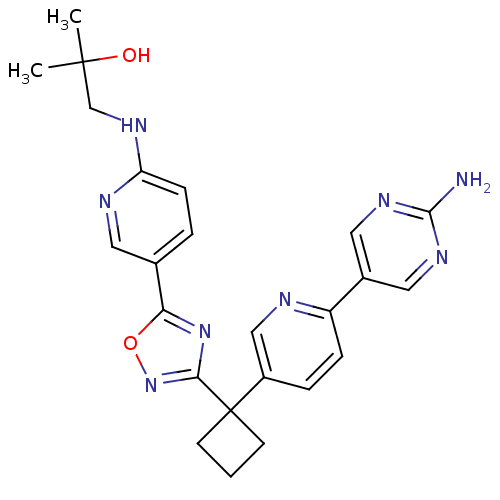 (US8575201, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Compounds area assessed for the ability to bind to FLAP in a binding assay that measures compound-specific displacement of an iodinated (125I) FLAP i... | US Patent US8575201 (2013) BindingDB Entry DOI: 10.7270/Q2ZK5FBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM104557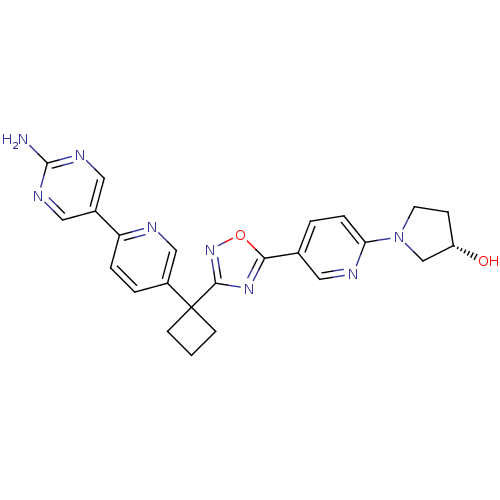 (US8575201, 48) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Compounds area assessed for the ability to bind to FLAP in a binding assay that measures compound-specific displacement of an iodinated (125I) FLAP i... | US Patent US8575201 (2013) BindingDB Entry DOI: 10.7270/Q2ZK5FBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM104542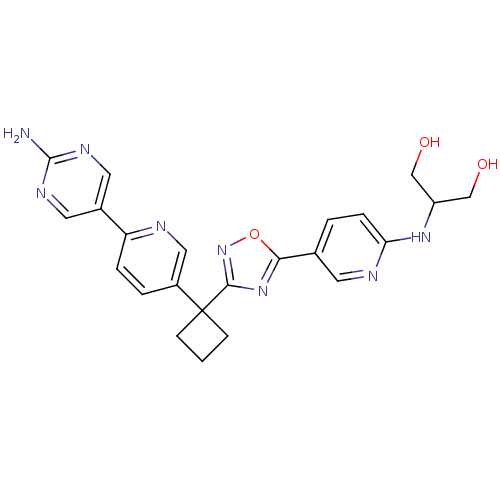 (US8575201, 33) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Compounds area assessed for the ability to bind to FLAP in a binding assay that measures compound-specific displacement of an iodinated (125I) FLAP i... | US Patent US8575201 (2013) BindingDB Entry DOI: 10.7270/Q2ZK5FBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50078052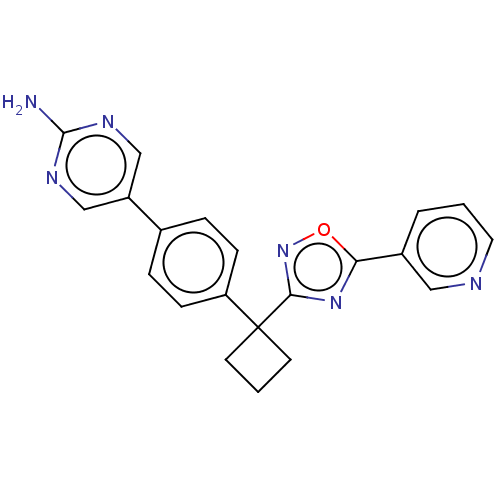 (CHEMBL3417415) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]-L-691831 from human FLAP expressed in insect SF9 cell membranes