

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A3 (Rattus norvegicus) | BDBM50453675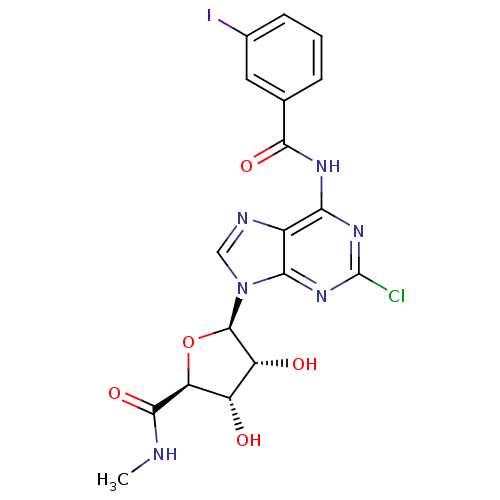 (CHEMBL2113400) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity against adenosine A3 receptor from rat brain. | J Med Chem 38: 1720-35 (1995) BindingDB Entry DOI: 10.7270/Q21Z453H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50088439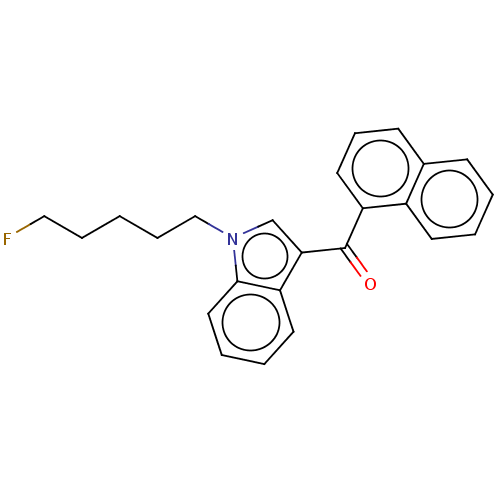 (CHEMBL3526578) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.395 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in B6SJL mouse brain membrane after 90 mins by liquid scintillation spectrophotometric analysis | Drug Metab Dispos 40: 2174-84 (2012) Article DOI: 10.1124/dmd.112.047530 BindingDB Entry DOI: 10.7270/Q23R0VK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Rattus norvegicus) | BDBM50118812 ((2S,3S,4R,5R)-3,4-Dihydroxy-5-[6-(3-iodo-benzylami...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity against adenosine A3 receptor from rat brain. | J Med Chem 38: 1720-35 (1995) BindingDB Entry DOI: 10.7270/Q21Z453H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM204859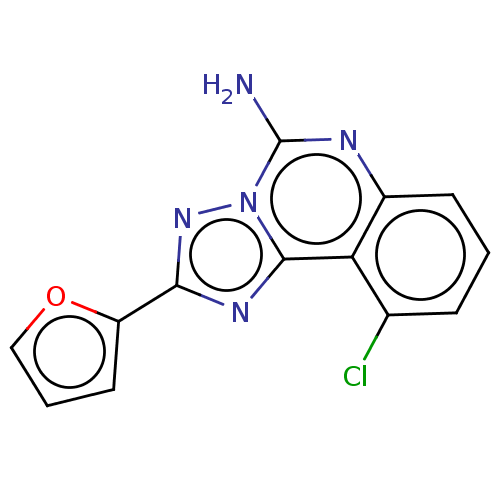 (US9227979, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by The Secretary, Department of Health and Human Services; Universita Degli Studi Di Trieste; Universita Degli Studi Di Padova US Patent | Assay Description Radioligand binding assays at hA1, hA2A, and hA3ARs were performed according to the procedures described in Gao, Z. G., et al., Biochem Pharmacol. 20... | US Patent US9227979 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50353747 (CHEMBL561013 | JWH-018) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in B6SJL mouse brain membrane after 90 mins by liquid scintillation spectrophotometric analysis | Drug Metab Dispos 40: 2174-84 (2012) Article DOI: 10.1124/dmd.112.047530 BindingDB Entry DOI: 10.7270/Q23R0VK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM86032 (trans-H2-PAT(-) | trans-PAT) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Binding affinity to human histamine H3 receptor expressed in HEK293 cells | Bioorg Med Chem 23: 1588-600 (2015) Article DOI: 10.1016/j.bmc.2015.01.060 BindingDB Entry DOI: 10.7270/Q2TF0010 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50065036 (CHEMBL3402656) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Binding affinity to human histamine H3 receptor expressed in HEK293 cells | Bioorg Med Chem 23: 1588-600 (2015) Article DOI: 10.1016/j.bmc.2015.01.060 BindingDB Entry DOI: 10.7270/Q2TF0010 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50065035 (CHEMBL3402657) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Binding affinity to human histamine H3 receptor expressed in HEK293 cells | Bioorg Med Chem 