

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM14312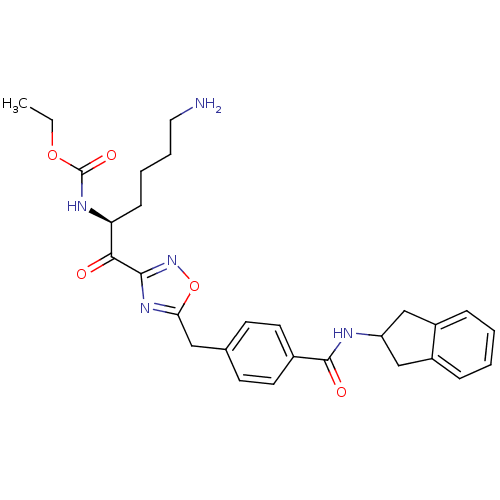 (CHEMBL214368 | CRA23 | ethyl N-[(2S)-6-amino-1-(5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM14311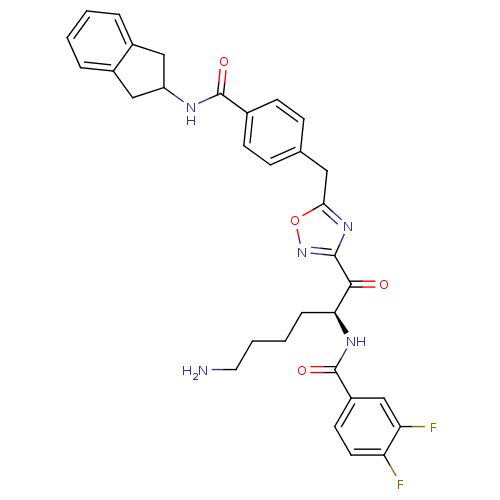 (CRA22 | N-[(2S)-6-amino-1-(5-{[4-(2,3-dihydro-1H-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM14316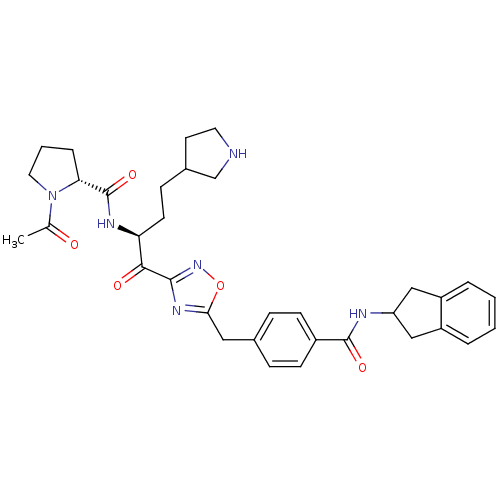 ((2R)-N-[(2S)-1-(5-{[4-(2,3-dihydro-1H-inden-2-ylca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM14317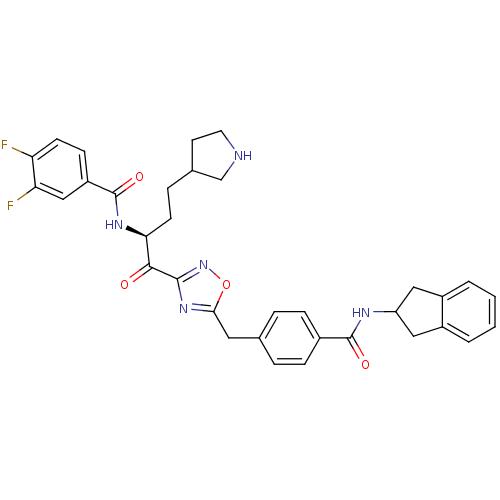 (CRA28 | N-[(2S)-1-(5-{[4-(2,3-dihydro-1H-inden-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM14307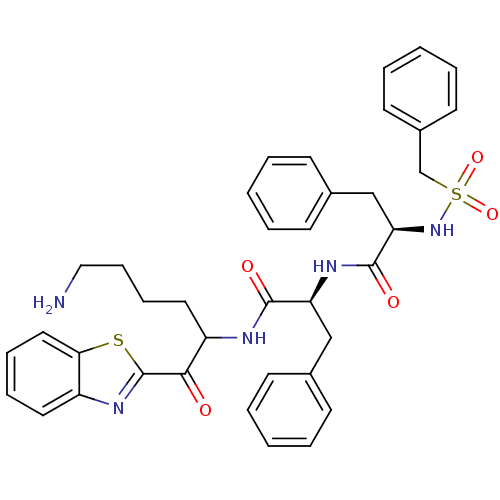 ((2R)-N-[(1S)-1-{[6-amino-1-(1,3-benzothiazol-2-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 23 | -43.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM14314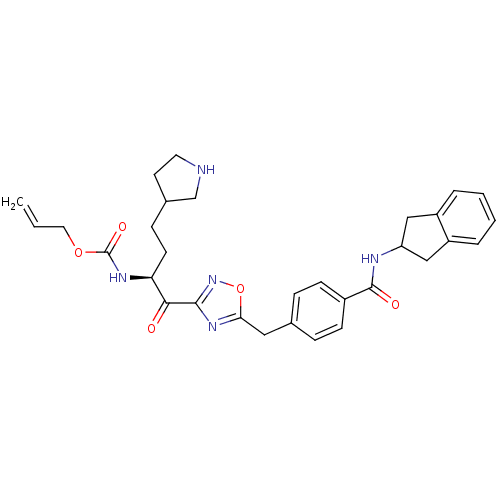 (CRA25 | prop-2-en-1-yl N-[(2S)-1-(5-{[4-(2,3-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14316 ((2R)-N-[(2S)-1-(5-{[4-(2,3-dihydro-1H-inden-2-ylca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM14315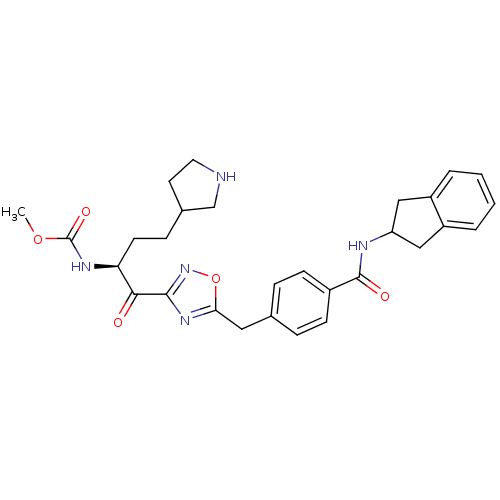 (CRA26 | methyl N-[(2S)-1-(5-{[4-(2,3-dihydro-1H-in...