

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM113742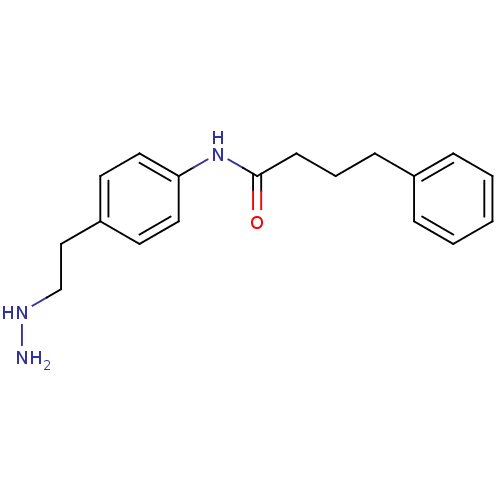 (N-[4-(2-Hydrazinylethyl)phenyl]-4-phenylbutanamide...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins University School of Medicine , Baltimore, Maryland 21205, United States | Assay Description Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM... | ACS Chem Biol 9: 1284-93 (2014) Article DOI: 10.1021/cb500018s BindingDB Entry DOI: 10.7270/Q2FN14VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM113750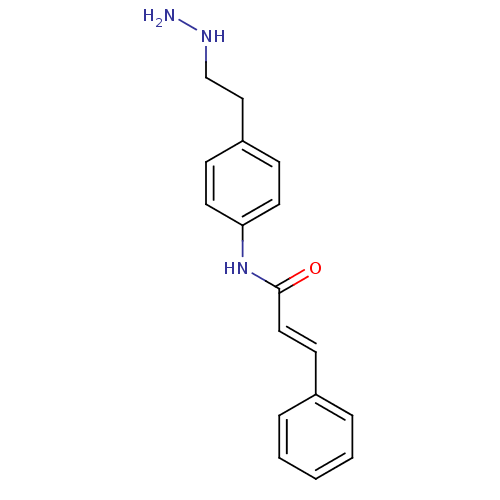 ((2E)-N-[4-(2-Hydrazinylethyl)phenyl]-3-phenylprop-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins University School of Medicine , Baltimore, Maryland 21205, United States | Assay Description Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM... | ACS Chem Biol 9: 1284-93 (2014) Article DOI: 10.1021/cb500018s BindingDB Entry DOI: 10.7270/Q2FN14VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM113753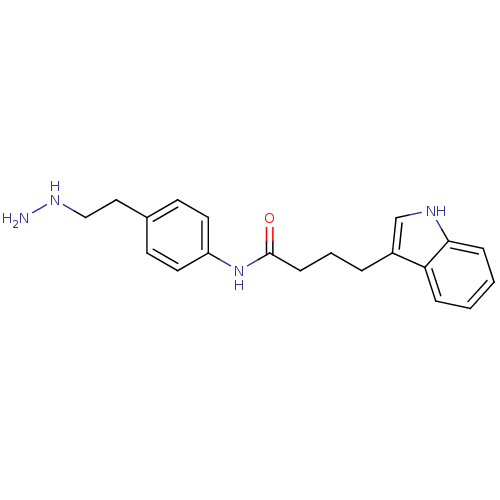 (N-[4-(2-Hydrazinylethyl)phenyl]-4-(1H-indol-3-yl)b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins University School of Medicine , Baltimore, Maryland 21205, United States | Assay Description Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM... | ACS Chem Biol 9: 1284-93 (2014) Article DOI: 10.1021/cb500018s BindingDB Entry DOI: 10.7270/Q2FN14VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM113745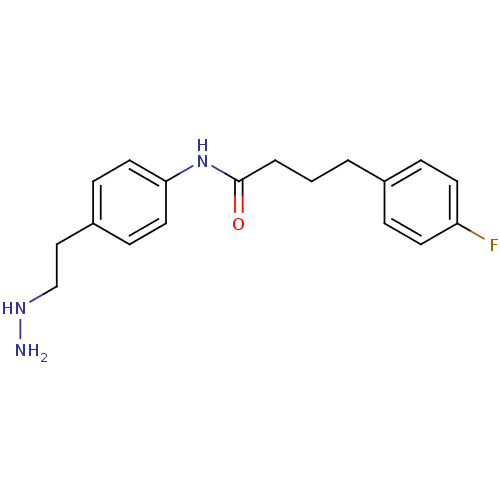 (4-(4-Fluorophenyl)-N-[4-(2-hydrazinylethyl)phenyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 138 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins University School of Medicine , Baltimore, Maryland 21205, United States | Assay Description Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM... | ACS Chem Biol 9: 1284-93 (2014) Article DOI: 10.1021/cb500018s BindingDB Entry DOI: 10.7270/Q2FN14VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM113744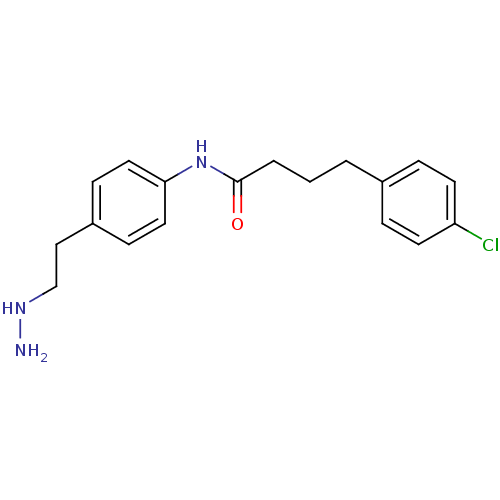 (4-(4-Chlorophenyl)-N-[4-(2-hydrazinylethyl)phenyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 156 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins University School of Medicine , Baltimore, Maryland 21205, United States | Assay Description Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM... | ACS Chem Biol 9: 1284-93 (2014) Article DOI: 10.1021/cb500018s BindingDB Entry DOI: 10.7270/Q2FN14VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM113748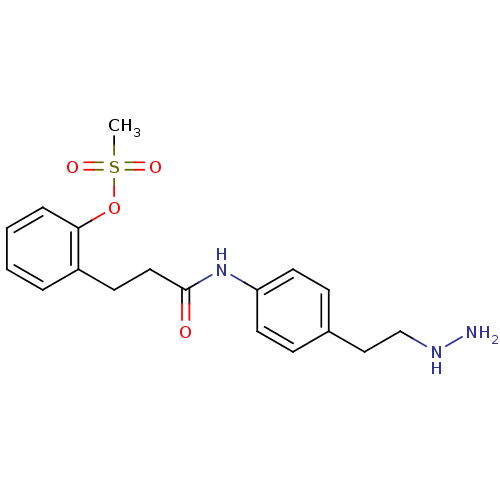 (2-(3-{[4-(2-Hydrazinylethyl)phenyl]amino}-3-oxopro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 