23: 1588-600 (2015) Article DOI: 10.1016/j.bmc.2015.01.060 BindingDB Entry DOI: 10.7270/Q2TF0010 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM204870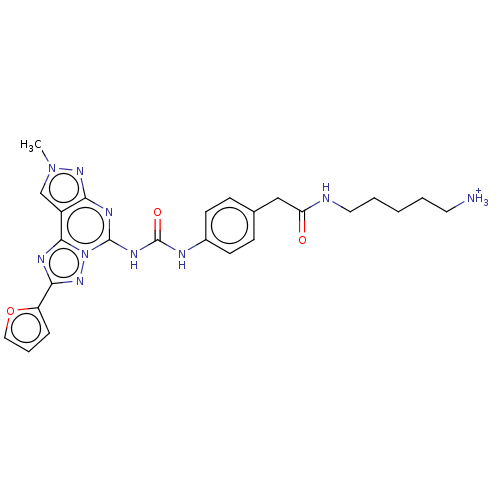 (EA7/MRS5816 | US9227979, 19) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by The Secretary, Department of Health and Human Services; Universita Degli Studi Di Trieste; Universita Degli Studi Di Padova US Patent | Assay Description Radioligand binding assays at hA1, hA2A, and hA3ARs were performed according to the procedures described in Gao, Z. G., et al., Biochem Pharmacol. 20... | US Patent US9227979 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM204863 (EA12/MRS5821 | US9227979, 12) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by The Secretary, Department of Health and Human Services; Universita Degli Studi Di Trieste; Universita Degli Studi Di Padova US Patent | Assay Description Radioligand binding assays at hA1, hA2A, and hA3ARs were performed according to the procedures described in Gao, Z. G., et al., Biochem Pharmacol. 20... | US Patent US9227979 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50065014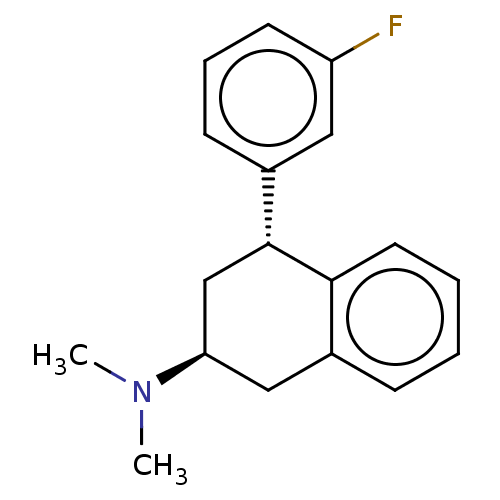 (CHEMBL3402677) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Binding affinity to human histamine H3 receptor expressed in HEK293 cells | Bioorg Med Chem 23: 1588-600 (2015) Article DOI: 10.1016/j.bmc.2015.01.060 BindingDB Entry DOI: 10.7270/Q2TF0010 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM204864 (EA15/MRS5824 | US9227979, 13) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by The Secretary, Department of Health and Human Services; Universita Degli Studi Di Trieste; Universita Degli Studi Di Padova US Patent | Assay Description Radioligand binding assays at hA1, hA2A, and hA3ARs were performed according to the procedures described in Gao, Z. G., et al., Biochem Pharmacol. 20... | US Patent US9227979 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM204859 (US9227979, 7) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by The Secretary, Department of Health and Human Services; Universita Degli Studi Di Trieste; Universita Degli Studi Di Padova US Patent | Assay Description Radioligand binding assays at hA1, hA2A, and hA3ARs were performed according to the procedures described in Gao, Z. G., et al., Biochem Pharmacol. 20... | US Patent US9227979 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50065018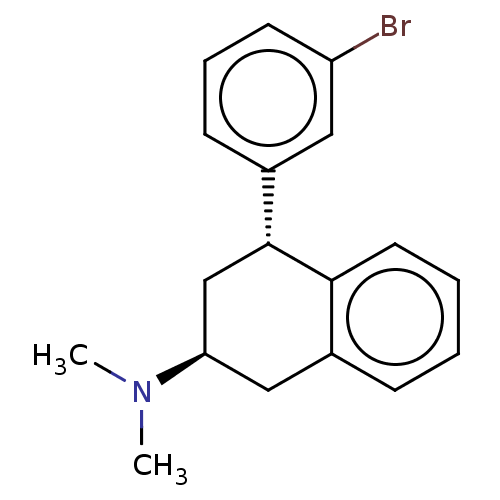 (CHEMBL3402673) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]Mesulergine from human 5-HT2C-INI receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem 23: 1588-600 (2015) Article DOI: 10.1016/j.bmc.2015.01.060 BindingDB Entry DOI: 10.7270/Q2TF0010 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50065024 (CHEMBL3402667) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Binding affinity to human histamine H3 receptor expressed in HEK293 cells | Bioorg Med Chem 23: 1588-600 (2015) Article DOI: 10.1016/j.bmc.2015.01.060 BindingDB Entry DOI: 10.7270/Q2TF0010 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM204879 (MRS5846/5762 5-hex-5-ynamide | US9227979, 37) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by The Secretary, Department of Health and Human Services; Universita Degli Studi Di Trieste; Universita Degli Studi Di Padova US Patent | Assay Description Radioligand binding assays at hA1, hA2A, and hA3ARs were performed according to the procedures described in Gao, Z. G., et al., Biochem Pharmacol. 20... | US Patent US9227979 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM204865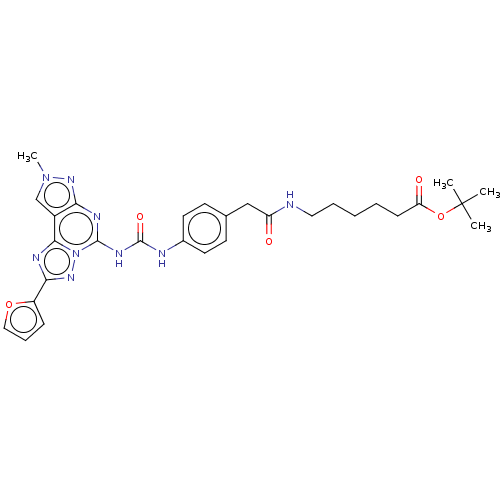 (EA6/MRS5815 | US9227979, 14) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by The Secretary, Department of Health and Human Services; Universita Degli Studi Di Trieste; Universita Degli Studi Di Padova US Patent | Assay Description Radioligand binding assays at hA1, hA2A, and hA3ARs were performed according to the procedures described in Gao, Z. G., et al., Biochem Pharmacol. 20... | US Patent US9227979 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50065040 (CHEMBL3402682) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]Mesulergine from human 5-HT2C-INI receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem 23: 1588-600 (2015) Article DOI: 10.1016/j.bmc.2015.01.060 BindingDB Entry DOI: 10.7270/Q2TF0010 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50065025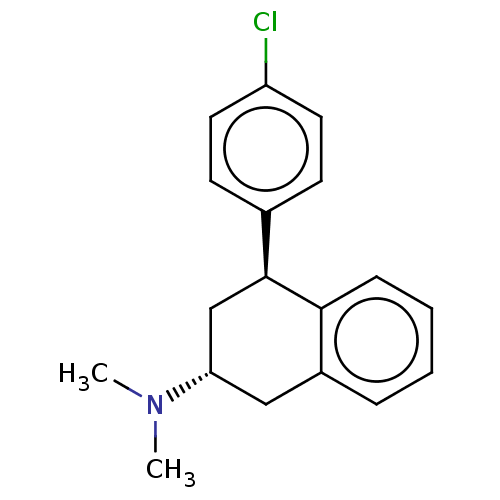 (CHEMBL3402666) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Binding affinity to human histamine H3 receptor expressed in HEK293 cells | Bioorg Med Chem 23: 1588-600 (2015) Article DOI: 10.1016/j.bmc.2015.01.060 BindingDB Entry DOI: 10.7270/Q2TF0010 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM204869 (EA16/MRS5825 | US9227979, 18) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by The Secretary, Department of Health and Human Services; Universita Degli Studi Di Trieste; Universita Degli Studi Di Padova US Patent | Assay Description Radioligand binding assays at hA1, hA2A, and hA3ARs were performed according to the procedures described in Gao, Z. G., et al., Biochem Pharmacol. 20... | US Patent US9227979 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM204861 (MRS5449 | US9227979, 9) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by The Secretary, Department of Health and Human Services; Universita Degli Studi Di Trieste; Universita Degli Studi Di Padova US Patent | Assay Description Radioligand binding assays at hA1, hA2A, and hA3ARs were performed according to the procedures described in Gao, Z. G., et al., Biochem Pharmacol. 