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14312 (CHEMBL214368 | CRA23 | ethyl N-[(2S)-6-amino-1-(5-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM14313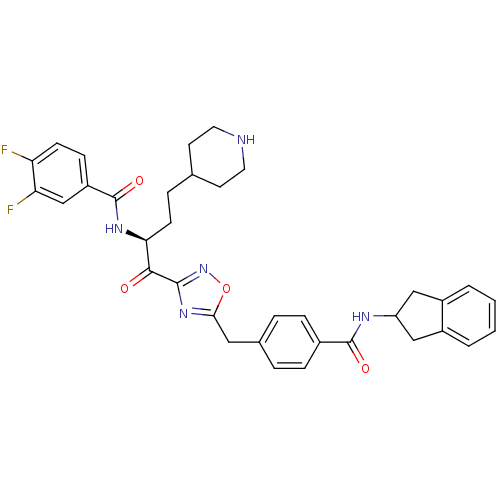 (CRA24 | N-[(2S)-1-(5-{[4-(2,3-dihydro-1H-inden-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14311 (CRA22 | N-[(2S)-6-amino-1-(5-{[4-(2,3-dihydro-1H-i...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM14318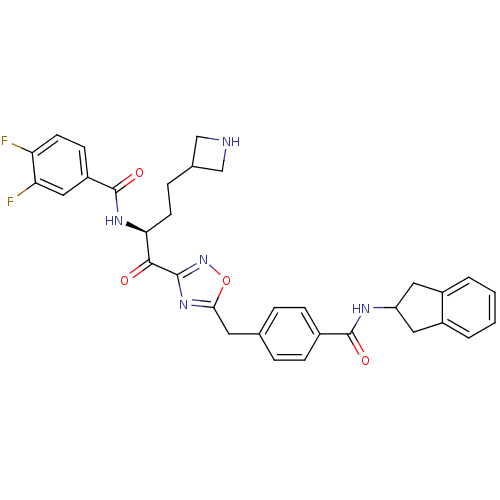 (CRA29 | N-[(2S)-4-(azetidin-3-yl)-1-(5-{[4-(2,3-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14297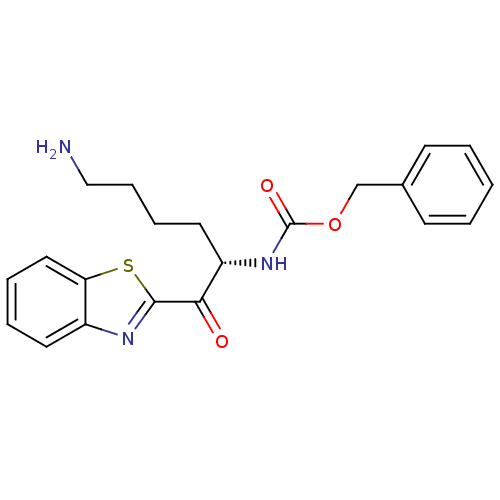 (US10676470, Compound 23 | US11332464, Compound 23 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14314 (CRA25 | prop-2-en-1-yl N-[(2S)-1-(5-{[4-(2,3-dihyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14300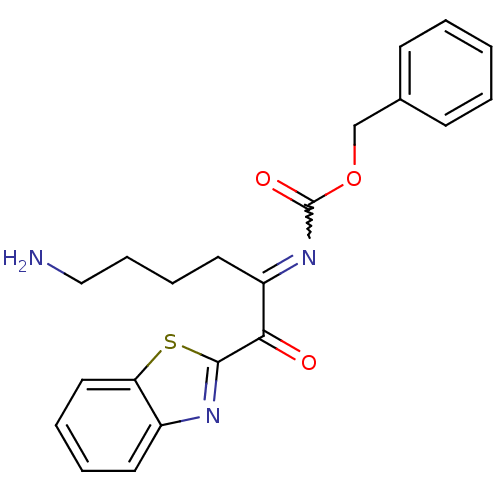 (benzthiazole analog 3 | benzyl N-[(2S,4E)-6-amino-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14315 (CRA26 | methyl N-[(2S)-1-(5-{[4-(2,3-dihydro-1H-in...