 Found 215 hits with Last Name = 'katayama' and Initial = 'n'
Found 215 hits with Last Name = 'katayama' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50095997
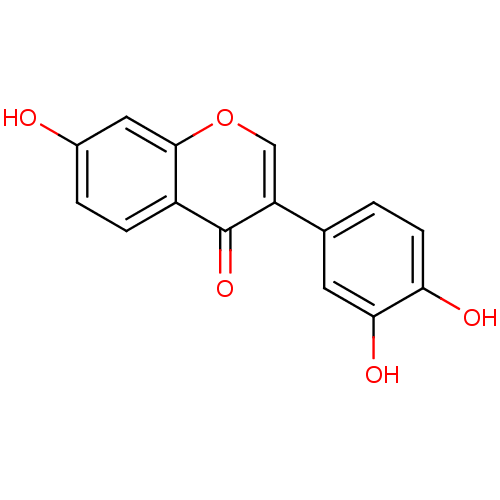
(3',4',7-trihydroxyisoflavone | CHEMBL13486)Show InChI InChI=1S/C15H10O5/c16-9-2-3-10-14(6-9)20-7-11(15(10)19)8-1-4-12(17)13(18)5-8/h1-7,16-18H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) |
J Med Chem 44: 540-7 (2001)
BindingDB Entry DOI: 10.7270/Q2W66MGR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50096004
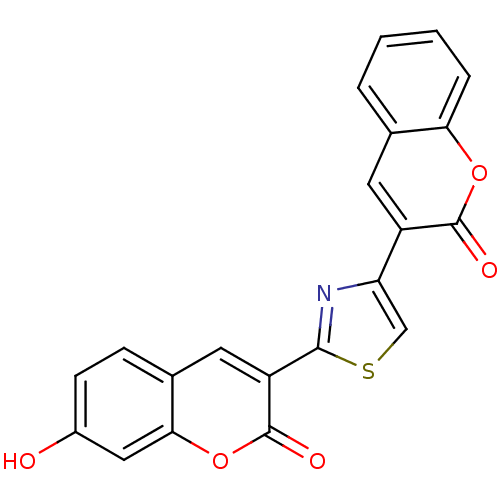
(7-Hydroxy-3-[4-(2-oxo-2H-chromen-3-yl)-thiazol-2-y...)Show SMILES Oc1ccc2cc(-c3nc(cs3)-c3cc4ccccc4oc3=O)c(=O)oc2c1 Show InChI InChI=1S/C21H11NO5S/c23-13-6-5-12-8-15(21(25)27-18(12)9-13)19-22-16(10-28-19)14-7-11-3-1-2-4-17(11)26-20(14)24/h1-10,23H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) |
J Med Chem 44: 540-7 (2001)
BindingDB Entry DOI: 10.7270/Q2W66MGR |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50096003
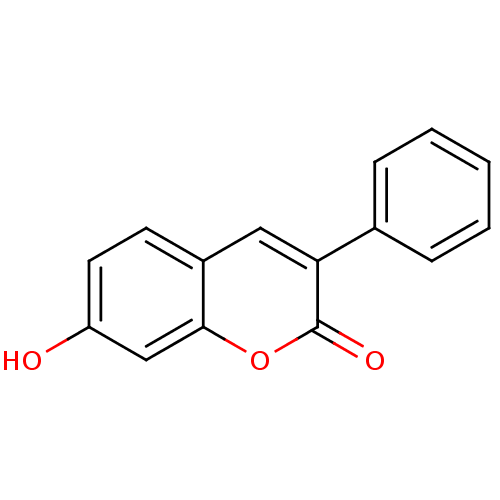
(7-Hydroxy-3-phenyl-chromen-2-one | 7-hydroxy-3-phe...)Show InChI InChI=1S/C15H10O3/c16-12-7-6-11-8-13(10-4-2-1-3-5-10)15(17)18-14(11)9-12/h1-9,16H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) |
J Med Chem 44: 540-7 (2001)
BindingDB Entry DOI: 10.7270/Q2W66MGR |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50096001
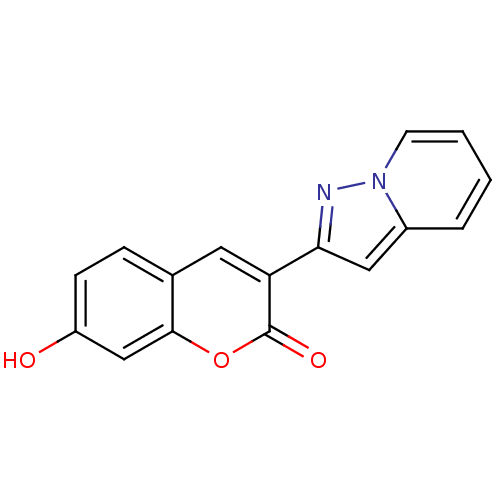
(7-Hydroxy-3-pyrazolo[1,5-a]pyridin-2-yl-chromen-2-...)Show InChI InChI=1S/C16H10N2O3/c19-12-5-4-10-7-13(16(20)21-15(10)9-12)14-8-11-3-1-2-6-18(11)17-14/h1-9,19H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) |
J Med Chem 44: 540-7 (2001)
BindingDB Entry DOI: 10.7270/Q2W66MGR |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50095993
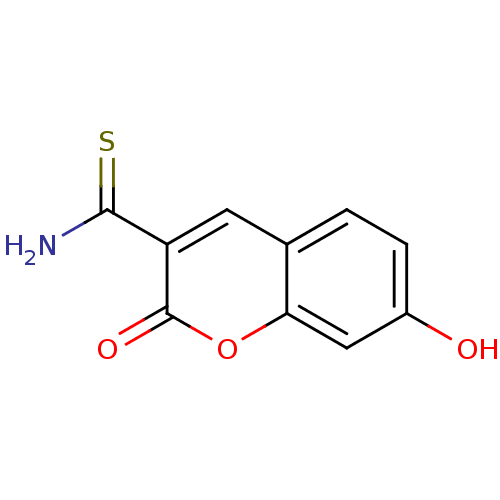
(7-Hydroxy-2-oxo-2H-chromene-3-carbothioic acid ami...)Show InChI InChI=1S/C10H7NO3S/c11-9(15)7-3-5-1-2-6(12)4-8(5)14-10(7)13/h1-4,12H,(H2,11,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) |
J Med Chem 44: 540-7 (2001)
