| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Tryptase beta-2 |
|---|
| Ligand | BDBM14302 |
|---|
| Substrate/Competitor | BDBM12679 |
|---|
| Meas. Tech. | Enzyme Assay and Determination of the Inhibition Constants |
|---|
| pH | 7.4±n/a |
|---|
| Temperature | 295.15±n/a K |
|---|
| Ki | >150000±n/a nM |
|---|
| Citation |  McGrath, ME; Sprengeler, PA; Hirschbein, B; Somoza, JR; Lehoux, I; Janc, JW; Gjerstad, E; Graupe, M; Estiarte, A; Venkataramani, C; Liu, Y; Yee, R; Ho, JD; Green, MJ; Lee, CS; Liu, L; Tai, V; Spencer, J; Sperandio, D; Katz, BA Structure-guided design of peptide-based tryptase inhibitors. Biochemistry45:5964-73 (2006) [PubMed] Article McGrath, ME; Sprengeler, PA; Hirschbein, B; Somoza, JR; Lehoux, I; Janc, JW; Gjerstad, E; Graupe, M; Estiarte, A; Venkataramani, C; Liu, Y; Yee, R; Ho, JD; Green, MJ; Lee, CS; Liu, L; Tai, V; Spencer, J; Sperandio, D; Katz, BA Structure-guided design of peptide-based tryptase inhibitors. Biochemistry45:5964-73 (2006) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Solution Info, Assay Method |
|---|
| |
| Tryptase beta-2 |
|---|
| Name: | Tryptase beta-2 |
|---|
| Synonyms: | TPS2 | TPSB2 | TRYB2_HUMAN | Tryptase | Tryptase II | Tryptase beta-1 | Tryptase-2 |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 30518.79 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_210702 |
|---|
| Residue: | 275 |
|---|
| Sequence: | MLNLLLLALPVLASRAYAAPAPGQALQRVGIVGGQEAPRSKWPWQVSLRVHGPYWMHFCG
GSLIHPQWVLTAAHCVGPDVKDLAALRVQLREQHLYYQDQLLPVSRIIVHPQFYTAQIGA
DIALLELEEPVNVSSHVHTVTLPPASETFPPGMPCWVTGWGDVDNDERLPPPFPLKQVKV
PIMENHICDAKYHLGAYTGDDVRIVRDDMLCAGNTRRDSCQGDSGGPLVCKVNGTWLQAG
VVSWGEGCAQPNRPGIYTRVTYYLDWIHHYVPKKP
|
|
|
|---|
| BDBM14302 |
|---|
| BDBM12679 |
|---|
| Name | BDBM14302 |
|---|
| Synonyms: | benzthiazole analog 5 | benzyl N-[(2S)-3-(6-aminopyridin-3-yl)-1-(1,3-benzothiazol-2-yl)-1-oxopropan-2-yl]carbamate |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C23H20N4O3S |
|---|
| Mol. Mass. | 432.495 |
|---|
| SMILES | Nc1ccc(C[C@H](NC(=O)OCc2ccccc2)C(=O)c2nc3ccccc3s2)cn1 |r| |
|---|
| Structure | 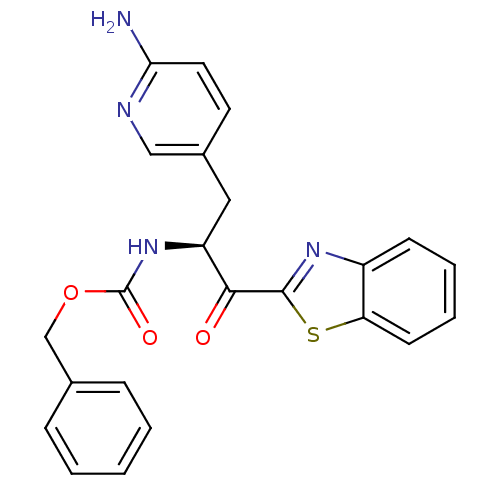
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 McGrath, ME; Sprengeler, PA; Hirschbein, B; Somoza, JR; Lehoux, I; Janc, JW; Gjerstad, E; Graupe, M; Estiarte, A; Venkataramani, C; Liu, Y; Yee, R; Ho, JD; Green, MJ; Lee, CS; Liu, L; Tai, V; Spencer, J; Sperandio, D; Katz, BA Structure-guided design of peptide-based tryptase inhibitors. Biochemistry45:5964-73 (2006) [PubMed] Article
McGrath, ME; Sprengeler, PA; Hirschbein, B; Somoza, JR; Lehoux, I; Janc, JW; Gjerstad, E; Graupe, M; Estiarte, A; Venkataramani, C; Liu, Y; Yee, R; Ho, JD; Green, MJ; Lee, CS; Liu, L; Tai, V; Spencer, J; Sperandio, D; Katz, BA Structure-guided design of peptide-based tryptase inhibitors. Biochemistry45:5964-73 (2006) [PubMed] Article