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14299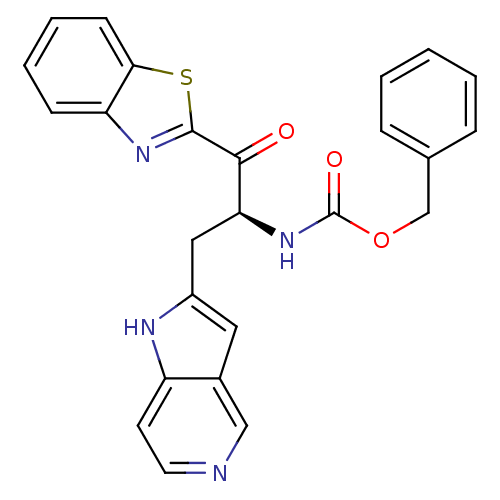 (benzthiazole analog 2 | benzyl N-[(2S)-1-(1,3-benz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM14297 (US10676470, Compound 23 | US11332464, Compound 23 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.30E+3 | -29.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14317 (CRA28 | N-[(2S)-1-(5-{[4-(2,3-dihydro-1H-inden-2-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM14299 (benzthiazole analog 2 | benzyl N-[(2S)-1-(1,3-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20E+4 | -27.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14301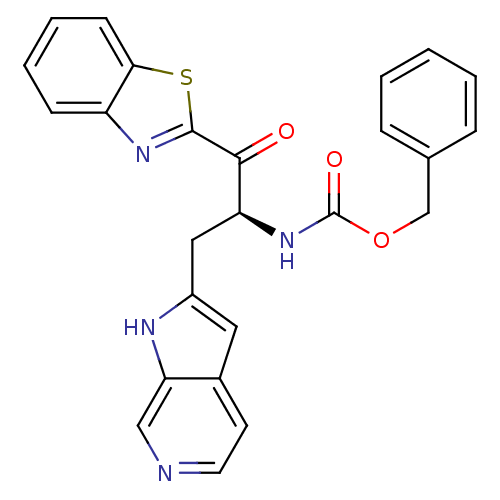 (benzthiazole analog 4 | benzyl N-[(2S)-1-(1,3-benz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM14308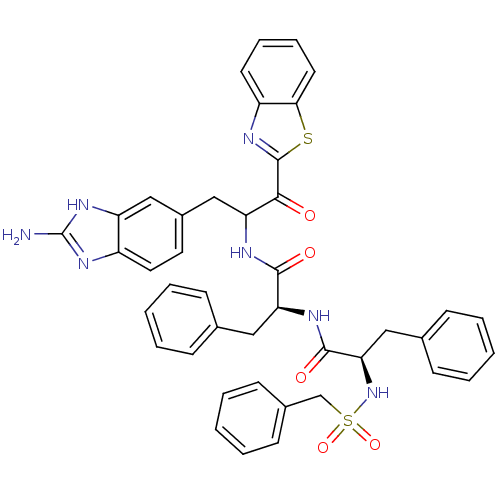 ((2S)-N-[3-(2-amino-1H-1,3-benzodiazol-5-yl)-1-(1,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.80E+4 | -26.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM14300 (benzthiazole analog 3 | benzyl N-[(2S,4E)-6-amino-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.50E+4 | -26.