BindingDB Entry DOI: 10.7270/Q2W66MGR |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50096002
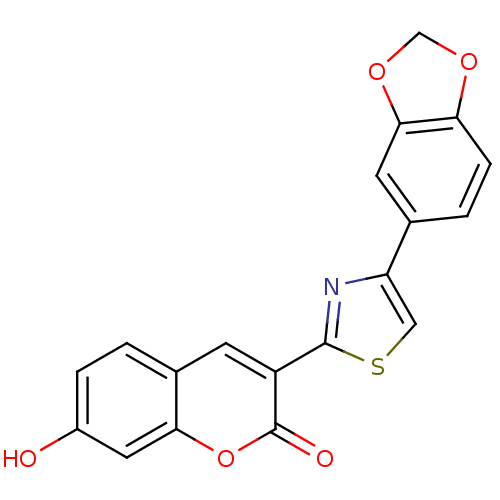
(3-(4-Benzo[1,3]dioxol-5-yl-thiazol-2-yl)-7-hydroxy...)Show SMILES Oc1ccc2cc(-c3nc(cs3)-c3ccc4OCOc4c3)c(=O)oc2c1 Show InChI InChI=1S/C19H11NO5S/c21-12-3-1-11-5-13(19(22)25-16(11)7-12)18-20-14(8-26-18)10-2-4-15-17(6-10)24-9-23-15/h1-8,21H,9H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) |
J Med Chem 44: 540-7 (2001)
BindingDB Entry DOI: 10.7270/Q2W66MGR |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50096007
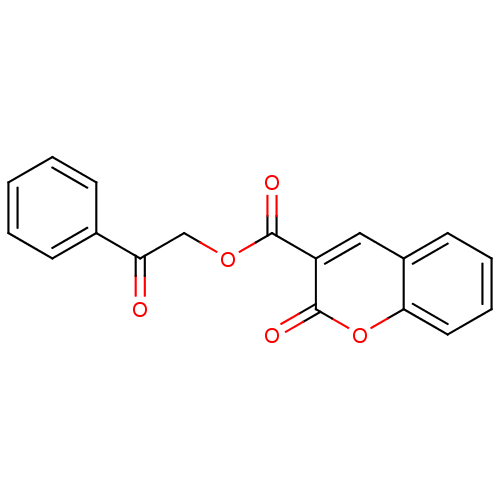
(2-Oxo-2H-chromene-3-carboxylic acid 2-oxo-2-phenyl...)Show InChI InChI=1S/C18H12O5/c19-15(12-6-2-1-3-7-12)11-22-17(20)14-10-13-8-4-5-9-16(13)23-18(14)21/h1-10H,11H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) |
J Med Chem 44: 540-7 (2001)
BindingDB Entry DOI: 10.7270/Q2W66MGR |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50096006
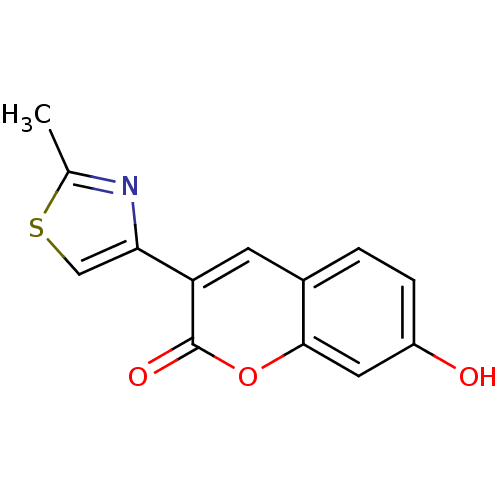
(7-Hydroxy-3-(2-methyl-thiazol-4-yl)-chromen-2-one ...)Show InChI InChI=1S/C13H9NO3S/c1-7-14-11(6-18-7)10-4-8-2-3-9(15)5-12(8)17-13(10)16/h2-6,15H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) |
J Med Chem 44: 540-7 (2001)
BindingDB Entry DOI: 10.7270/Q2W66MGR |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50096000
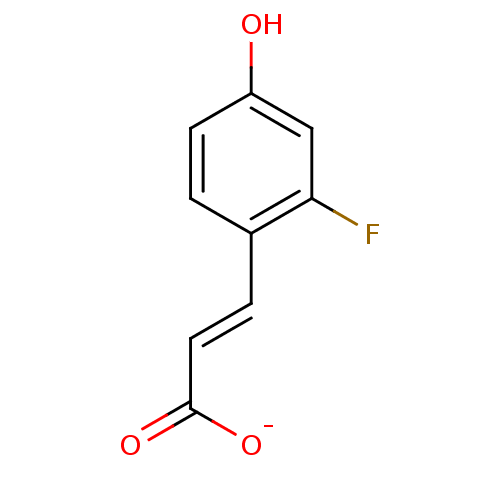
(3-(2-Fluoro-4-hydroxy-phenyl)-acrylic acid anion)Show InChI InChI=1S/C9H7FO3/c10-8-5-7(11)3-1-6(8)2-4-9(12)13/h1-5,11H,(H,12,13)/p-1/b4-2+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| PubMed
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) |
J Med Chem 44: 540-7 (2001)
BindingDB Entry DOI: 10.7270/Q2W66MGR |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50095994
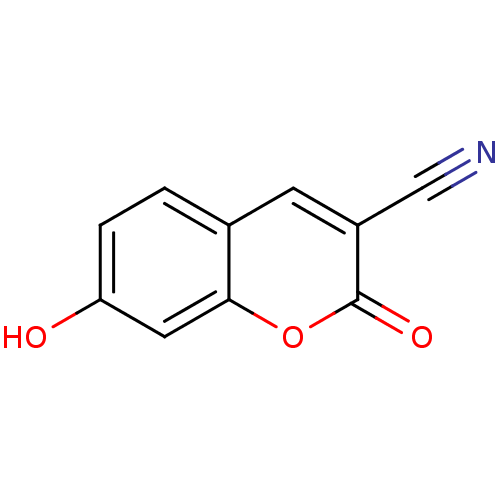
(3-Cyano-7-hydroxycoumarin (2) | 7-Hydroxy-2-oxo-2H...)Show InChI InChI=1S/C10H5NO3/c11-5-7-3-6-1-2-8(12)4-9(6)14-10(7)13/h1-4,12H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) |
J Med Chem 44: 540-7 (2001)
BindingDB Entry DOI: 10.7270/Q2W66MGR |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50096005