204 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins University School of Medicine , Baltimore, Maryland 21205, United States | Assay Description Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM... | ACS Chem Biol 9: 1284-93 (2014) Article DOI: 10.1021/cb500018s BindingDB Entry DOI: 10.7270/Q2FN14VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM113746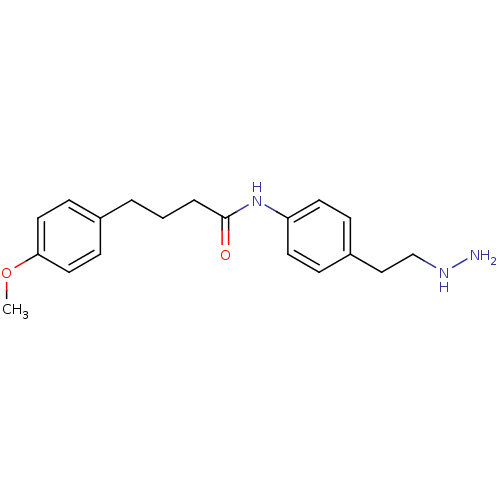 (N-[4-(2-Hydrazinylethyl)phenyl]-4-(4-methoxyphenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 207 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins University School of Medicine , Baltimore, Maryland 21205, United States | Assay Description Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM... | ACS Chem Biol 9: 1284-93 (2014) Article DOI: 10.1021/cb500018s BindingDB Entry DOI: 10.7270/Q2FN14VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM113752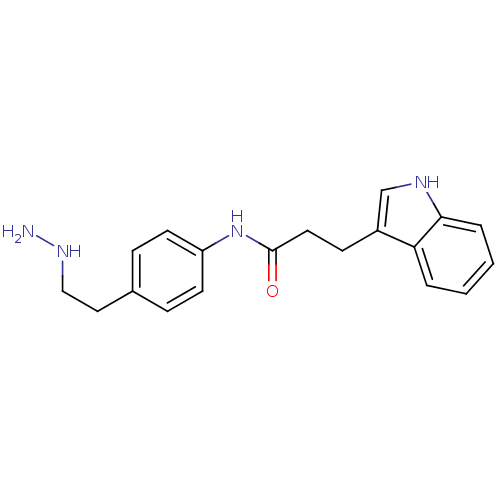 (N-[4-(2-Hydrazinylethyl)phenyl]-3-(1H-indol-3-yl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins University School of Medicine , Baltimore, Maryland 21205, United States | Assay Description Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM... | ACS Chem Biol 9: 1284-93 (2014) Article DOI: 10.1021/cb500018s BindingDB Entry DOI: 10.7270/Q2FN14VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM113749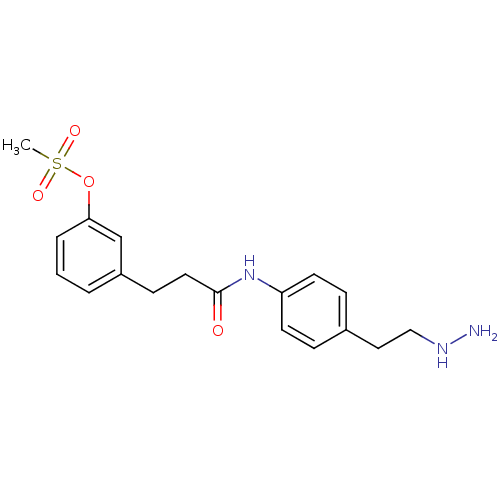 (3-(3-{[4-(2-Hydrazinylethyl)phenyl]amino}-3-oxopro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 223 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins University School of Medicine , Baltimore, Maryland 21205, United States | Assay Description Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM... | ACS Chem Biol 9: 1284-93 (2014) Article DOI: 10.1021/cb500018s BindingDB Entry DOI: 10.7270/Q2FN14VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM113741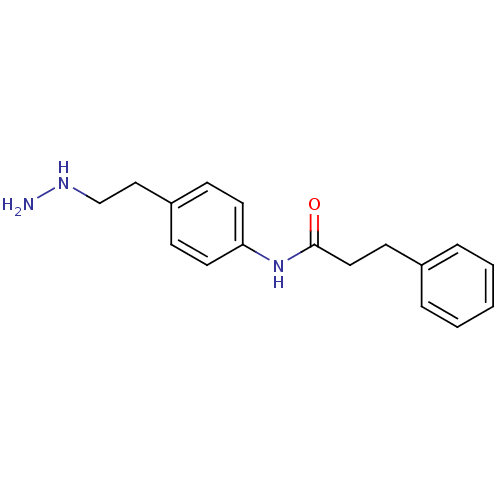 (N-[4-(2-Hydrazinylethyl)phenyl]-3-phenylpropanamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins University School of Medicine , Baltimore, Maryland 21205, United States | Assay Description Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM... | ACS Chem Biol 9: 1284-93 (2014) Article DOI: 10.1021/cb500018s BindingDB Entry DOI: 10.7270/Q2FN14VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM113743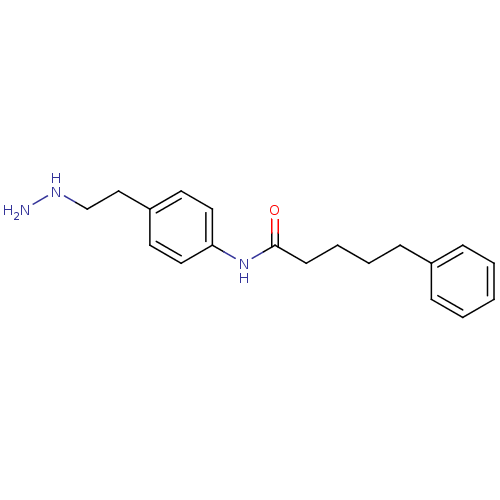 (N-[4-(2-Hydrazinylethyl)phenyl]-5-phenylpentanamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins University School of Medicine , Baltimore, Maryland 21205, United States | Assay Description Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM... | ACS Chem Biol 9: 1284-93 (2014) Article DOI: 10.1021/cb500018s BindingDB Entry DOI: 10.7270/Q2FN14VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM113739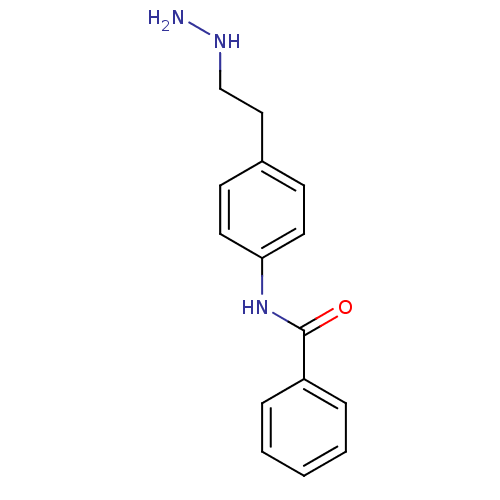 (N-[4-(2-Hydrazinylethyl)phenyl]benzamide dihydroch...