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM14309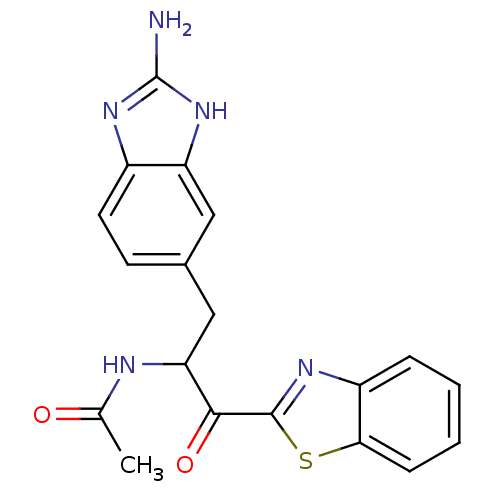 (2-Aminobenzimidazole Compound 13 | N-[3-(2-amino-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.10E+4 | -25.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM14301 (benzthiazole analog 4 | benzyl N-[(2S)-1-(1,3-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.50E+4 | -24.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14313 (CRA24 | N-[(2S)-1-(5-{[4-(2,3-dihydro-1H-inden-2-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM14310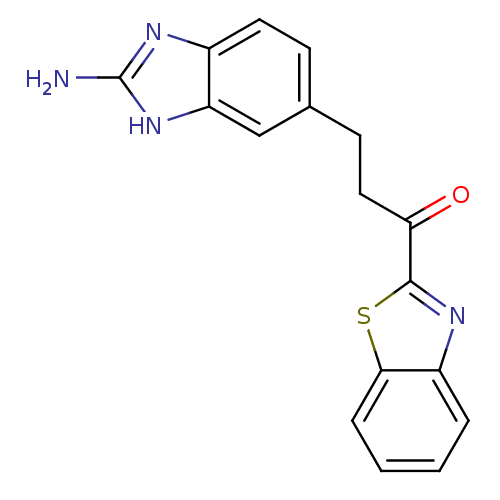 (2-Aminobenzimidazole Compound 14 | 3-(2-amino-1H-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.40E+4 | -22.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM7960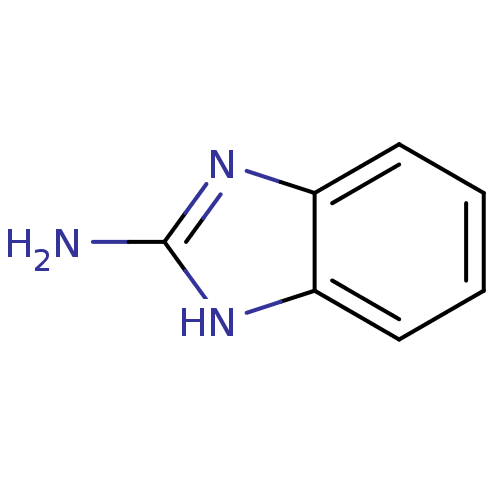 (1H-1,3-benzodiazol-2-amine | 2-Aminobenzimidazole ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | 1.10E+5 | -22.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM14303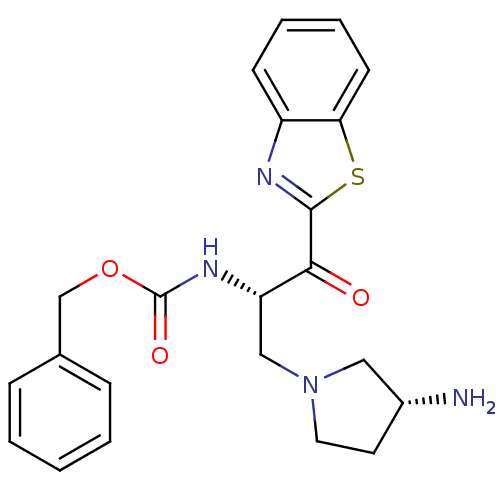 (benzthiazole analog 6 | benzyl N-[(2S)-3-[(3R)-3-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.50E+5 | >-21.