(7-Hydroxy-3-(4-methyl-thiazol-2-yl)-chromen-2-one ...)Show InChI InChI=1S/C13H9NO3S/c1-7-6-18-12(14-7)10-4-8-2-3-9(15)5-11(8)17-13(10)16/h2-6,15H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) |
J Med Chem 44: 540-7 (2001)
BindingDB Entry DOI: 10.7270/Q2W66MGR |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50096008
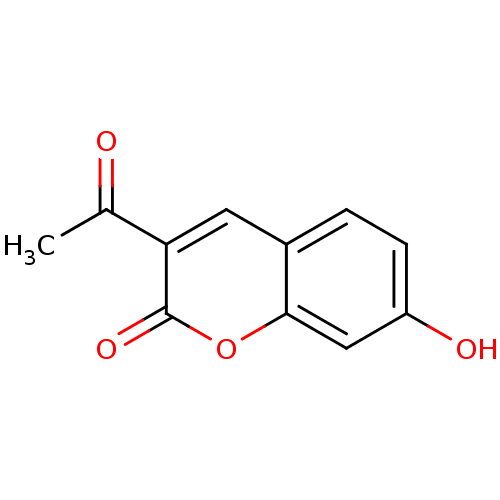
(3-Acetyl-7-hydroxy-chromen-2-one | 3-acetyl-7-hydr...)Show InChI InChI=1S/C11H8O4/c1-6(12)9-4-7-2-3-8(13)5-10(7)15-11(9)14/h2-5,13H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) |
J Med Chem 44: 540-7 (2001)
BindingDB Entry DOI: 10.7270/Q2W66MGR |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50095996
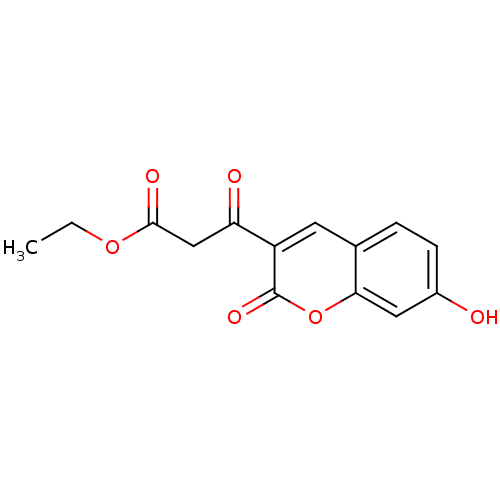
(3-(7-Hydroxy-2-oxo-2H-chromen-3-yl)-3-oxo-propioni...)Show InChI InChI=1S/C14H12O6/c1-2-19-13(17)7-11(16)10-5-8-3-4-9(15)6-12(8)20-14(10)18/h3-6,15H,2,7H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) |
J Med Chem 44: 540-7 (2001)
BindingDB Entry DOI: 10.7270/Q2W66MGR |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50096009
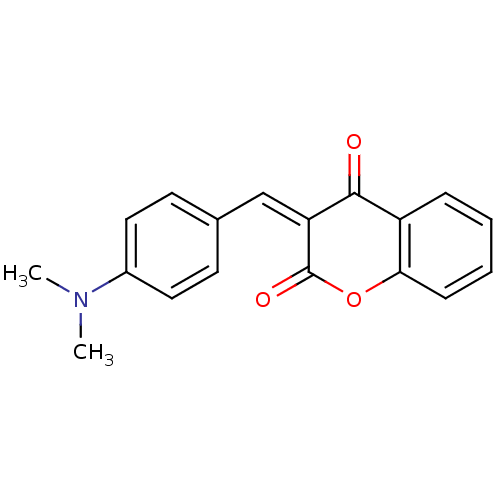
(3-(4-Dimethylamino-benzylidene)-chroman-2,4-dione ...)Show InChI InChI=1S/C18H15NO3/c1-19(2)13-9-7-12(8-10-13)11-15-17(20)14-5-3-4-6-16(14)22-18(15)21/h3-11H,1-2H3/b15-11- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) |
J Med Chem 44: 540-7 (2001)
BindingDB Entry DOI: 10.7270/Q2W66MGR |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50095995
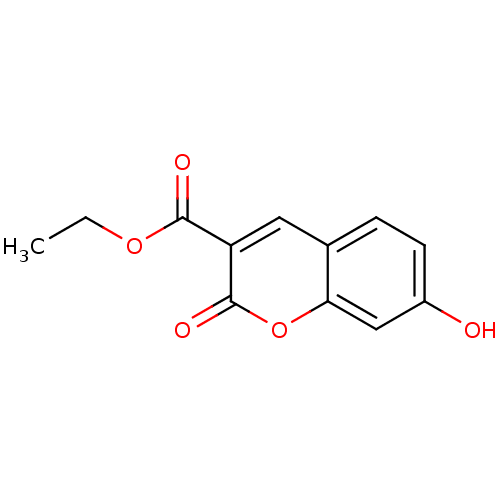
(7-HYDROXY-2-OXO-CHROMENE-3-CARBOXYLIC ACID ETHYL E...)Show InChI InChI=1S/C12H10O5/c1-2-16-11(14)9-5-7-3-4-8(13)6-10(7)17-12(9)15/h3-6,13H,2H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against tautomerase macrophage migration inhibitory factor (MIF) |
J Med Chem 44: 540-7 (2001)
BindingDB Entry DOI: 10.7270/Q2W66MGR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50246785
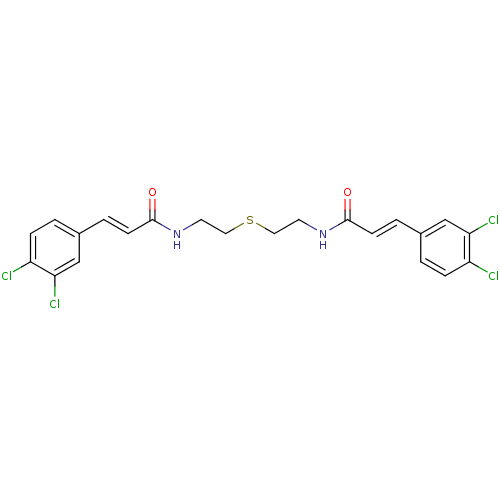
((2E,2'E)-N,N'-(Thiodiethane-2,1-diyl)bis[3-(3,4-di...)Show SMILES Clc1ccc(\C=C\C(=O)NCCSCCNC(=O)\C=C\c2ccc(Cl)c(Cl)c2)cc1Cl Show InChI InChI=1S/C22H20Cl4N2O2S/c23-17-5-1-15(13-19(17)25)3-7-21(29)27-9-11-31-12-10-28-22(30)8-4-16-2-6-18(24)20(26)14-16/h1-8,13-14H,9-12H2,(H,27,29)(H,28,30)/b7-3+,8-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase a in presence of glucose |
Bioorg Med Chem 16: 8627-34 (2008)
Article DOI: 10.1016/j.bmc.2008.08.010
BindingDB Entry DOI: 10.7270/Q2T72H81 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50361337
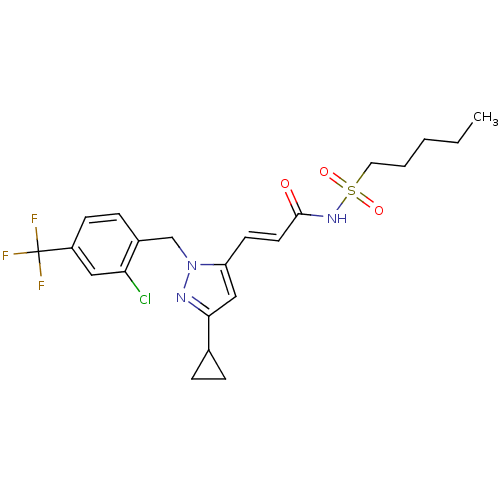
(CHEMBL1933845)Show SMILES CCCCCS(=O)(=O)NC(=O)\C=C\c1cc(nn1Cc1ccc(cc1Cl)C(F)(F)F)C1CC1 Show InChI InChI=1S/C22H25ClF3N3O3S/c1-2-3-4-11-33(31,32)28-21(30)10-9-18-13-20(15-5-6-15)27-29(18)14-16-7-8-17(12-19(16)23)22(24,25)26/h7-10,12-13,15H,2-6,11,14H2,1H3,(H,28,30)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AD-5061 from human GST-tagged PPARgamma |
Bioorg Med Chem 20: 714-33 (2012)
Article DOI: 10.1016/j.bmc.2011.12.008
BindingDB Entry DOI: 10.7270/Q2PK0GK3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50361338
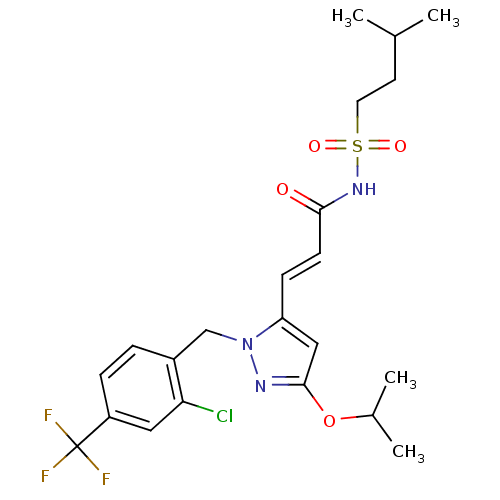
(CHEMBL1933842)Show SMILES CC(C)CCS(=O)(=O)NC(=O)\C=C\c1cc(OC(C)C)nn1Cc1ccc(cc1Cl)C(F)(F)F Show InChI InChI=1S/C22H27ClF3N3O4S/c1-14(2)9-10-34(31,32)28-20(30)8-7-18-12-21(33-15(3)4)27-29(18)13-16-5-6-17(11-19(16)23)22(24,25)26/h5-8,11-12,14-15H,9-10,13H2,1-4H3,(H,28,30)/b8-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AD-5061 from human GST-tagged PPARgamma |
Bioorg Med Chem 20: 714-33 (2012)
Article DOI: 10.1016/j.bmc.2011.12.008
BindingDB Entry DOI: 10.7270/Q2PK0GK3 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM35353