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins University School of Medicine , Baltimore, Maryland 21205, United States | Assay Description Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM... | ACS Chem Biol 9: 1284-93 (2014) Article DOI: 10.1021/cb500018s BindingDB Entry DOI: 10.7270/Q2FN14VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM113747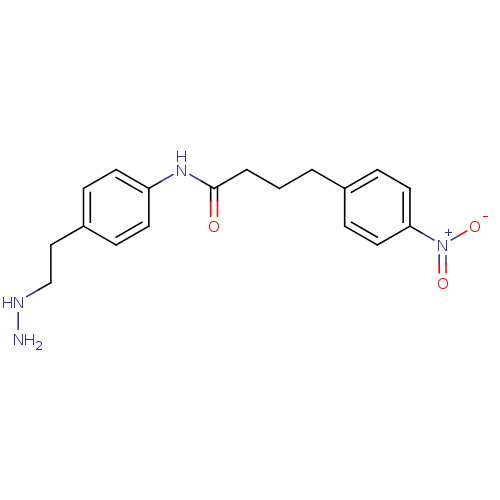 (N-[4-(2-Hydrazinylethyl)phenyl]-4-(4-nitrophenyl)b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 282 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins University School of Medicine , Baltimore, Maryland 21205, United States | Assay Description Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM... | ACS Chem Biol 9: 1284-93 (2014) Article DOI: 10.1021/cb500018s BindingDB Entry DOI: 10.7270/Q2FN14VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM113740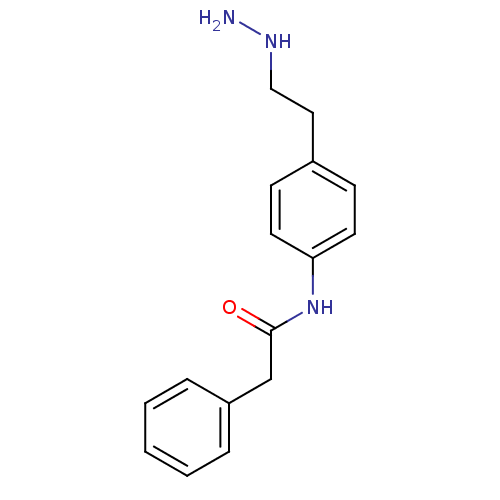 (N-[4-(2-Hydrazinylethyl)phenyl]-2-phenylacetamide ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 370 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins University School of Medicine , Baltimore, Maryland 21205, United States | Assay Description Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM... | ACS Chem Biol 9: 1284-93 (2014) Article DOI: 10.1021/cb500018s BindingDB Entry DOI: 10.7270/Q2FN14VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50105417 (CHEMBL1089 | Nardil | PHENELZINE | Phenethyl-hydra...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 820 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins University School of Medicine , Baltimore, Maryland 21205, United States | Assay Description Reactions (100 µL) were initiated by the addition of 2 µL MAO-A/B (final concentrations were 100-200 nM and 0.837 µM for MAO-A and MAO... | ACS Chem Biol 9: 1284-93 (2014) Article DOI: 10.1021/cb500018s BindingDB Entry DOI: 10.7270/Q2FN14VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM113751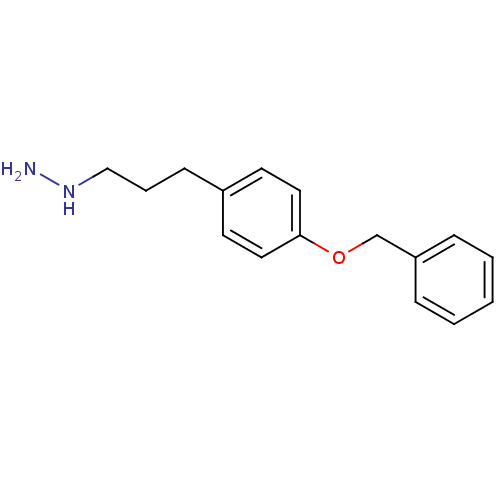 ({3-[4-(Benxyloxy)phenyl]propyl}hydrazine dihydroch...