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14303 (benzthiazole analog 6 | benzyl N-[(2S)-3-[(3R)-3-a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14318 (CRA29 | N-[(2S)-4-(azetidin-3-yl)-1-(5-{[4-(2,3-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14302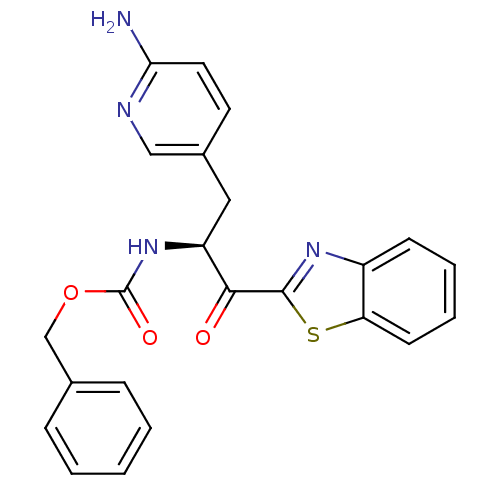 (benzthiazole analog 5 | benzyl N-[(2S)-3-(6-aminop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM14304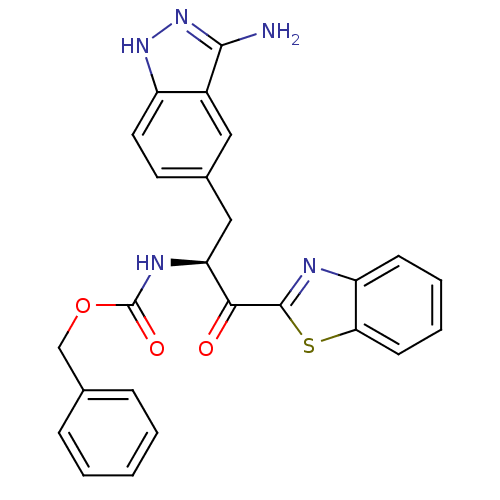 (benzthiazole analog 7 | benzyl N-[(2S)-3-(3-amino-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.50E+5 | >-21.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14304 (benzthiazole analog 7 | benzyl N-[(2S)-3-(3-amino-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14305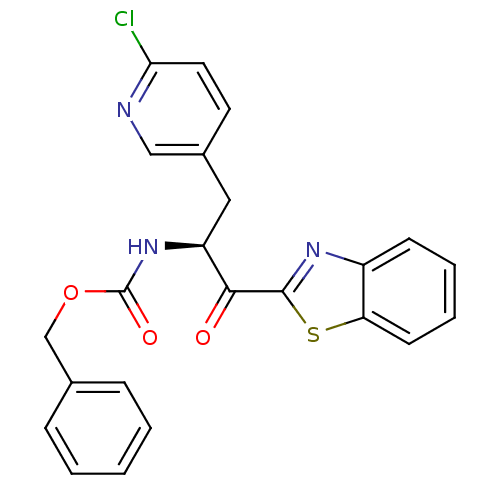 (benzthiazole analog 8 | benzyl N-[(2S)-1-(1,3-benz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM14305 (benzthiazole analog 8 | benzyl N-[(2S)-1-(1,3-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.50E+5 | >-21.