20... | US Patent US9227979 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50065036 (CHEMBL3402656) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]Mesulergine from human 5-HT2C-INI receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem 23: 1588-600 (2015) Article DOI: 10.1016/j.bmc.2015.01.060 BindingDB Entry DOI: 10.7270/Q2TF0010 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM204880 (EA18(5-hept-6-ynamide)/MRS5838 | US9227979, 38) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by The Secretary, Department of Health and Human Services; Universita Degli Studi Di Trieste; Universita Degli Studi Di Padova US Patent | Assay Description Radioligand binding assays at hA1, hA2A, and hA3ARs were performed according to the procedures described in Gao, Z. G., et al., Biochem Pharmacol. 20... | US Patent US9227979 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50065018 (CHEMBL3402673) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]Mesulergine from human 5-HT2B receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem 23: 1588-600 (2015) Article DOI: 10.1016/j.bmc.2015.01.060 BindingDB Entry DOI: 10.7270/Q2TF0010 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50065016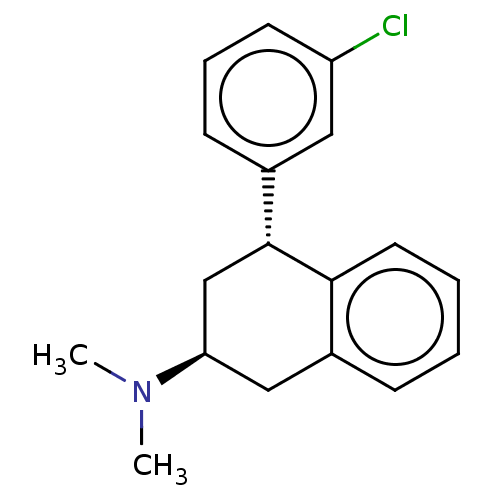 (CHEMBL3402675) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]Mesulergine from human 5-HT2C-INI receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem 23: 1588-600 (2015) Article DOI: 10.1016/j.bmc.2015.01.060 BindingDB Entry DOI: 10.7270/Q2TF0010 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50065026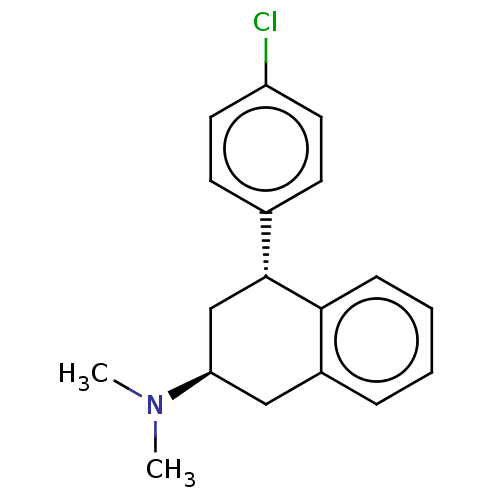 (CHEMBL3402665) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Binding affinity to human histamine H3 receptor expressed in HEK293 cells | Bioorg Med Chem 23: 1588-600 (2015) Article DOI: 10.1016/j.bmc.2015.01.060 BindingDB Entry DOI: 10.7270/Q2TF0010 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50065023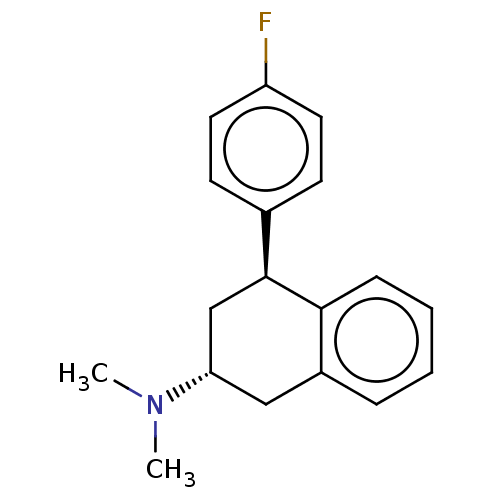 (CHEMBL3402668) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Binding affinity to human histamine H3 receptor expressed in HEK293 cells | Bioorg Med Chem 23: 1588-600 (2015) Article DOI: 10.1016/j.bmc.2015.01.060 BindingDB Entry DOI: 10.7270/Q2TF0010 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM204874 (EA13/MRS5822 | US9227979, 17) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8.06 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by The Secretary, Department of Health and Human Services; Universita Degli Studi Di Trieste; Universita Degli Studi Di Padova US Patent | Assay Description Radioligand binding assays at hA1, hA2A, and hA3ARs were performed according to the procedures described in Gao, Z. G., et al., Biochem Pharmacol. 