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins University School of Medicine , Baltimore, Maryland 21205, United States | Assay Description Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM... | ACS Chem Biol 9: 1284-93 (2014) Article DOI: 10.1021/cb500018s BindingDB Entry DOI: 10.7270/Q2FN14VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM113755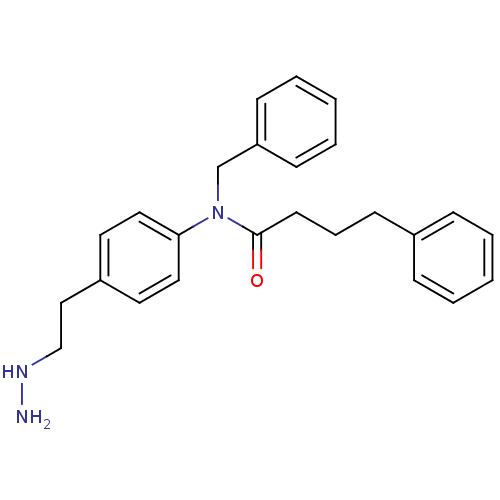 (N-Benzyl-N-[4-(2-hydrazinylethyl)phenyl]-4-phenylb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins University School of Medicine , Baltimore, Maryland 21205, United States | Assay Description Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM... | ACS Chem Biol 9: 1284-93 (2014) Article DOI: 10.1021/cb500018s BindingDB Entry DOI: 10.7270/Q2FN14VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM113754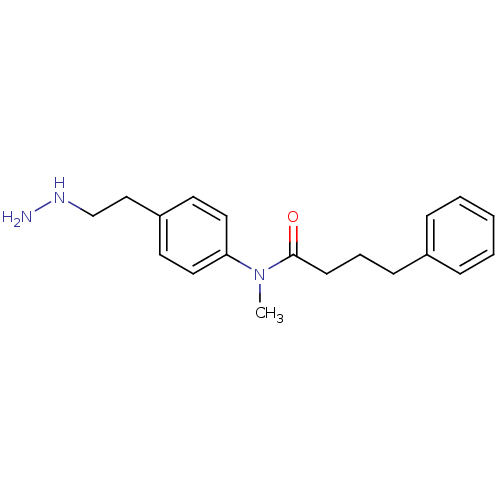 (N-[4-(2-Hydrazinylethyl)phenyl]-N-methyl-4-phenylb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins University School of Medicine , Baltimore, Maryland 21205, United States | Assay Description Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM... | ACS Chem Biol 9: 1284-93 (2014) Article DOI: 10.1021/cb500018s BindingDB Entry DOI: 10.7270/Q2FN14VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM113742 (N-[4-(2-Hydrazinylethyl)phenyl]-4-phenylbutanamide...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins University School of Medicine , Baltimore, Maryland 21205, United States | Assay Description Reactions (100 µL) were initiated by the addition of 2 µL MAO-A/B (final concentrations were 100-200 nM and 0.837 µM for MAO-A and MAO... | ACS Chem Biol 9: 1284-93 (2014) Article DOI: 10.1021/cb500018s BindingDB Entry DOI: 10.7270/Q2FN14VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50105417 (CHEMBL1089 | Nardil | PHENELZINE | Phenethyl-hydra...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins University School of Medicine , Baltimore, Maryland 21205, United States | Assay Description Reactions (100 µL) were initiated by the addition of 2 µL MAO-A/B (final concentrations were 100-200 nM and 0.837 µM for MAO-A and MAO... | ACS Chem Biol 9: 1284-93 (2014) Article DOI: 10.1021/cb500018s BindingDB Entry DOI: 10.7270/Q2FN14VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM113730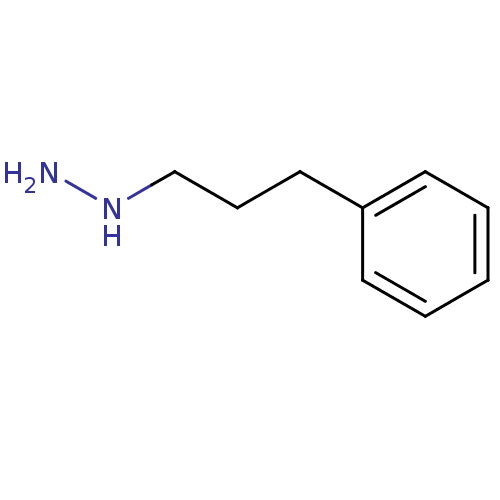 ((3-Phenylpropyl)hydrazine dihydrochloride salt (9c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins University School of Medicine , Baltimore, Maryland 21205, United States | Assay Description Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM... | ACS Chem Biol 9: 1284-93 (2014) Article DOI: 10.1021/cb500018s BindingDB Entry DOI: 10.7270/Q2FN14VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50105417 (CHEMBL1089 | Nardil | PHENELZINE | Phenethyl-hydra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins University School of Medicine , Baltimore, Maryland 21205, United States | Assay Description Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM... | ACS Chem Biol 9: 1284-93 (2014) Article DOI: 10.1021/cb500018s BindingDB Entry DOI: 10.7270/Q2FN14VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM113742 (N-[4-(2-Hydrazinylethyl)phenyl]-4-phenylbutanamide...