(indole-2-carboxamide derivative, 25e (R-isomer))Show SMILES O[C@@H]1c2cc(F)c(NC(=O)c3cc4cc(Cl)ccc4[nH]3)c(F)c2CCC1(F)F |r| Show InChI InChI=1S/C19H13ClF4N2O2/c20-9-1-2-13-8(5-9)6-14(25-13)18(28)26-16-12(21)7-11-10(15(16)22)3-4-19(23,24)17(11)27/h1-2,5-7,17,25,27H,3-4H2,(H,26,28)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Astellas Pharma Inc.
| Assay Description
The activity of recombinant human liver GPa in the forward direction was measured by monitoring the production of NADPH. Enzyme activity was assayed... |
Bioorg Med Chem 16: 10001-12 (2008)
Article DOI: 10.1016/j.bmc.2008.10.021
BindingDB Entry DOI: 10.7270/Q2K072MF |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50361340
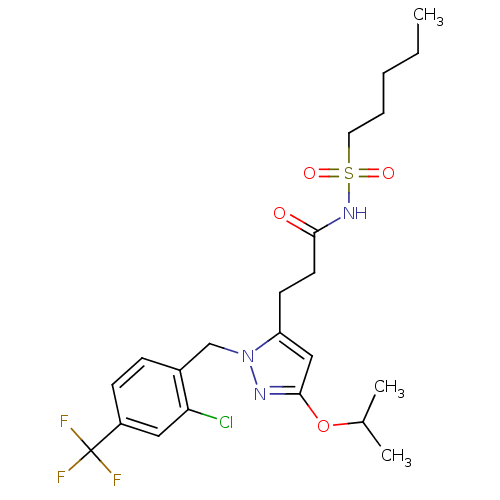
(CHEMBL1933822)Show SMILES CCCCCS(=O)(=O)NC(=O)CCc1cc(OC(C)C)nn1Cc1ccc(cc1Cl)C(F)(F)F Show InChI InChI=1S/C22H29ClF3N3O4S/c1-4-5-6-11-34(31,32)28-20(30)10-9-18-13-21(33-15(2)3)27-29(18)14-16-7-8-17(12-19(16)23)22(24,25)26/h7-8,12-13,15H,4-6,9-11,14H2,1-3H3,(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AD-5061 from human GST-tagged PPARgamma |
Bioorg Med Chem 20: 714-33 (2012)
Article DOI: 10.1016/j.bmc.2011.12.008
BindingDB Entry DOI: 10.7270/Q2PK0GK3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50361339
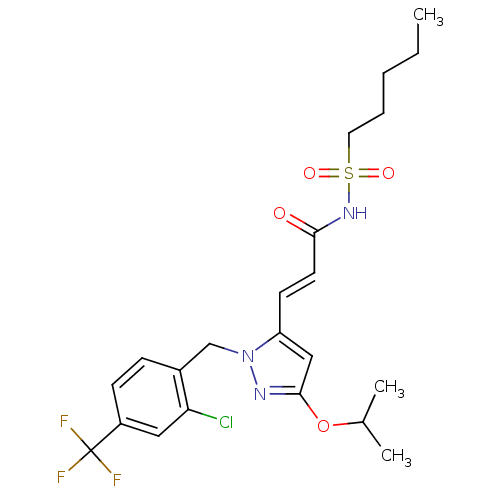
(CHEMBL1933841)Show SMILES CCCCCS(=O)(=O)NC(=O)\C=C\c1cc(OC(C)C)nn1Cc1ccc(cc1Cl)C(F)(F)F Show InChI InChI=1S/C22H27ClF3N3O4S/c1-4-5-6-11-34(31,32)28-20(30)10-9-18-13-21(33-15(2)3)27-29(18)14-16-7-8-17(12-19(16)23)22(24,25)26/h7-10,12-13,15H,4-6,11,14H2,1-3H3,(H,28,30)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AD-5061 from human GST-tagged PPARgamma |
Bioorg Med Chem 20: 714-33 (2012)
Article DOI: 10.1016/j.bmc.2011.12.008
BindingDB Entry DOI: 10.7270/Q2PK0GK3 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50246632
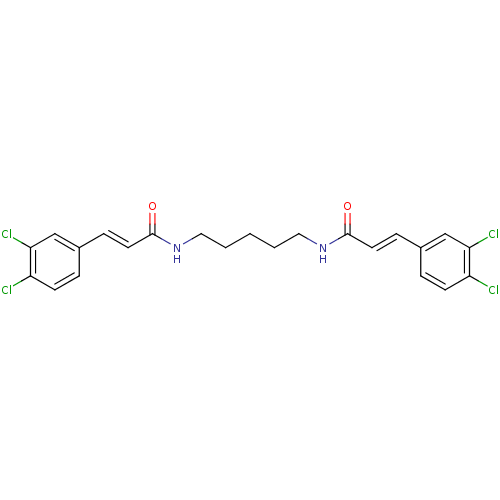
((2E,2'E)-N,N'-Pentane-1,5-diylbis[3-(3,4-dichlorop...)Show SMILES Clc1ccc(\C=C\C(=O)NCCCCCNC(=O)\C=C\c2ccc(Cl)c(Cl)c2)cc1Cl Show InChI InChI=1S/C23H22Cl4N2O2/c24-18-8-4-16(14-20(18)26)6-10-22(30)28-12-2-1-3-13-29-23(31)11-7-17-5-9-19(25)21(27)15-17/h4-11,14-15H,1-3,12-13H2,(H,28,30)(H,29,31)/b10-6+,11-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase a in presence of glucose |
Bioorg Med Chem 16: 8627-34 (2008)
Article DOI: 10.1016/j.bmc.2008.08.010
BindingDB Entry DOI: 10.7270/Q2T72H81 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50246752