20... | US Patent US9227979 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50065020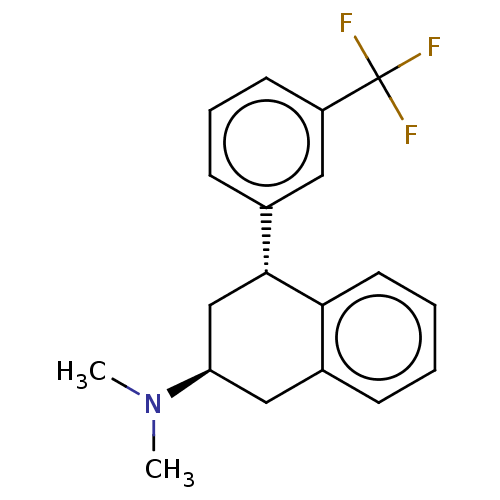 (CHEMBL3402671) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]Mesulergine from human 5-HT2C-INI receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem 23: 1588-600 (2015) Article DOI: 10.1016/j.bmc.2015.01.060 BindingDB Entry DOI: 10.7270/Q2TF0010 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50065022 (CHEMBL3402669) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]Mesulergine from human 5-HT2C-INI receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem 23: 1588-600 (2015) Article DOI: 10.1016/j.bmc.2015.01.060 BindingDB Entry DOI: 10.7270/Q2TF0010 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50065016 (CHEMBL3402675) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Binding affinity to human histamine H3 receptor expressed in HEK293 cells | Bioorg Med Chem 23: 1588-600 (2015) Article DOI: 10.1016/j.bmc.2015.01.060 BindingDB Entry DOI: 10.7270/Q2TF0010 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50065038 (CHEMBL3402680) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]Mesulergine from human 5-HT2B receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem 23: 1588-600 (2015) Article DOI: 10.1016/j.bmc.2015.01.060 BindingDB Entry DOI: 10.7270/Q2TF0010 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM204872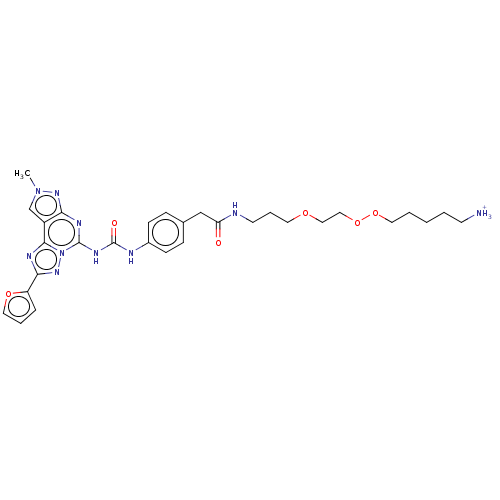 (EA10/MRS5819 | US9227979, 21) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 10.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by The Secretary, Department of Health and Human Services; Universita Degli Studi Di Trieste; Universita Degli Studi Di Padova US Patent | Assay Description Radioligand binding assays at hA1, hA2A, and hA3ARs were performed according to the procedures described in Gao, Z. G., et al., Biochem Pharmacol. 20... | US Patent US9227979 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM204878 (EA19(5-pent-4-ynamide)/MRS5811 | US9227979, 36) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by The Secretary, Department of Health and Human Services; Universita Degli Studi Di Trieste; Universita Degli Studi Di Padova US Patent | Assay Description Radioligand binding assays at hA1, hA2A, and hA3ARs were performed according to the procedures described in Gao, Z. G., et al., Biochem Pharmacol. 20... | US Patent US9227979 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Rattus norvegicus) | BDBM50453671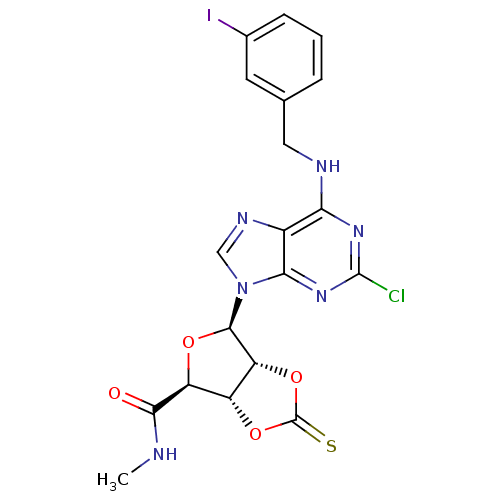 (CHEMBL2113701) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity against adenosine A3 receptor from rat brain. | J Med Chem 38: 1720-35 (1995) BindingDB Entry DOI: 10.7270/Q21Z453H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM204881 (EA20(5-oct-7-ynamide)/MRS5840 | US9227979, 39) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 12.