after 2 hrs by Topcount analysis | J Med Chem 58: 1669-90 (2015) Article DOI: 10.1021/jm501185j BindingDB Entry DOI: 10.7270/Q2SB47GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50078036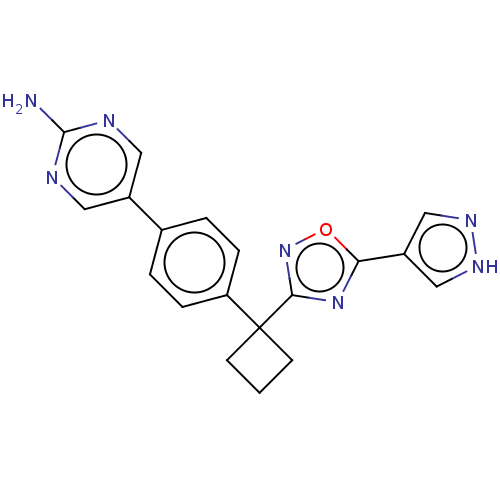 (CHEMBL3417428) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]-L-691831 from human FLAP expressed in insect SF9 cell membranes after 2 hrs by Topcount analysis | J Med Chem 58: 1669-90 (2015) Article DOI: 10.1021/jm501185j BindingDB Entry DOI: 10.7270/Q2SB47GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50078040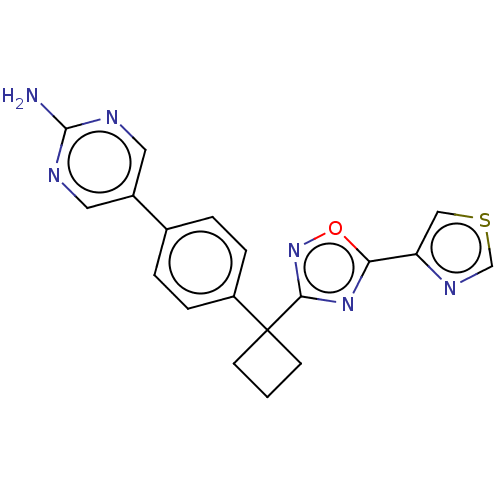 (CHEMBL3417425) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]-L-691831 from human FLAP expressed in insect SF9 cell membranes after 2 hrs by Topcount analysis | J Med Chem 58: 1669-90 (2015) Article DOI: 10.1021/jm501185j BindingDB Entry DOI: 10.7270/Q2SB47GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM104566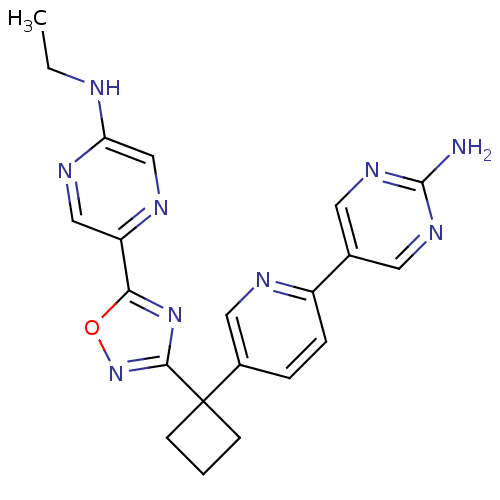 (US8575201, 57) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Compounds area assessed for the ability to bind to FLAP in a binding assay that measures compound-specific displacement of an iodinated (125I) FLAP i... | US Patent US8575201 (2013) BindingDB Entry DOI: 10.7270/Q2ZK5FBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM104555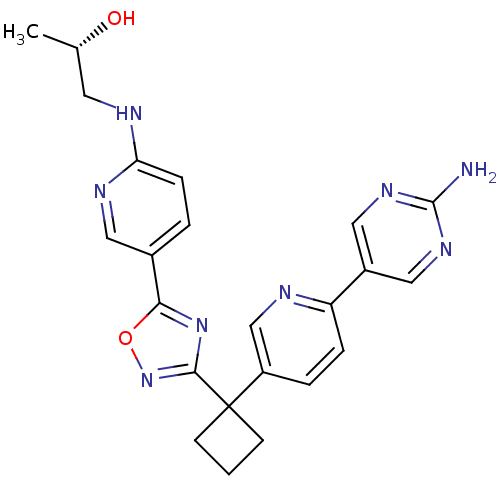 (US8575201, 46) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Compounds area assessed for the ability to bind to FLAP in a binding assay that measures compound-specific displacement of an iodinated (125I) FLAP i... | US Patent US8575201 (2013) BindingDB Entry DOI: 10.7270/Q2ZK5FBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50078033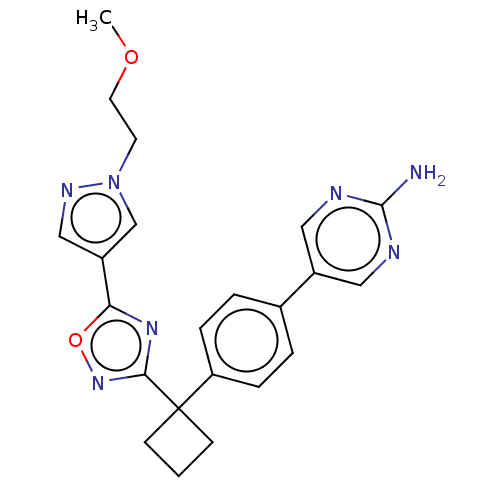 (CHEMBL3417431) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]-L-691831 from human FLAP expressed in insect SF9 cell membranes after 2 hrs by Topcount analysis | J Med Chem 58: 1669-90 (2015) Article DOI: 10.1021/jm501185j BindingDB Entry DOI: 10.7270/Q2SB47GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50078038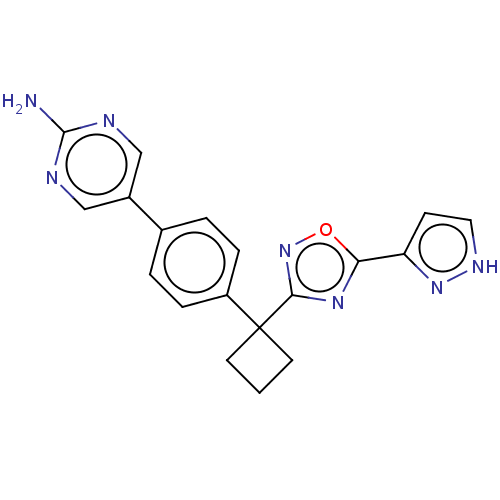 (CHEMBL3417427) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]-L-691831 from human FLAP expressed in insect SF9 cell membranes after 2 hrs by Topcount analysis | J Med Chem 58: 1669-90 (2015) Article DOI: 10.1021/jm501185j BindingDB Entry DOI: 10.7270/Q2SB47GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM104622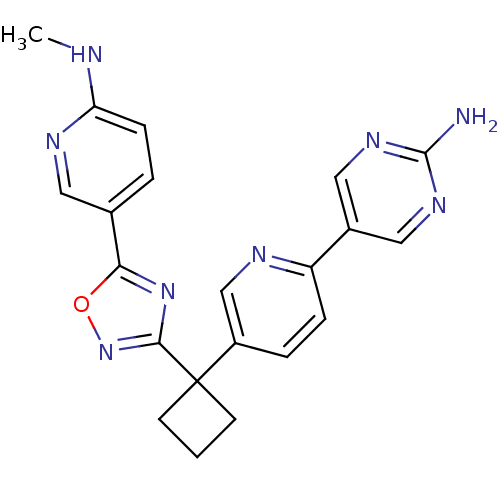 (US8575201, 113) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Compounds area assessed for the ability to bind to FLAP in a binding assay that measures compound-specific displacement