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM14302 (benzthiazole analog 5 | benzyl N-[(2S)-3-(6-aminop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.50E+5 | >-21.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM7960 (1H-1,3-benzodiazol-2-amine | 2-Aminobenzimidazole ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | MMDB Article PubMed | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM14324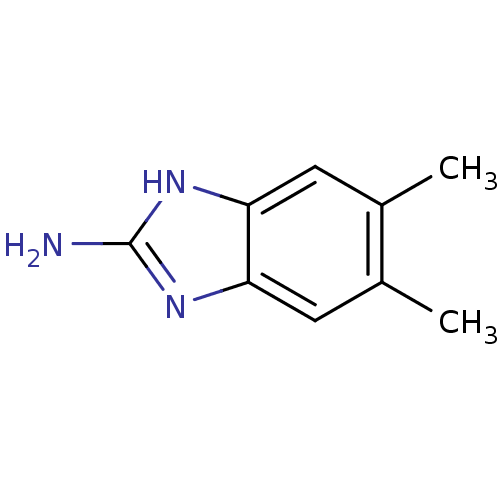 (5,6-dimethyl-1H-1,3-benzodiazol-2-amine | Fragment...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM14322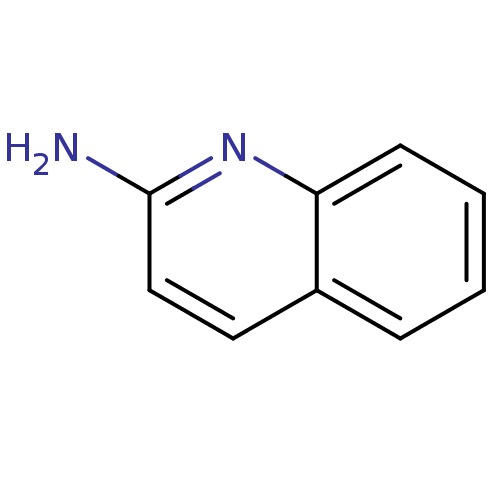 (2-Aminoquinoline 1 | 2-aminoquinoline | CHEMBL6123...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14319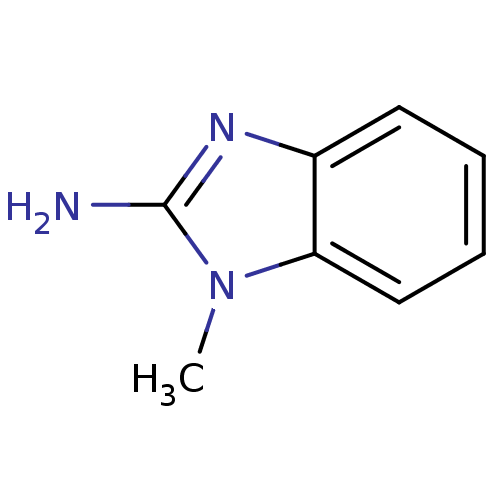 (1-methyl-1H-1,3-benzodiazol-2-amine | Fragment 16) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM14320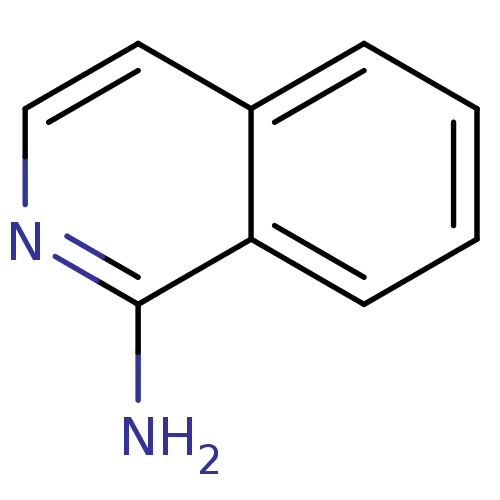 (1-amino-isoquinoline | CHEMBL62083 | Fragment 17 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14322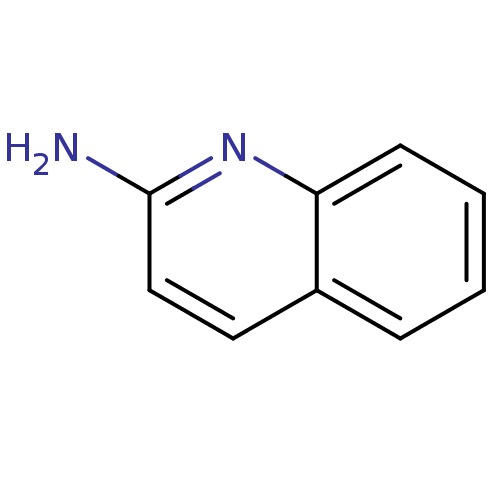 (2-Aminoquinoline 1 | 2-aminoquinoline | CHEMBL6123...