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins University School of Medicine , Baltimore, Maryland 21205, United States | Assay Description Reactions (100 µL) were initiated by the addition of 2 µL MAO-A/B (final concentrations were 100-200 nM and 0.837 µM for MAO-A and MAO... | ACS Chem Biol 9: 1284-93 (2014) Article DOI: 10.1021/cb500018s BindingDB Entry DOI: 10.7270/Q2FN14VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM113732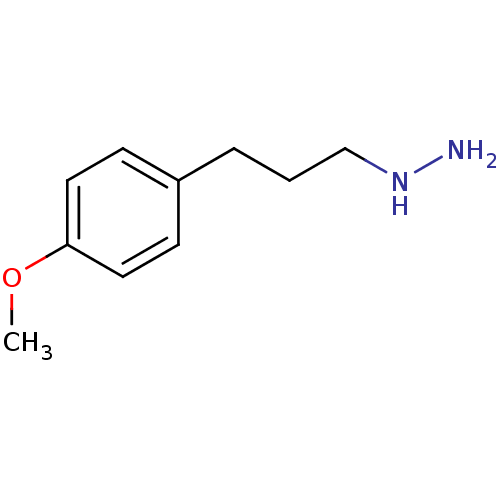 ([3-(4-Methoxyphenyl)propyl]hydrazine dihydrochlori...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins University School of Medicine , Baltimore, Maryland 21205, United States | Assay Description Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM... | ACS Chem Biol 9: 1284-93 (2014) Article DOI: 10.1021/cb500018s BindingDB Entry DOI: 10.7270/Q2FN14VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM113737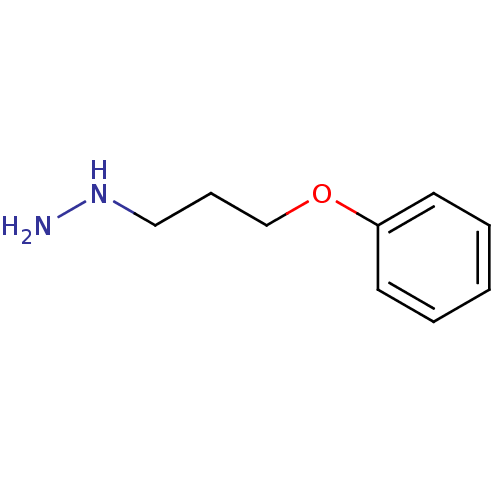 ((3-Phenoxypropyl)hydrazine dihydrochloride salt (1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins University School of Medicine , Baltimore, Maryland 21205, United States | Assay Description Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM... | ACS Chem Biol 9: 1284-93 (2014) Article DOI: 10.1021/cb500018s BindingDB Entry DOI: 10.7270/Q2FN14VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM113735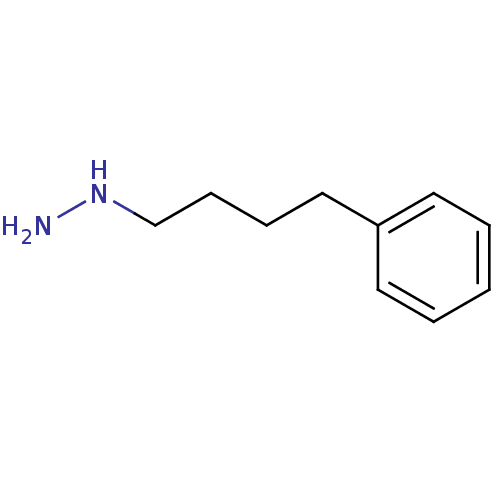 ((4-Phenylbutyl)hydrazine dihydrochloride salt (9h)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins University School of Medicine , Baltimore, Maryland 21205, United States | Assay Description Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM... | ACS Chem Biol 9: 1284-93 (2014) Article DOI: 10.1021/cb500018s BindingDB Entry DOI: 10.7270/Q2FN14VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM113736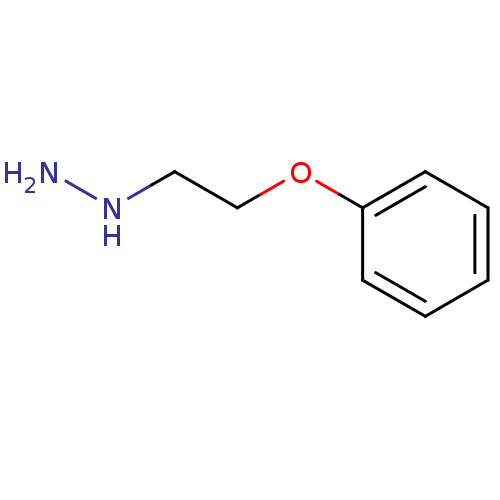 ((2-Phenoxyethyl)hydrazine dihydrochloride salt (10...