of an iodinated (125I) FLAP i... | US Patent US8575201 (2013) BindingDB Entry DOI: 10.7270/Q2ZK5FBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM104619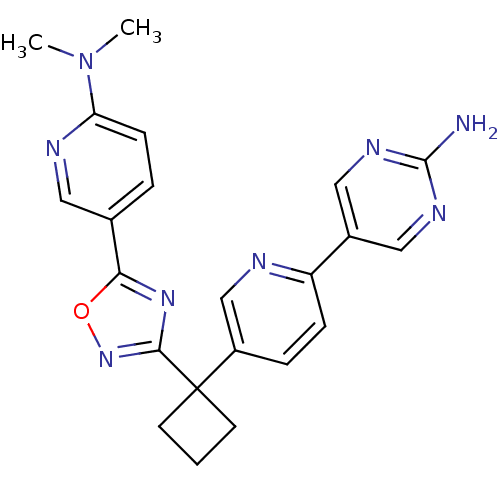 (US8575201, 110) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Compounds area assessed for the ability to bind to FLAP in a binding assay that measures compound-specific displacement of an iodinated (125I) FLAP i... | US Patent US8575201 (2013) BindingDB Entry DOI: 10.7270/Q2ZK5FBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50078043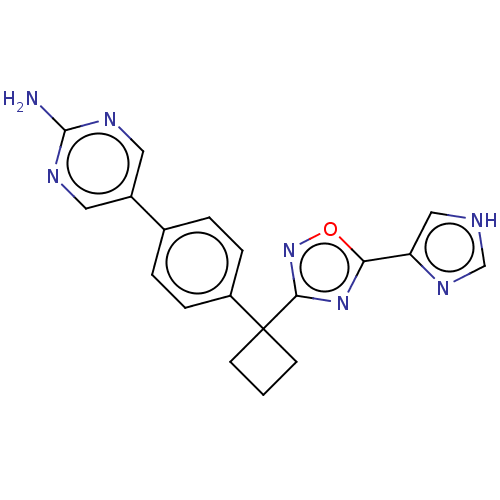 (CHEMBL3417422) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]-L-691831 from human FLAP expressed in insect SF9 cell membranes after 2 hrs by Topcount analysis | J Med Chem 58: 1669-90 (2015) Article DOI: 10.1021/jm501185j BindingDB Entry DOI: 10.7270/Q2SB47GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM104531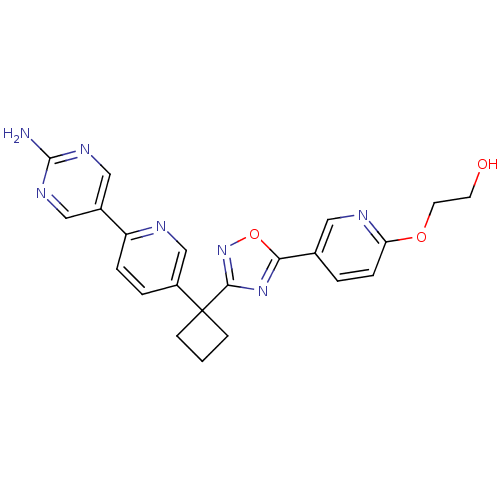 (US8575201, 22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Compounds area assessed for the ability to bind to FLAP in a binding assay that measures compound-specific displacement of an iodinated (125I) FLAP i... | US Patent US8575201 (2013) BindingDB Entry DOI: 10.7270/Q2ZK5FBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50078031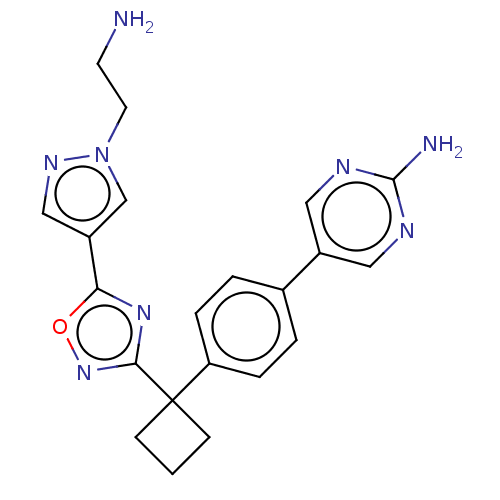 (CHEMBL3417508) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]-L-691831 from human FLAP expressed in insect SF9 cell membranes after 2 hrs by Topcount analysis | J Med Chem 58: 1669-90 (2015) Article DOI: 10.1021/jm501185j BindingDB Entry DOI: 10.7270/Q2SB47GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50078115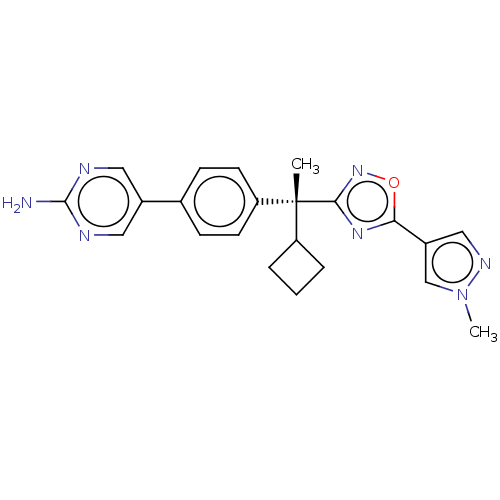 (CHEMBL3417522) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]-L-691831 from human FLAP expressed in insect SF9 cell membranes after 2 hrs by Topcount analysis | J Med Chem 58: 1669-90 (2015) Article DOI: 10.1021/jm501185j BindingDB Entry DOI: 10.7270/Q2SB47GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM104562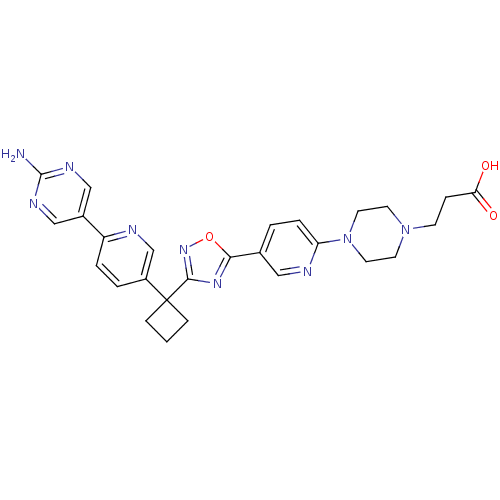 (US8575201, 53) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Compounds area assessed for the ability to bind to FLAP in a binding assay that measures compound-specific displacement of an iodinated (125I) FLAP i... | US Patent US8575201 (2013) BindingDB Entry DOI: 10.7270/Q2ZK5FBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM104571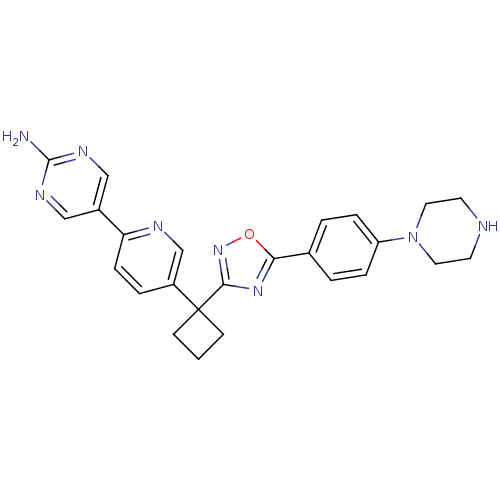 (US8575201, 62) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Compounds area assessed for the ability to bind to FLAP in a binding assay that measures compound-specific displacement of an