((2E,2'E)-N,N'-(Oxydiethane-2,1-diyl)bis[3-(3,4-dic...)Show SMILES Clc1ccc(\C=C\C(=O)NCCOCCNC(=O)\C=C\c2ccc(Cl)c(Cl)c2)cc1Cl Show InChI InChI=1S/C22H20Cl4N2O3/c23-17-5-1-15(13-19(17)25)3-7-21(29)27-9-11-31-12-10-28-22(30)8-4-16-2-6-18(24)20(26)14-16/h1-8,13-14H,9-12H2,(H,27,29)(H,28,30)/b7-3+,8-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase a in presence of glucose |
Bioorg Med Chem 16: 8627-34 (2008)
Article DOI: 10.1016/j.bmc.2008.08.010
BindingDB Entry DOI: 10.7270/Q2T72H81 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50246786

((2E,2'E)-N,N'-(Sulfinyldiethane-2,1-diyl)bis[3-(3,...)Show SMILES Clc1ccc(\C=C\C(=O)NCCS(=O)CCNC(=O)\C=C\c2ccc(Cl)c(Cl)c2)cc1Cl Show InChI InChI=1S/C22H20Cl4N2O3S/c23-17-5-1-15(13-19(17)25)3-7-21(29)27-9-11-32(31)12-10-28-22(30)8-4-16-2-6-18(24)20(26)14-16/h1-8,13-14H,9-12H2,(H,27,29)(H,28,30)/b7-3+,8-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase a in presence of glucose |
Bioorg Med Chem 16: 8627-34 (2008)
Article DOI: 10.1016/j.bmc.2008.08.010
BindingDB Entry DOI: 10.7270/Q2T72H81 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM35351
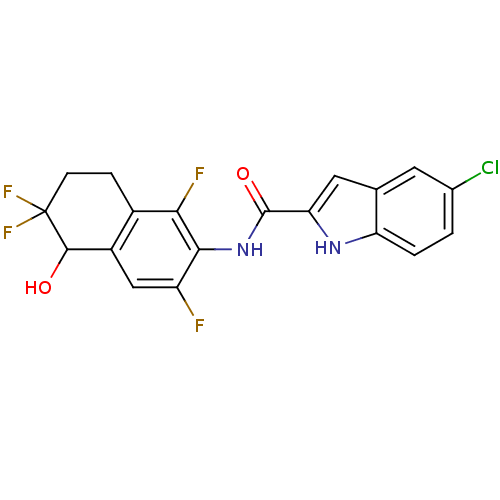
(indole-2-carboxamide derivative, 25e)Show SMILES OC1c2cc(F)c(NC(=O)c3cc4cc(Cl)ccc4[nH]3)c(F)c2CCC1(F)F Show InChI InChI=1S/C19H13ClF4N2O2/c20-9-1-2-13-8(5-9)6-14(25-13)18(28)26-16-12(21)7-11-10(15(16)22)3-4-19(23,24)17(11)27/h1-2,5-7,17,25,27H,3-4H2,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Astellas Pharma Inc.
| Assay Description
The activity of recombinant human liver GPa in the forward direction was measured by monitoring the production of NADPH. Enzyme activity was assayed... |
Bioorg Med Chem 16: 10001-12 (2008)
Article DOI: 10.1016/j.bmc.2008.10.021
BindingDB Entry DOI: 10.7270/Q2K072MF |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50246788