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by The Secretary, Department of Health and Human Services; Universita Degli Studi Di Trieste; Universita Degli Studi Di Padova US Patent | Assay Description Radioligand binding assays at hA1, hA2A, and hA3ARs were performed according to the procedures described in Gao, Z. G., et al., Biochem Pharmacol. 20... | US Patent US9227979 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50088440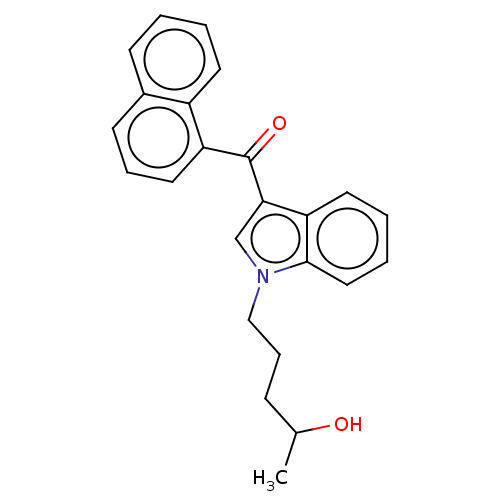 (CHEMBL3526291) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in B6SJL mouse brain membrane after 90 mins by liquid scintillation spectrophotometric analysis | Drug Metab Dispos 40: 2174-84 (2012) Article DOI: 10.1124/dmd.112.047530 BindingDB Entry DOI: 10.7270/Q23R0VK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM60994 ((10R,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in B6SJL mouse brain membrane after 90 mins by liquid scintillation spectrophotometric analysis | Drug Metab Dispos 40: 2174-84 (2012) Article DOI: 10.1124/dmd.112.047530 BindingDB Entry DOI: 10.7270/Q23R0VK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM204871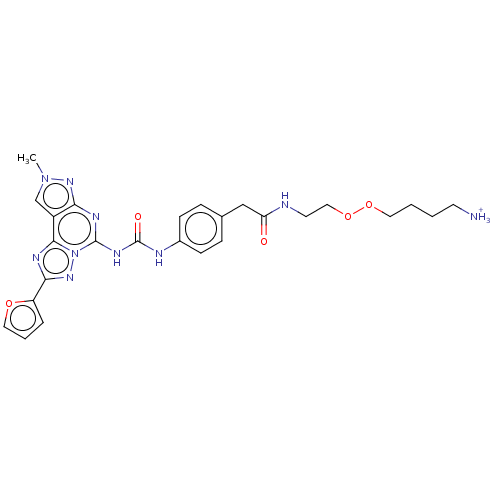 (EA4/MRS5813 | US9227979, 20) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 19.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by The Secretary, Department of Health and Human Services; Universita Degli Studi Di Trieste; Universita Degli Studi Di Padova US Patent | Assay Description Radioligand binding assays at hA1, hA2A, and hA3ARs were performed according to the procedures described in Gao, Z. G., et al., Biochem Pharmacol. 20... | US Patent US9227979 (2016) BindingDB Entry DOI: 10.7270/Q2JD4VMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50065036 (CHEMBL3402656) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]Ketanserin from human 5-HT2A receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem 23: 1588-600 (2015) Article DOI: 10.1016/j.bmc.2015.01.060 BindingDB Entry DOI: 10.7270/Q2TF0010 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50065036 (CHEMBL3402656) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]Mesulergine from human 5-HT2B receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem 23: 1588-600 (2015) Article DOI: 10.1016/j.bmc.2015.01.060 BindingDB Entry DOI: 10.7270/Q2TF0010 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50065018 (CHEMBL3402673) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]Ketanserin from human 5-HT2A receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem 23: 1588-600 (2015) Article DOI: 10.1016/j.bmc.2015.01.060 