| Citation |  Tucker, TJ; Saggar, S; Sisko, JT; Tynebor, RM; Williams, TM; Felock, PJ; Flynn, JA; Lai, MT; Liang, Y; McGaughey, G; Liu, M; Miller, M; Moyer, G; Munshi, V; Perlow-Poehnelt, R; Prasad, S; Sanchez, R; Torrent, M; Vacca, JP; Wan, BL; Yan, Y The design and synthesis of diaryl ether second generation HIV-1 non-nucleoside reverse transcriptase inhibitors (NNRTIs) with enhanced potency versus key clinical mutations. Bioorg Med Chem Lett18:2959-66 (2008) [PubMed] Article Tucker, TJ; Saggar, S; Sisko, JT; Tynebor, RM; Williams, TM; Felock, PJ; Flynn, JA; Lai, MT; Liang, Y; McGaughey, G; Liu, M; Miller, M; Moyer, G; Munshi, V; Perlow-Poehnelt, R; Prasad, S; Sanchez, R; Torrent, M; Vacca, JP; Wan, BL; Yan, Y The design and synthesis of diaryl ether second generation HIV-1 non-nucleoside reverse transcriptase inhibitors (NNRTIs) with enhanced potency versus key clinical mutations. Bioorg Med Chem Lett18:2959-66 (2008) [PubMed] Article |
|---|
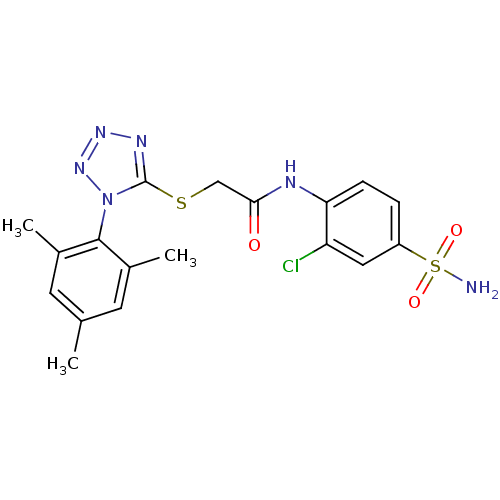
| SMILES | Cc1cc(C)c(c(C)c1)-n1nnnc1SCC(=O)Nc1ccc(cc1Cl)S(N)(=O)=O |(15.06,-12.69,;15.39,-11.18,;14.25,-10.14,;14.59,-8.64,;13.45,-7.6,;16.05,-8.17,;17.19,-9.2,;18.66,-8.73,;16.86,-10.72,;16.38,-6.66,;15.35,-5.51,;16.12,-4.18,;17.63,-4.5,;17.78,-6.04,;19.12,-6.81,;20.45,-6.04,;21.78,-6.81,;21.78,-8.35,;23.12,-6.05,;24.45,-6.82,;24.44,-8.36,;25.77,-9.13,;27.11,-8.36,;27.11,-6.82,;25.78,-6.05,;25.77,-4.51,;28.44,-9.13,;29.77,-9.9,;27.67,-10.47,;29.22,-7.8,)| |
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Tucker, TJ; Saggar, S; Sisko, JT; Tynebor, RM; Williams, TM; Felock, PJ; Flynn, JA; Lai, MT; Liang, Y; McGaughey, G; Liu, M; Miller, M; Moyer, G; Munshi, V; Perlow-Poehnelt, R; Prasad, S; Sanchez, R; Torrent, M; Vacca, JP; Wan, BL; Yan, Y The design and synthesis of diaryl ether second generation HIV-1 non-nucleoside reverse transcriptase inhibitors (NNRTIs) with enhanced potency versus key clinical mutations. Bioorg Med Chem Lett18:2959-66 (2008) [PubMed] Article
Tucker, TJ; Saggar, S; Sisko, JT; Tynebor, RM; Williams, TM; Felock, PJ; Flynn, JA; Lai, MT; Liang, Y; McGaughey, G; Liu, M; Miller, M; Moyer, G; Munshi, V; Perlow-Poehnelt, R; Prasad, S; Sanchez, R; Torrent, M; Vacca, JP; Wan, BL; Yan, Y The design and synthesis of diaryl ether second generation HIV-1 non-nucleoside reverse transcriptase inhibitors (NNRTIs) with enhanced potency versus key clinical mutations. Bioorg Med Chem Lett18:2959-66 (2008) [PubMed] Article