((2E,2'E)-N,N'-Butane-1,4-diylbis[3-(3,4-dichloroph...)Show SMILES Clc1ccc(\C=C\C(=O)NCCCCNC(=O)\C=C\c2ccc(Cl)c(Cl)c2)cc1Cl Show InChI InChI=1S/C22H20Cl4N2O2/c23-17-7-3-15(13-19(17)25)5-9-21(29)27-11-1-2-12-28-22(30)10-6-16-4-8-18(24)20(26)14-16/h3-10,13-14H,1-2,11-12H2,(H,27,29)(H,28,30)/b9-5+,10-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase a in presence of glucose |
Bioorg Med Chem 16: 8627-34 (2008)
Article DOI: 10.1016/j.bmc.2008.08.010
BindingDB Entry DOI: 10.7270/Q2T72H81 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50106720
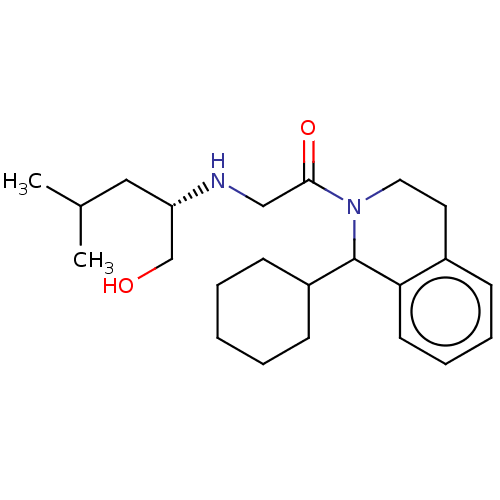
(CHEMBL3600880)Show SMILES CC(C)C[C@@H](CO)NCC(=O)N1CCc2ccccc2C1C1CCCCC1 |r| Show InChI InChI=1S/C23H36N2O2/c1-17(2)14-20(16-26)24-15-22(27)25-13-12-18-8-6-7-11-21(18)23(25)19-9-4-3-5-10-19/h6-8,11,17,19-20,23-24,26H,3-5,9-10,12-16H2,1-2H3/t20-,23?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) by fluorescence-based assay |
Bioorg Med Chem 23: 4638-48 (2015)
Article DOI: 10.1016/j.bmc.2015.05.053
BindingDB Entry DOI: 10.7270/Q27D2WXJ |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM35350
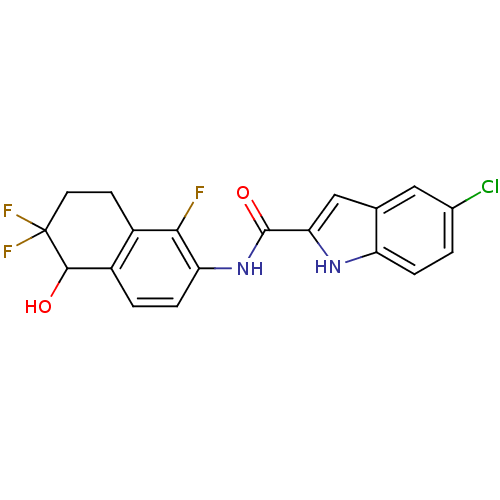
(indole-2-carboxamide derivative, 25d)Show SMILES OC1c2ccc(NC(=O)c3cc4cc(Cl)ccc4[nH]3)c(F)c2CCC1(F)F Show InChI InChI=1S/C19H14ClF3N2O2/c20-10-1-3-13-9(7-10)8-15(24-13)18(27)25-14-4-2-12-11(16(14)21)5-6-19(22,23)17(12)26/h1-4,7-8,17,24,26H,5-6H2,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Astellas Pharma Inc.
| Assay Description
The activity of recombinant human liver GPa in the forward direction was measured by monitoring the production of NADPH. Enzyme activity was assayed... |
Bioorg Med Chem 16: 10001-12 (2008)
Article DOI: 10.1016/j.bmc.2008.10.021
BindingDB Entry DOI: 10.7270/Q2K072MF |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM35349
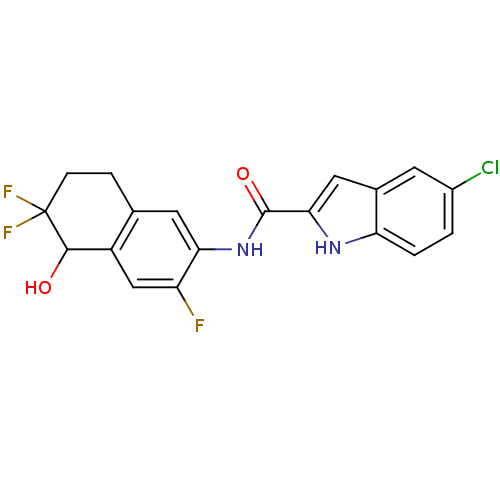
(indole-2-carboxamide derivative, 25c)Show SMILES OC1c2cc(F)c(NC(=O)c3cc4cc(Cl)ccc4[nH]3)cc2CCC1(F)F Show InChI InChI=1S/C19H14ClF3N2O2/c20-11-1-2-14-10(5-11)7-16(24-14)18(27)25-15-6-9-3-4-19(22,23)17(26)12(9)8-13(15)21/h1-2,5-8,17,24,26H,3-4H2,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Astellas Pharma Inc.
| Assay Description
The activity of recombinant human liver GPa in the forward direction was measured by monitoring the production of NADPH. Enzyme activity was assayed... |
Bioorg Med Chem 16: 10001-12 (2008)
Article DOI: 10.1016/j.bmc.2008.10.021
BindingDB Entry DOI: 10.7270/Q2K072MF |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM35345
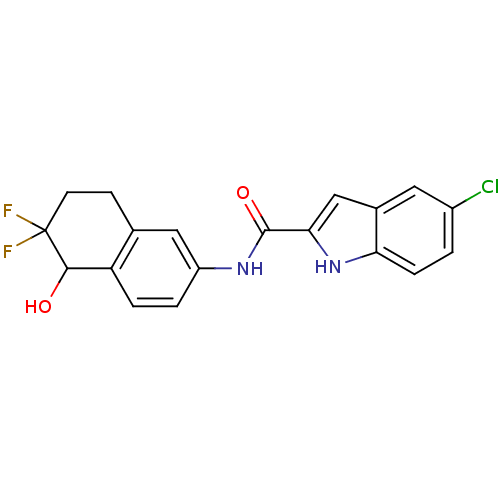
(indole-2-carboxamide derivative, 15)Show SMILES OC1c2ccc(NC(=O)c3cc4cc(Cl)ccc4[nH]3)cc2CCC1(F)F Show InChI InChI=1S/C19H15ClF2N2O2/c20-12-1-4-15-11(7-12)9-16(24-15)18(26)23-13-2-3-14-10(8-13)5-6-19(21,22)17(14)25/h1-4,7-9,17,24-25H,5-6H2,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Astellas Pharma Inc.
| Assay Description
The activity of recombinant human liver GPa in the forward direction was measured by monitoring the production of NADPH. Enzyme activity was assayed... |
Bioorg Med Chem 16: 10001-12 (2008)
Article DOI: 10.1016/j.bmc.2008.10.021
BindingDB Entry DOI: 10.7270/Q2K072MF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50106719
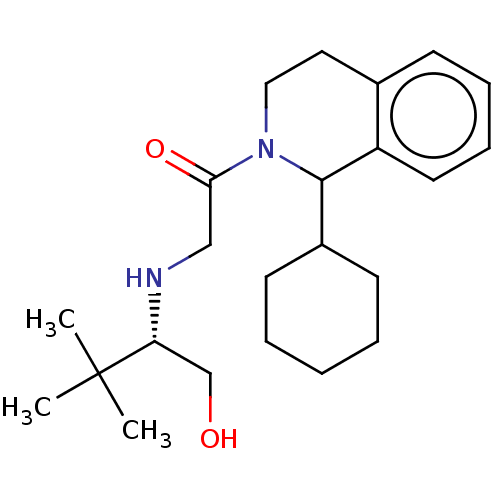
(CHEMBL3600879)Show SMILES CC(C)(C)[C@@H](CO)NCC(=O)N1CCc2ccccc2C1C1CCCCC1 |r| Show InChI InChI=1S/C23H36N2O2/c1-23(2,3)20(16-26)24-15-21(27)25-14-13-17-9-7-8-12-19(17)22(25)18-10-5-4-6-11-18/h7-9,12,18,20,22,24,26H,4-6,10-11,13-16H2,1-3H3/t20-,22?/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) by fluorescence-based assay |
Bioorg Med Chem 23: 4638-48 (2015)
Article DOI: 10.1016/j.bmc.2015.05.053
BindingDB Entry DOI: 10.7270/Q27D2WXJ |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM35352
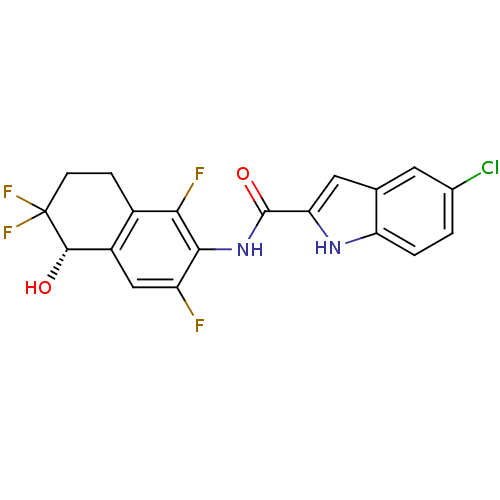
(indole-2-carboxamide derivative, 25e (S-isomer))Show SMILES O[C@H]1c2cc(F)c(NC(=O)c3cc4cc(Cl)ccc4[nH]3)c(F)c2CCC1(F)F |r| Show InChI InChI=1S/C19H13ClF4N2O2/c20-9-1-2-13-8(5-9)6-14(25-13)18(28)26-16-12(21)7-11-10(15(16)22)3-4-19(23,24)17(11)27/h1-2,5-7,17,25,27H,3-4H2,(H,26,28)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Astellas Pharma Inc.
| Assay Description
The activity of recombinant human liver GPa in the forward direction was measured by monitoring the production of NADPH. Enzyme activity was assayed... |
Bioorg Med Chem 16: 10001-12 (2008)
Article DOI: 10.1016/j.bmc.2008.10.021
BindingDB Entry DOI: 10.7270/Q2K072MF |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM35636

(5-chloroindolecarboxamide, 13a)Show InChI InChI=1S/C16H14ClN3O3/c17-11-2-3-12-10(5-11)6-13(19-12)16(23)20-15-4-1-9(7-18-15)14(22)8-21/h1-7,14,19,21-22H,8H2,(H,18,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Astellas Pharma Inc.
| Assay Description
The activity of recombinant human liver GPa in the forward direction was measured by monitoring the production of NADPH. Enzyme activity was assayed... |
Bioorg Med Chem 16: 5452-64 (2008)
Article DOI: 10.1016/j.bmc.2008.04.010
BindingDB Entry DOI: 10.7270/Q2J101H2 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50246632