BindingDB Entry DOI: 10.7270/Q2TF0010 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50065021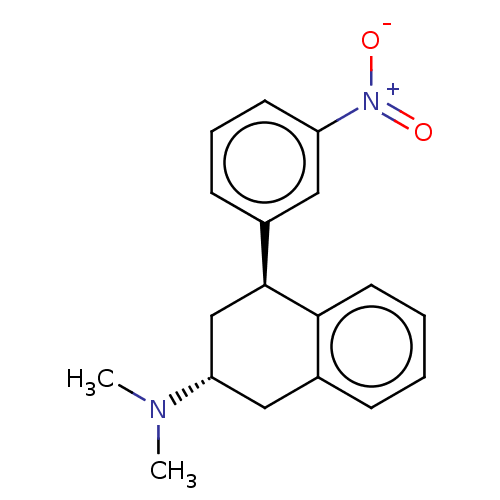 (CHEMBL3402670) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]Mesulergine from human 5-HT2B receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem 23: 1588-600 (2015) Article DOI: 10.1016/j.bmc.2015.01.060 BindingDB Entry DOI: 10.7270/Q2TF0010 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM86032 (trans-H2-PAT(-) | trans-PAT) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]Mesulergine from human 5-HT2C-INI receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem 23: 1588-600 (2015) Article DOI: 10.1016/j.bmc.2015.01.060 BindingDB Entry DOI: 10.7270/Q2TF0010 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50065038 (CHEMBL3402680) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]Ketanserin from human 5-HT2A receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem 23: 1588-600 (2015) Article DOI: 10.1016/j.bmc.2015.01.060 BindingDB Entry DOI: 10.7270/Q2TF0010 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50065016 (CHEMBL3402675) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]Mesulergine from human 5-HT2B receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem 23: 1588-600 (2015) Article DOI: 10.1016/j.bmc.2015.01.060 BindingDB Entry DOI: 10.7270/Q2TF0010 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50065028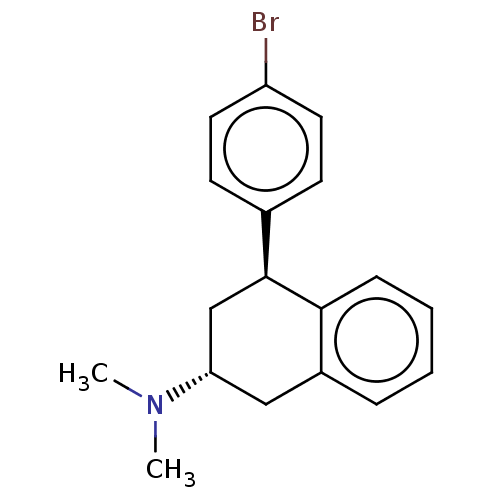 (CHEMBL3402664) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]Mesulergine from human 5-HT2B receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem 23: 1588-600 (2015) Article DOI: 10.1016/j.bmc.2015.01.060 BindingDB Entry DOI: 10.7270/Q2TF0010 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50065038 (CHEMBL3402680) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]Mesulergine from human 5-HT2C-INI receptor expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem 23: 1588-600 (2015) Article DOI: 10.1016/j.bmc.2015.01.060 BindingDB Entry DOI: 10.7270/Q2TF0010 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM86029 (trans-H2-PAT(+)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Binding affinity to human histamine H3 receptor expressed in HEK293 cells | Bioorg Med Chem 23: 1588-600 (2015) Article DOI: 10.1016/j.bmc.2015.01.060 BindingDB Entry DOI: 10.7270/Q2TF0010 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50065018 (CHEMBL3402673) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Binding affinity to human histamine H3 receptor expressed in HEK293 cells | Bioorg Med Chem 23: 1588-600 (2015) Article DOI: 10.1016/j.bmc.2015.01.060 BindingDB Entry DOI: 10.7270/Q2TF0010 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 996 total ) | Next | Last >> |