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins University School of Medicine , Baltimore, Maryland 21205, United States | Assay Description Reactions (100 µL) were initiated by the addition of 2 µL of GST-LSD1 (final concentration 96-154 nM). The reaction mixture contained 50 mM... | ACS Chem Biol 9: 1284-93 (2014) Article DOI: 10.1021/cb500018s BindingDB Entry DOI: 10.7270/Q2FN14VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50506506 (CHEMBL4465833) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of EL in human HT1080 cells using A10070 as substrate preincubated for 20 min followed by DMPG vesicle doped substrate addition and measur... | J Med Chem 63: 1660-1670 (2020) Article DOI: 10.1021/acs.jmedchem.9b01831 BindingDB Entry DOI: 10.7270/Q28G8PZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50506498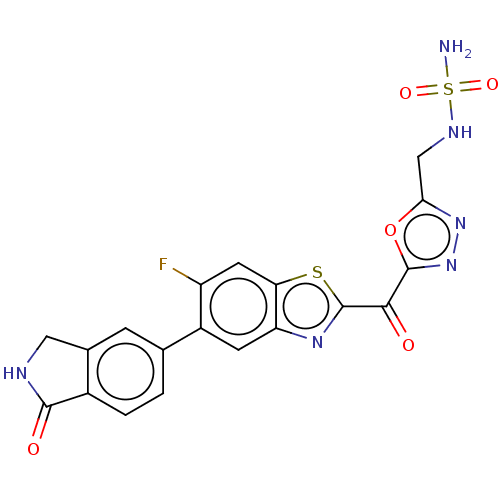 (CHEMBL4581886) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of EL in human HT1080 cells using A10070 as substrate preincubated for 20 min followed by DMPG vesicle doped substrate addition and measur... | J Med Chem 63: 1660-1670 (2020) Article DOI: 10.1021/acs.jmedchem.9b01831 BindingDB Entry DOI: 10.7270/Q28G8PZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50506505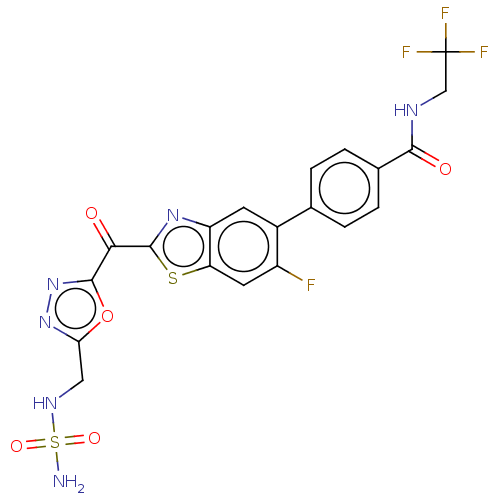 (CHEMBL4460376) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of EL in human HT1080 cells using A10070 as substrate preincubated for 20 min followed by DMPG vesicle doped substrate addition and measur... | J Med Chem 63: 1660-1670 (2020) Article DOI: 10.1021/acs.jmedchem.9b01831 BindingDB Entry DOI: 10.7270/Q28G8PZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50506504 (CHEMBL4460663) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of EL in human HT1080 cells using A10070 as substrate preincubated for 20 min followed by DMPG vesicle doped substrate addition and measur... | J Med Chem 63: 1660-1670 (2020) Article DOI: 10.1021/acs.jmedchem.9b01831 BindingDB Entry DOI: 10.7270/Q28G8PZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50506501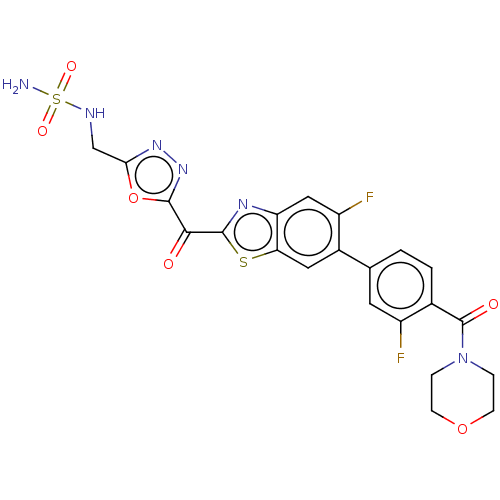 (CHEMBL4563019) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of EL in human HT1080 cells using A10070 as substrate preincubated for 20 min followed by DMPG vesicle doped substrate addition and measur... | J Med Chem 63: 1660-1670 (2020) Article DOI: 10.1021/acs.jmedchem.9b01831 BindingDB Entry DOI: 10.7270/Q28G8PZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50554034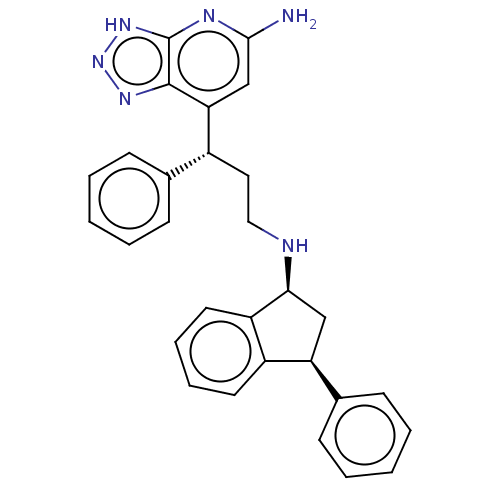 (CHEMBL4747269) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human MPO incubated for 10 mins in presence of 120 mM NaCl by aminophenyl fluorescein based assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115723 BindingDB Entry DOI: 10.7270/Q21Z4829 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endothelial lipase (Homo sapiens (Human)) | BDBM50506500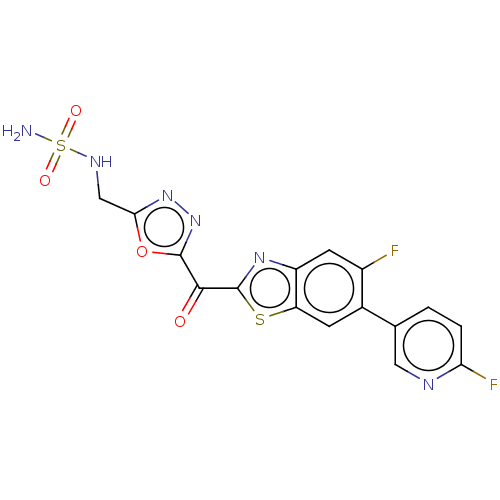 (CHEMBL4471611) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of EL in human HT1080 cells using A10070 as substrate preincubated for 20 min followed by DMPG vesicle doped substrate addition and measur... | J Med Chem 63: 1660-1670 (2020) Article DOI: 10.1021/acs.jmedchem.9b01831 BindingDB Entry DOI: 10.7270/Q28G8PZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20024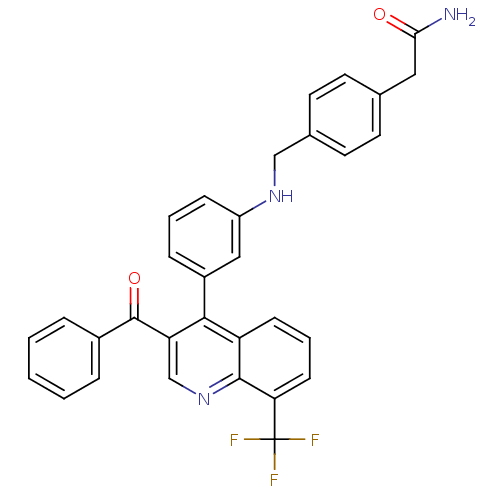 (2-{4-[({3-[3-benzoyl-8-(trifluoromethyl)quinolin-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | 316 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50554035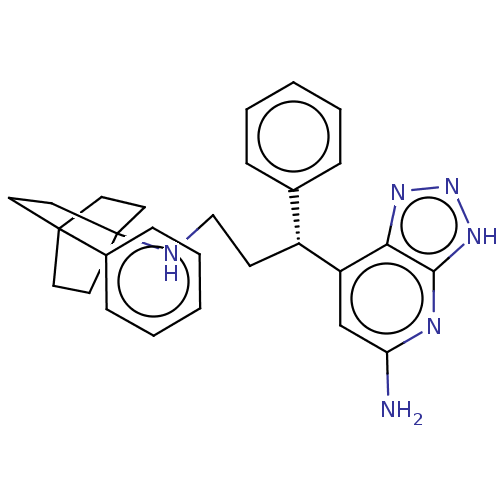 (CHEMBL4790231) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human MPO incubated for 10 mins in presence of 120 mM NaCl by aminophenyl fluorescein based assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115723 BindingDB Entry DOI: 10.7270/Q21Z4829 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20015 (2-[4-({[3-(3-benzyl-8-chloroquinolin-4-yl)phenyl]a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | 23 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20001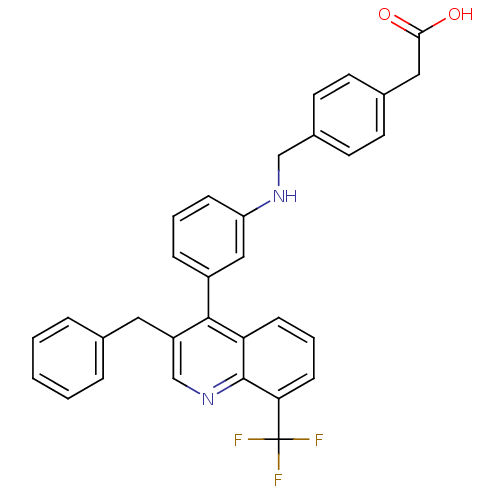 (2-{4-[({3-[3-benzyl-8-(trifluoromethyl)quinolin-4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | 33 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 49: 6151-4 (2006) Article DOI: 10.1021/jm0609566 BindingDB Entry DOI: 10.7270/Q20863KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20001 (2-{4-[({3-[3-benzyl-8-(trifluoromethyl)quinolin-4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | 33 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatic triacylglycerol lipase (Homo sapiens (Human)) | BDBM50506499 (CHEMBL4463265) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human HL expressed in human COS7 cells using A10070 as substrate preincubated for 20 min followed by DMPG vesicle doped substrate addit... | J Med Chem 63: 1660-1670 (2020) Article DOI: 10.1021/acs.jmedchem.9b01831 BindingDB Entry DOI: 10.7270/Q28G8PZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50554052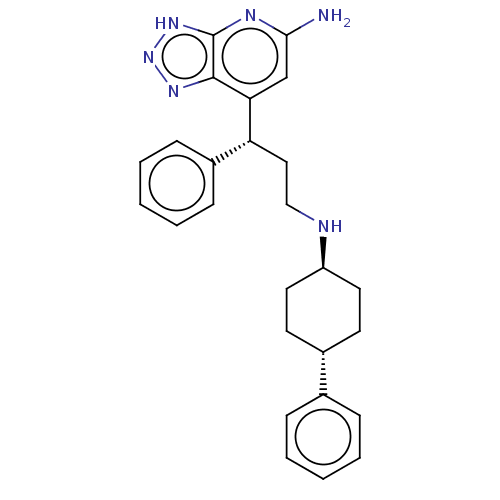 (CHEMBL4763667) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human MPO incubated for 10 mins in presence of 120 mM NaCl by aminophenyl fluorescein based assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115723 BindingDB Entry DOI: 10.7270/Q21Z4829 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20001 (2-{4-[({3-[3-benzyl-8-(trifluoromethyl)quinolin-4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | 90 