| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Glycogen phosphorylase, liver form |
|---|
| Ligand | BDBM35634 |
|---|
| Substrate/Competitor | BDBM24362 |
|---|
| Meas. Tech. | Glycogen Phosphorylase Activity Assay |
|---|
| pH | 6.8±n/a |
|---|
| Temperature | 295.15±n/a K |
|---|
| IC50 | 2100±n/a nM |
|---|
| Citation |  Onda, K; Suzuki, T; Shiraki, R; Yonetoku, Y; Negoro, K; Momose, K; Katayama, N; Orita, M; Yamaguchi, T; Ohta, M; Tsukamoto, S Synthesis of 5-chloro-N-aryl-1H-indole-2-carboxamide derivatives as inhibitors of human liver glycogen phosphorylase a. Bioorg Med Chem16:5452-64 (2008) [PubMed] Article Onda, K; Suzuki, T; Shiraki, R; Yonetoku, Y; Negoro, K; Momose, K; Katayama, N; Orita, M; Yamaguchi, T; Ohta, M; Tsukamoto, S Synthesis of 5-chloro-N-aryl-1H-indole-2-carboxamide derivatives as inhibitors of human liver glycogen phosphorylase a. Bioorg Med Chem16:5452-64 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Solution Info, Assay Method |
|---|
| |
| Glycogen phosphorylase, liver form |
|---|
| Name: | Glycogen phosphorylase, liver form |
|---|
| Synonyms: | Glycogen Phosphorylase (PYGL) | Glycogen Phosphorylase, liver form | Liver glycogen phosphorylase | PYGL | PYGL_HUMAN |
|---|
| Type: | Homodimer |
|---|
| Mol. Mass.: | 97153.98 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Dimers associate into a tetramer to form the enzymatically active phosphorylase A. |
|---|
| Residue: | 847 |
|---|
| Sequence: | MAKPLTDQEKRRQISIRGIVGVENVAELKKSFNRHLHFTLVKDRNVATTRDYYFALAHTV
RDHLVGRWIRTQQHYYDKCPKRVYYLSLEFYMGRTLQNTMINLGLQNACDEAIYQLGLDI
EELEEIEEDAGLGNGGLGRLAACFLDSMATLGLAAYGYGIRYEYGIFNQKIRDGWQVEEA
DDWLRYGNPWEKSRPEFMLPVHFYGKVEHTNTGTKWIDTQVVLALPYDTPVPGYMNNTVN
TMRLWSARAPNDFNLRDFNVGDYIQAVLDRNLAENISRVLYPNDNFFEGKELRLKQEYFV
VAATLQDIIRRFKASKFGSTRGAGTVFDAFPDQVAIQLNDTHPALAIPELMRIFVDIEKL
PWSKAWELTQKTFAYTNHTVLPEALERWPVDLVEKLLPRHLEIIYEINQKHLDRIVALFP
KDVDRLRRMSLIEEEGSKRINMAHLCIVGSHAVNGVAKIHSDIVKTKVFKDFSELEPDKF
QNKTNGITPRRWLLLCNPGLAELIAEKIGEDYVKDLSQLTKLHSFLGDDVFLRELAKVKQ
ENKLKFSQFLETEYKVKINPSSMFDVQVKRIHEYKRQLLNCLHVITMYNRIKKDPKKLFV
PRTVIIGGKAAPGYHMAKMIIKLITSVADVVNNDPMVGSKLKVIFLENYRVSLAEKVIPA
TDLSEQISTAGTEASGTGNMKFMLNGALTIGTMDGANVEMAEEAGEENLFIFGMRIDDVA
ALDKKGYEAKEYYEALPELKLVIDQIDNGFFSPKQPDLFKDIINMLFYHDRFKVFADYEA
YVKCQDKVSQLYMNPKAWNTMVLKNIAASGKFSSDRTIKEYAQNIWNVEPSDLKISLSNE
SNKVNGN
|
|
|
|---|
| BDBM35634 |
|---|
| BDBM24362 |
|---|
| Name | BDBM35634 |
|---|
| Synonyms: | 5-chloroindolecarboxamide, 9i |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C17H14ClN3O5 |
|---|
| Mol. Mass. | 375.763 |
|---|
| SMILES | OCC(O)c1ccc(NC(=O)c2cc3cc(Cl)ccc3[nH]2)cc1[N+]([O-])=O |
|---|
| Structure | 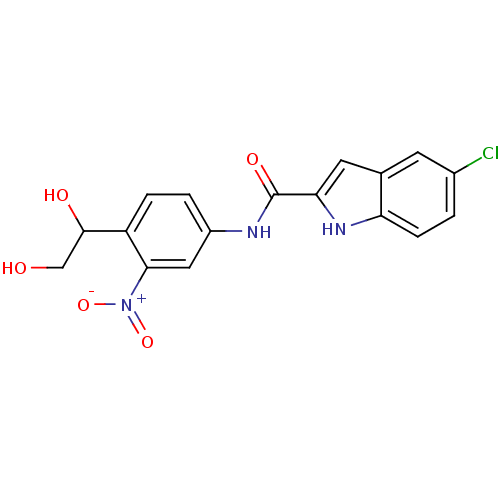
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Onda, K; Suzuki, T; Shiraki, R; Yonetoku, Y; Negoro, K; Momose, K; Katayama, N; Orita, M; Yamaguchi, T; Ohta, M; Tsukamoto, S Synthesis of 5-chloro-N-aryl-1H-indole-2-carboxamide derivatives as inhibitors of human liver glycogen phosphorylase a. Bioorg Med Chem16:5452-64 (2008) [PubMed] Article
Onda, K; Suzuki, T; Shiraki, R; Yonetoku, Y; Negoro, K; Momose, K; Katayama, N; Orita, M; Yamaguchi, T; Ohta, M; Tsukamoto, S Synthesis of 5-chloro-N-aryl-1H-indole-2-carboxamide derivatives as inhibitors of human liver glycogen phosphorylase a. Bioorg Med Chem16:5452-64 (2008) [PubMed] Article