| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Oxysterols receptor LXR-alpha [197-447] |
|---|
| Ligand | BDBM26065 |
|---|
| Substrate/Competitor | BDBM19993 |
|---|
| Meas. Tech. | LXR Binding Assay and hLXR Reporter Assay |
|---|
| IC50 | 78±n/a nM |
|---|
| EC50 | 1810±n/a nM |
|---|
| Citation |  Wrobel, J; Steffan†, R; Bowen, SM; Magolda, R; Matelan†, E; Unwalla†, R; Basso, M; Clerin, V; Gardell, SJ; Nambi, P; Quinet, E; Reminick, JI; Vlasuk, GP; Wang, S; Feingold, I; Huselton, C; Bonn, T Indazole-Based Liver X Receptor (LXR) Modulators with Maintained Atherosclerotic Lesion Reduction Activity but Diminished Stimulation of Hepatic Triglyceride Synthesis J Med Chem51:7161-8 (2008) [PubMed] Article Wrobel, J; Steffan†, R; Bowen, SM; Magolda, R; Matelan†, E; Unwalla†, R; Basso, M; Clerin, V; Gardell, SJ; Nambi, P; Quinet, E; Reminick, JI; Vlasuk, GP; Wang, S; Feingold, I; Huselton, C; Bonn, T Indazole-Based Liver X Receptor (LXR) Modulators with Maintained Atherosclerotic Lesion Reduction Activity but Diminished Stimulation of Hepatic Triglyceride Synthesis J Med Chem51:7161-8 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Solution Info, Assay Method |
|---|
| |
| Oxysterols receptor LXR-alpha [197-447] |
|---|
| Name: | Oxysterols receptor LXR-alpha [197-447] |
|---|
| Synonyms: | LXRA | Liver X Receptor alpha (LXR-alpha) | NR1H3 | NR1H3_HUMAN | Nuclear orphan receptor LXR-alpha | Nuclear receptor subfamily 1 group H member 3 | Oxysterols receptor LXR-alpha |
|---|
| Type: | Receptor |
|---|
| Mol. Mass.: | 28986.41 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | LXR alpha ligand binding domain (amino acid residues 197-447) with an N-terminal biotinylation tag expressed in E.coli, was used for the binding assays. |
|---|
| Residue: | 251 |
|---|
| Sequence: | SSPPQILPQLSPEQLGMIEKLVAAQQQCNRRSFSDRLRVTPWPMAPDPHSREARQQRFAH
FTELAIVSVQEIVDFAKQLPGFLQLSREDQIALLKTSAIEVMLLETSRRYNPGSESITFL
KDFSYNREDFAKAGLQVEFINPIFEFSRAMNELQLNDAEFALLIAISIFSADRPNVQDQL
QVERLQHTYVEALHAYVSIHHPHDRLMFPRMLMKLVSLRTLSSVHSEQVFALRLQDKKLP
PLLSEIWDVHE
|
|
|
|---|
| BDBM26065 |
|---|
| BDBM19993 |
|---|
| Name | BDBM26065 |
|---|
| Synonyms: | 2-[(2,4-dimethylphenyl)methyl]-3-(4-fluorophenyl)-7-(trifluoromethyl)-2H-indazole | 2-benzyl-3-aryl-7-trifluoromethylindazole, 11 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C23H18F4N2 |
|---|
| Mol. Mass. | 398.396 |
|---|
| SMILES | Cc1ccc(Cn2nc3c(cccc3c2-c2ccc(F)cc2)C(F)(F)F)c(C)c1 |
|---|
| Structure | 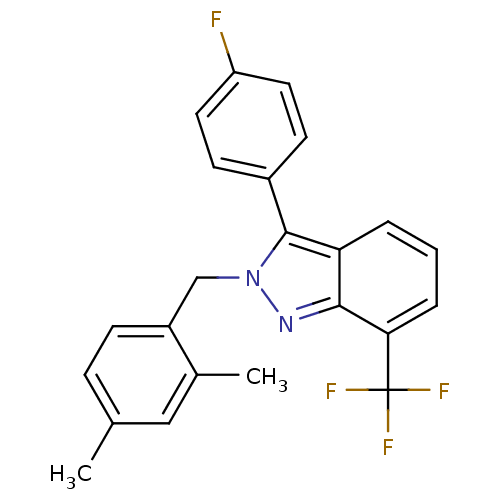
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Wrobel, J; Steffan†, R; Bowen, SM; Magolda, R; Matelan†, E; Unwalla†, R; Basso, M; Clerin, V; Gardell, SJ; Nambi, P; Quinet, E; Reminick, JI; Vlasuk, GP; Wang, S; Feingold, I; Huselton, C; Bonn, T Indazole-Based Liver X Receptor (LXR) Modulators with Maintained Atherosclerotic Lesion Reduction Activity but Diminished Stimulation of Hepatic Triglyceride Synthesis J Med Chem51:7161-8 (2008) [PubMed] Article
Wrobel, J; Steffan†, R; Bowen, SM; Magolda, R; Matelan†, E; Unwalla†, R; Basso, M; Clerin, V; Gardell, SJ; Nambi, P; Quinet, E; Reminick, JI; Vlasuk, GP; Wang, S; Feingold, I; Huselton, C; Bonn, T Indazole-Based Liver X Receptor (LXR) Modulators with Maintained Atherosclerotic Lesion Reduction Activity but Diminished Stimulation of Hepatic Triglyceride Synthesis J Med Chem51:7161-8 (2008) [PubMed] Article