iodinated (125I) FLAP i... | US Patent US8575201 (2013) BindingDB Entry DOI: 10.7270/Q2ZK5FBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM104530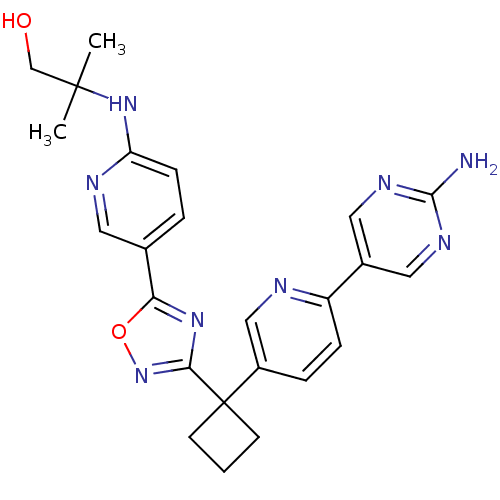 (US8575201, 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Compounds area assessed for the ability to bind to FLAP in a binding assay that measures compound-specific displacement of an iodinated (125I) FLAP i... | US Patent US8575201 (2013) BindingDB Entry DOI: 10.7270/Q2ZK5FBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM104561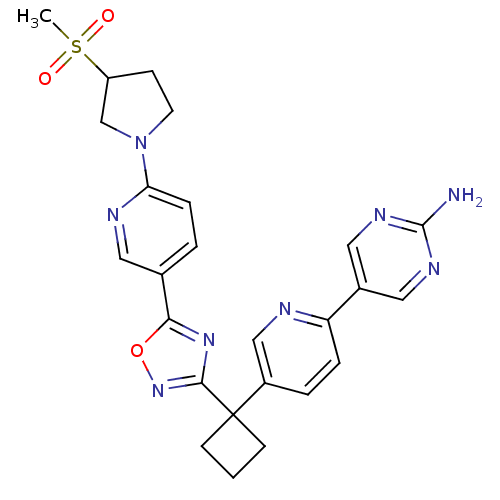 (US8575201, 52) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Compounds area assessed for the ability to bind to FLAP in a binding assay that measures compound-specific displacement of an iodinated (125I) FLAP i... | US Patent US8575201 (2013) BindingDB Entry DOI: 10.7270/Q2ZK5FBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM104598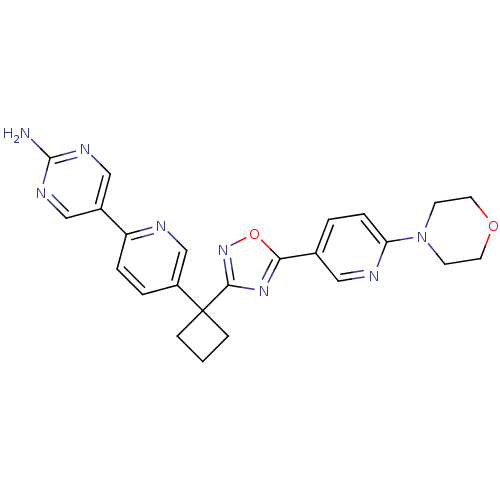 (US8575201, 89) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Compounds area assessed for the ability to bind to FLAP in a binding assay that measures compound-specific displacement of an iodinated (125I) FLAP i... | US Patent US8575201 (2013) BindingDB Entry DOI: 10.7270/Q2ZK5FBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 978 total ) | Next | Last >> |