((2E,2'E)-N,N'-Pentane-1,5-diylbis[3-(3,4-dichlorop...)Show SMILES Clc1ccc(\C=C\C(=O)NCCCCCNC(=O)\C=C\c2ccc(Cl)c(Cl)c2)cc1Cl Show InChI InChI=1S/C23H22Cl4N2O2/c24-18-8-4-16(14-20(18)26)6-10-22(30)28-12-2-1-3-13-29-23(31)11-7-17-5-9-19(25)21(27)15-17/h4-11,14-15H,1-3,12-13H2,(H,28,30)(H,29,31)/b10-6+,11-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase a in absence of glucose |
Bioorg Med Chem 16: 8627-34 (2008)
Article DOI: 10.1016/j.bmc.2008.08.010
BindingDB Entry DOI: 10.7270/Q2T72H81 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50106714

(CHEMBL3600876)Show SMILES CC[C@H](O)CNCC(=O)N1CCc2ccccc2C1C1CCCCC1 |r| Show InChI InChI=1S/C21H32N2O2/c1-2-18(24)14-22-15-20(25)23-13-12-16-8-6-7-11-19(16)21(23)17-9-4-3-5-10-17/h6-8,11,17-18,21-22,24H,2-5,9-10,12-15H2,1H3/t18-,21?/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <300 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) by fluorescence-based assay |
Bioorg Med Chem 23: 4638-48 (2015)
Article DOI: 10.1016/j.bmc.2015.05.053
BindingDB Entry DOI: 10.7270/Q27D2WXJ |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50246786

((2E,2'E)-N,N'-(Sulfinyldiethane-2,1-diyl)bis[3-(3,...)Show SMILES Clc1ccc(\C=C\C(=O)NCCS(=O)CCNC(=O)\C=C\c2ccc(Cl)c(Cl)c2)cc1Cl Show InChI InChI=1S/C22H20Cl4N2O3S/c23-17-5-1-15(13-19(17)25)3-7-21(29)27-9-11-32(31)12-10-28-22(30)8-4-16-2-6-18(24)20(26)14-16/h1-8,13-14H,9-12H2,(H,27,29)(H,28,30)/b7-3+,8-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase a in absence of glucose |
Bioorg Med Chem 16: 8627-34 (2008)
Article DOI: 10.1016/j.bmc.2008.08.010
BindingDB Entry DOI: 10.7270/Q2T72H81 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM35339

(indole-2-carboxamide derivative, 5b)Show SMILES OC1CCCc2cc(NC(=O)c3cc4cc(Cl)ccc4[nH]3)ccc12 Show InChI InChI=1S/C19H17ClN2O2/c20-13-4-7-16-12(8-13)10-17(22-16)19(24)21-14-5-6-15-11(9-14)2-1-3-18(15)23/h4-10,18,22-23H,1-3H2,(H,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Astellas Pharma Inc.
| Assay Description
The activity of recombinant human liver GPa in the forward direction was measured by monitoring the production of NADPH. Enzyme activity was assayed... |
Bioorg Med Chem 16: 10001-12 (2008)
Article DOI: 10.1016/j.bmc.2008.10.021
BindingDB Entry DOI: 10.7270/Q2K072MF |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50382055
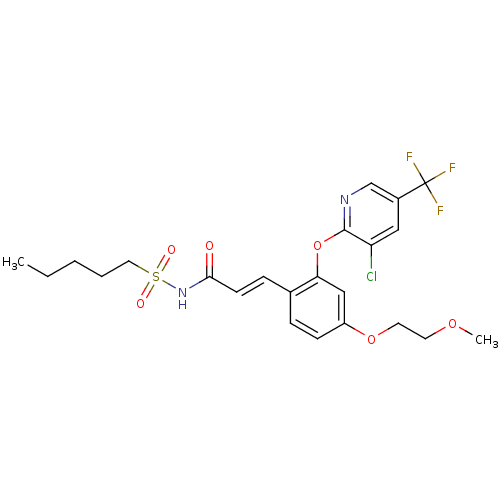
(CHEMBL2024606)Show SMILES CCCCCS(=O)(=O)NC(=O)\C=C\c1ccc(OCCOC)cc1Oc1ncc(cc1Cl)C(F)(F)F Show InChI InChI=1S/C23H26ClF3N2O6S/c1-3-4-5-12-36(31,32)29-21(30)9-7-16-6-8-18(34-11-10-33-2)14-20(16)35-22-19(24)13-17(15-28-22)23(25,26)27/h6-9,13-15H,3-5,10-12H2,1-2H3,(H,29,30)/b9-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AD-5061 from human GST-tagged PPARgamma |
Bioorg Med Chem 20: 3332-58 (2012)
Article DOI: 10.1016/j.bmc.2012.03.036
BindingDB Entry DOI: 10.7270/Q2NK3G26 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM35633

(5-chloroindolecarboxamide, 9h)Show SMILES OCC(O)c1ccc(NC(=O)c2cc3cc(Cl)ccc3[nH]2)cc1C(F)(F)F Show InChI InChI=1S/C18H14ClF3N2O3/c19-10-1-4-14-9(5-10)6-15(24-14)17(27)23-11-2-3-12(16(26)8-25)13(7-11)18(20,21)22/h1-7,16,24-26H,8H2,(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Astellas Pharma Inc.
| Assay Description
The activity of recombinant human liver GPa in the forward direction was measured by monitoring the production of NADPH. Enzyme activity was assayed... |
Bioorg Med Chem 16: 5452-64 (2008)
Article DOI: 10.1016/j.bmc.2008.04.010
BindingDB Entry DOI: 10.7270/Q2J101H2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50095969

(CHEMBL3593436)Show SMILES OC1(CNCC(=O)N2CCc3ccccc3[C@@H]2c2ccccc2)CCCCC1 |r| Show InChI InChI=1S/C16H17N5O5/c17-13-10-14(20-16(19-13)25-8-4-2-1-3-5-8)21(7-18-10)15-12(24)11(23)9(6-22)26-15/h1-5,7,9,11-12,15,22-24H,6H2,(H2,17,19,20)/t9-,11+,12+,15?/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <390 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) by fluorescence-based assay |
Bioorg Med Chem 23: 4638-48 (2015)
Article DOI: 10.1016/j.bmc.2015.05.053
BindingDB Entry DOI: 10.7270/Q27D2WXJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50106722

(CHEMBL3600882)Show SMILES C[C@@H](O)CNCC(=O)N1CCc2c(F)cccc2C1C1CCCCC1 |r| Show InChI InChI=1S/C20H29FN2O2/c1-14(24)12-22-13-19(25)23-11-10-16-17(8-5-9-18(16)21)20(23)15-6-3-2-4-7-15/h5,8-9,14-15,20,22,24H,2-4,6-7,10-13H2,1H3/t14-,20?/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <390 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) by fluorescence-based assay |
Bioorg Med Chem 23: 4638-48 (2015)
Article DOI: 10.1016/j.bmc.2015.05.053
BindingDB Entry DOI: 10.7270/Q27D2WXJ |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50246788