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14320 (1-amino-isoquinoline | CHEMBL62083 | Fragment 17 |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14324 (5,6-dimethyl-1H-1,3-benzodiazol-2-amine | Fragment...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM14323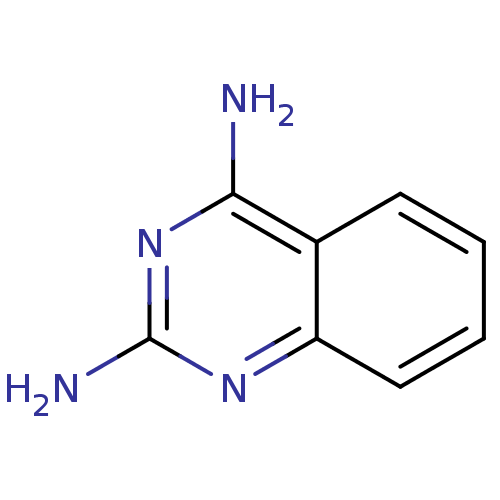 (Fragment 20 | quinazoline-2,4-diamine) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM14319 (1-methyl-1H-1,3-benzodiazol-2-amine | Fragment 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14321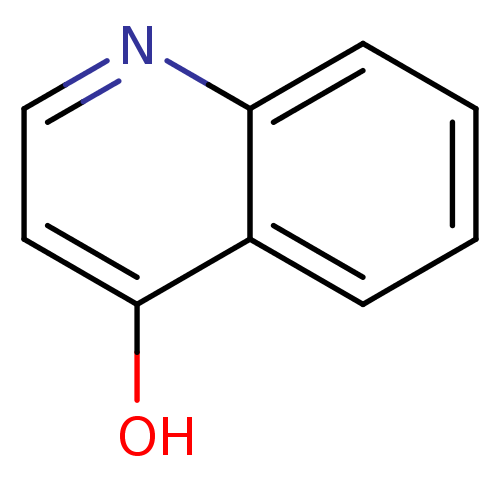 (Fragment 18 | quinolin-4-ol) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM14321 (Fragment 18 | quinolin-4-ol) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM14323 (Fragment 20 | quinazoline-2,4-diamine) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera | Assay Description Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... | Biochemistry 45: 5964-73 (2006) Article DOI: 10.1021/bi060173m BindingDB Entry DOI: 10.7270/Q2W09450 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 7687 total ) | Next | Last >> |