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20000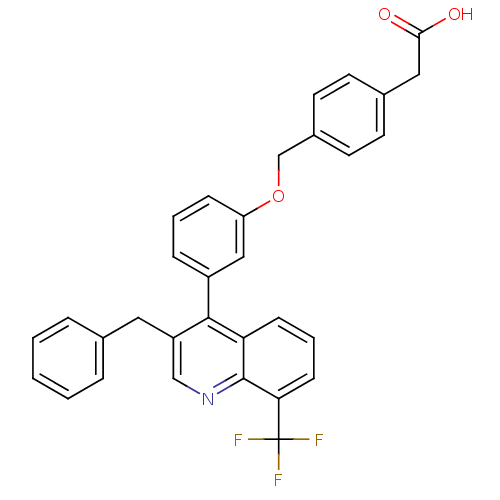 (2-(4-{3-[3-benzyl-8-(trifluoromethyl)quinolin-4-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | 90 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 51: 7161-8 (2008) Article DOI: 10.1021/jm800799q BindingDB Entry DOI: 10.7270/Q2XW4H4G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20000 (2-(4-{3-[3-benzyl-8-(trifluoromethyl)quinolin-4-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.10 | n/a | 71 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 49: 6151-4 (2006) Article DOI: 10.1021/jm0609566 BindingDB Entry DOI: 10.7270/Q20863KH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20004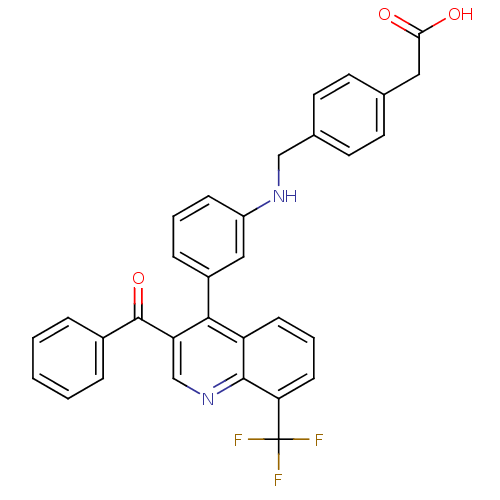 (2-{4-[({3-[3-benzoyl-8-(trifluoromethyl)quinolin-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | 29 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20020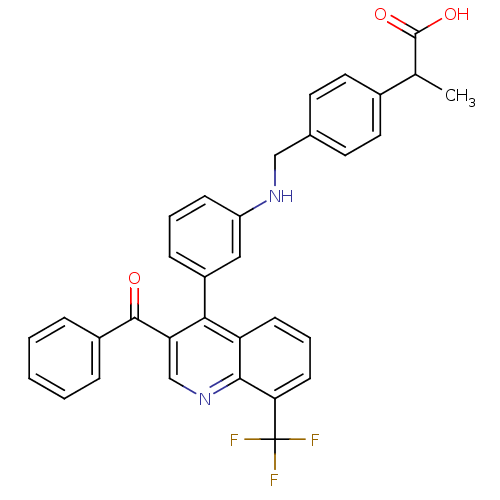 (2-{4-[({3-[3-benzoyl-8-(trifluoromethyl)quinolin-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | 58 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20014 (2-[4-({[3-(3-benzoyl-8-chloroquinolin-4-yl)phenyl]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | 39 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50554050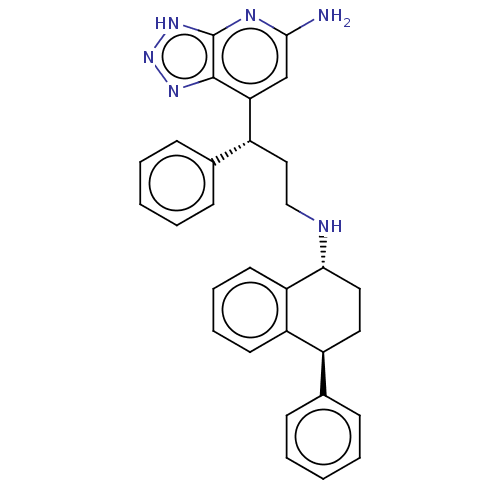 (CHEMBL4752248) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human MPO incubated for 10 mins in presence of 120 mM NaCl by aminophenyl fluorescein based assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115723 BindingDB Entry DOI: 10.7270/Q21Z4829 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatic triacylglycerol lipase (Homo sapiens (Human)) | BDBM24567 ((2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridecan-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of recombinant human HL expressed in COS7 cells using PED-A1 containing DMPG vesicles as substrate pretreated for 20 mins followed by subs... | ACS Med Chem Lett 9: 1263-1268 (2018) Article DOI: 10.1021/acsmedchemlett.8b00424 BindingDB Entry DOI: 10.7270/Q2TF01QC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatic triacylglycerol lipase (Homo sapiens (Human)) | BDBM187287 (US9169240, 27) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human HL expressed in human COS7 cells using A10070 as substrate preincubated for 20 min followed by DMPG vesicle doped substrate addit... | J Med Chem 63: 1660-1670 (2020) Article DOI: 10.1021/acs.jmedchem.9b01831 BindingDB Entry DOI: 10.7270/Q28G8PZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 539 total ) | Next | Last >> |