((2E,2'E)-N,N'-Butane-1,4-diylbis[3-(3,4-dichloroph...)Show SMILES Clc1ccc(\C=C\C(=O)NCCCCNC(=O)\C=C\c2ccc(Cl)c(Cl)c2)cc1Cl Show InChI InChI=1S/C22H20Cl4N2O2/c23-17-7-3-15(13-19(17)25)5-9-21(29)27-11-1-2-12-28-22(30)10-6-16-4-8-18(24)20(26)14-16/h3-10,13-14H,1-2,11-12H2,(H,27,29)(H,28,30)/b9-5+,10-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase a in absence of glucose |
Bioorg Med Chem 16: 8627-34 (2008)
Article DOI: 10.1016/j.bmc.2008.08.010
BindingDB Entry DOI: 10.7270/Q2T72H81 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM35628

(5-chloroindolecarboxamide, 9c)Show SMILES OCC(O)c1ccc(NC(=O)c2cc3cc(Cl)ccc3[nH]2)c(Cl)c1 Show InChI InChI=1S/C17H14Cl2N2O3/c18-11-2-4-13-10(5-11)7-15(20-13)17(24)21-14-3-1-9(6-12(14)19)16(23)8-22/h1-7,16,20,22-23H,8H2,(H,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Astellas Pharma Inc.
| Assay Description
The activity of recombinant human liver GPa in the forward direction was measured by monitoring the production of NADPH. Enzyme activity was assayed... |
Bioorg Med Chem 16: 5452-64 (2008)
Article DOI: 10.1016/j.bmc.2008.04.010
BindingDB Entry DOI: 10.7270/Q2J101H2 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50246752

((2E,2'E)-N,N'-(Oxydiethane-2,1-diyl)bis[3-(3,4-dic...)Show SMILES Clc1ccc(\C=C\C(=O)NCCOCCNC(=O)\C=C\c2ccc(Cl)c(Cl)c2)cc1Cl Show InChI InChI=1S/C22H20Cl4N2O3/c23-17-5-1-15(13-19(17)25)3-7-21(29)27-9-11-31-12-10-28-22(30)8-4-16-2-6-18(24)20(26)14-16/h1-8,13-14H,9-12H2,(H,27,29)(H,28,30)/b7-3+,8-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase a in absence of glucose |
Bioorg Med Chem 16: 8627-34 (2008)
Article DOI: 10.1016/j.bmc.2008.08.010
BindingDB Entry DOI: 10.7270/Q2T72H81 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM35638

(5-chloroindolecarboxamide, 13c)Show InChI InChI=1S/C15H13ClN4O3/c16-9-1-2-10-8(3-9)4-11(19-10)15(23)20-14-6-17-12(5-18-14)13(22)7-21/h1-6,13,19,21-22H,7H2,(H,18,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Astellas Pharma Inc.
| Assay Description
The activity of recombinant human liver GPa in the forward direction was measured by monitoring the production of NADPH. Enzyme activity was assayed... |
Bioorg Med Chem 16: 5452-64 (2008)
Article DOI: 10.1016/j.bmc.2008.04.010
BindingDB Entry DOI: 10.7270/Q2J101H2 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM35338
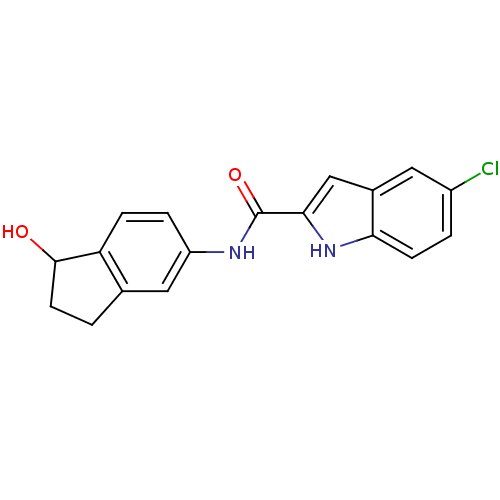
(indole-2-carboxamide derivative, 3)Show InChI InChI=1S/C18H15ClN2O2/c19-12-2-5-15-11(7-12)9-16(21-15)18(23)20-13-3-4-14-10(8-13)1-6-17(14)22/h2-5,7-9,17,21-22H,1,6H2,(H,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Astellas Pharma Inc.
| Assay Description
The activity of recombinant human liver GPa in the forward direction was measured by monitoring the production of NADPH. Enzyme activity was assayed... |
Bioorg Med Chem 16: 10001-12 (2008)
Article DOI: 10.1016/j.bmc.2008.10.021
BindingDB Entry DOI: 10.7270/Q2K072MF |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50246785

((2E,2'E)-N,N'-(Thiodiethane-2,1-diyl)bis[3-(3,4-di...)Show SMILES Clc1ccc(\C=C\C(=O)NCCSCCNC(=O)\C=C\c2ccc(Cl)c(Cl)c2)cc1Cl Show InChI InChI=1S/C22H20Cl4N2O2S/c23-17-5-1-15(13-19(17)25)3-7-21(29)27-9-11-31-12-10-28-22(30)8-4-16-2-6-18(24)20(26)14-16/h1-8,13-14H,9-12H2,(H,27,29)(H,28,30)/b7-3+,8-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase a in absence of glucose |
Bioorg Med Chem 16: 8627-34 (2008)
Article DOI: 10.1016/j.bmc.2008.08.010
BindingDB Entry DOI: 10.7270/Q2T72H81 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM35348

(indole-2-carboxamide derivative, 25b)Show SMILES COc1c(NC(=O)c2cc3cc(Cl)ccc3[nH]2)ccc2C(O)C(F)(F)CCc12 Show InChI InChI=1S/C20H17ClF2N2O3/c1-28-17-12-6-7-20(22,23)18(26)13(12)3-5-15(17)25-19(27)16-9-10-8-11(21)2-4-14(10)24-16/h2-5,8-9,18,24,26H,6-7H2,1H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | 6.8 | 22 |
Astellas Pharma Inc.
| Assay Description
The activity of recombinant human liver GPa in the forward direction was measured by monitoring the production of NADPH. Enzyme activity was assayed... |
Bioorg Med Chem 16: 10001-12 (2008)
Article DOI: 10.1016/j.bmc.2008.10.021
BindingDB Entry DOI: 10.7270/Q2K072MF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50037212

(CHEMBL3356251)Show SMILES OC1(CNCC(=O)N2CCc3ccccc3[C@@H]2C2CCCCC2)CCCCC1 |r| Show InChI InChI=1S/C24H36N2O2/c27-22(17-25-18-24(28)14-7-2-8-15-24)26-16-13-19-9-5-6-12-21(19)23(26)20-10-3-1-4-11-20/h5-6,9,12,20,23,25,28H,1-4,7-8,10-11,13-18H2/t23-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) by fluorescence-based assay |
Bioorg Med Chem 23: 4638-48 (2015)
Article DOI: 10.1016/j.bmc.2015.05.053
BindingDB Entry DOI: 10.7270/Q27D2WXJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50037209

(CHEMBL3356247)Show InChI InChI=1S/C24H36N2O2/c27-22(17-25-18-24(28)14-7-2-8-15-24)26-16-13-19-9-5-6-12-21(19)23(26)20-10-3-1-4-11-20/h5-6,9,12,20,23,25,28H,1-4,7-8,10-11,13-18H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) by fluorescence-based assay |
Bioorg Med Chem 23: 4638-48 (2015)
Article DOI: 10.1016/j.bmc.2015.05.053
BindingDB Entry DOI: 10.7270/Q27D2WXJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data