| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Reverse transcriptase/RNaseH |
|---|
| Ligand | BDBM50064009 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_196382 (CHEMBL802554) |
|---|
| IC50 | 22±n/a nM |
|---|
| Citation |  Wishka, DG; Graber, DR; Kopta, LA; Olmsted, RA; Friis, JM; Hosley, JD; Adams, WJ; Seest, EP; Castle, TM; Dolak, LA; Keiser, BJ; Yagi, Y; Jeganathan, A; Schlachter, ST; Murphy, MJ; Cleek, GJ; Nugent, RA; Poppe, SM; Swaney, SM; Han, F; Watt, W; White, WL; Poel, TJ; Thomas, RC; Morris, J (-)-6-Chloro-2-[(1-furo[2, 3-c]pyridin-5-ylethyl)thio]-4-pyrimidinamine, PNU-142721, a new broad spectrum HIV-1 non-nucleoside reverse transcriptase inhibitor. J Med Chem41:1357-60 (1998) [PubMed] Article Wishka, DG; Graber, DR; Kopta, LA; Olmsted, RA; Friis, JM; Hosley, JD; Adams, WJ; Seest, EP; Castle, TM; Dolak, LA; Keiser, BJ; Yagi, Y; Jeganathan, A; Schlachter, ST; Murphy, MJ; Cleek, GJ; Nugent, RA; Poppe, SM; Swaney, SM; Han, F; Watt, W; White, WL; Poel, TJ; Thomas, RC; Morris, J (-)-6-Chloro-2-[(1-furo[2, 3-c]pyridin-5-ylethyl)thio]-4-pyrimidinamine, PNU-142721, a new broad spectrum HIV-1 non-nucleoside reverse transcriptase inhibitor. J Med Chem41:1357-60 (1998) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Reverse transcriptase/RNaseH |
|---|
| Name: | Reverse transcriptase/RNaseH |
|---|
| Synonyms: | HIV-1 Reverse Transcriptase RNase H | Human immunodeficiency virus type 1 reverse transcriptase | Reverse transcriptase/RNaseH |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 65229.15 |
|---|
| Organism: | Human immunodeficiency virus 1 |
|---|
| Description: | ChEMBL_1473730 |
|---|
| Residue: | 566 |
|---|
| Sequence: | PISPIETVPVKLKPGMDGPKVKQWPLTEEKIKALVEICTEMEKEGKISKIGPENPYNTPV
FAIKKKDSTKWRKLVDFRELNKRTQDFWEVQLGIPHPAGLKKRKSVTVLDVGDAYFSVPL
DEDFRKYTAFTIPSINNETPGIRYQYNVLPQGWKGSPAIFQSSMTKILEPFRKQNPDIVI
YQYMDDLYVGSDLEIGQHRTKIEELRQHLLRWGLTTPDKKHQKEPPFLWMGYELHPDKWT
VQPIVLPEKDSWTVNDIQKLVGKLNWASQIYPGIRVRQLCKLLRGTKALTEVIPLTEEAE
LELAENREILKEPVHGVYYDPSKDLIAEIQKQGQGQWTYQIYQEPFKNLRTGKYARMRGA
HTNDVKQLTEAVQKITTESIVIWGKTPKFKLPIQKETWETWWTEYWQATWIPEWEFVNTP
PLVKLWYQLEKEPIVGAETFYVDGAANRETKLGKAGYVTNRGRQKVVTLTDTTNQKTELQ
AIYLALQDSGLEVNIVTDSQYALGIIQAQPDQSESELVNQIIEQLIKKEKVYLAWVPAHK
GIGGNEQVDKLVSAGIRKVLFLDGID
|
|
|
|---|
| BDBM50064009 |
|---|
| n/a |
|---|
| Name | BDBM50064009 |
|---|
| Synonyms: | 6-Chloro-2-(1-furo[2,3-c]pyridin-5-yl-ethylsulfanyl)-pyrimidin-4-ylamine | CHEMBL280752 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C13H11ClN4OS |
|---|
| Mol. Mass. | 306.771 |
|---|
| SMILES | CC(Sc1nc(N)cc(Cl)n1)c1cc2ccoc2cn1 |
|---|
| Structure | 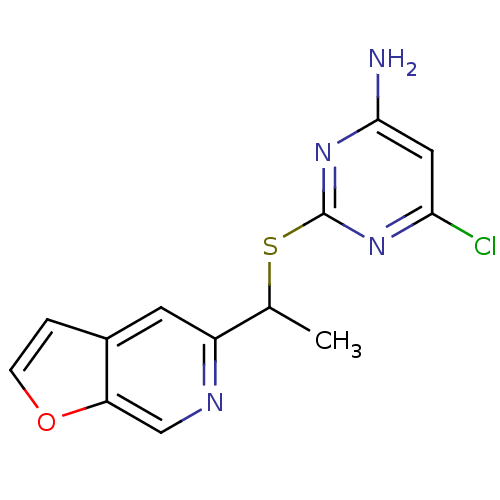
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Wishka, DG; Graber, DR; Kopta, LA; Olmsted, RA; Friis, JM; Hosley, JD; Adams, WJ; Seest, EP; Castle, TM; Dolak, LA; Keiser, BJ; Yagi, Y; Jeganathan, A; Schlachter, ST; Murphy, MJ; Cleek, GJ; Nugent, RA; Poppe, SM; Swaney, SM; Han, F; Watt, W; White, WL; Poel, TJ; Thomas, RC; Morris, J (-)-6-Chloro-2-[(1-furo[2, 3-c]pyridin-5-ylethyl)thio]-4-pyrimidinamine, PNU-142721, a new broad spectrum HIV-1 non-nucleoside reverse transcriptase inhibitor. J Med Chem41:1357-60 (1998) [PubMed] Article
Wishka, DG; Graber, DR; Kopta, LA; Olmsted, RA; Friis, JM; Hosley, JD; Adams, WJ; Seest, EP; Castle, TM; Dolak, LA; Keiser, BJ; Yagi, Y; Jeganathan, A; Schlachter, ST; Murphy, MJ; Cleek, GJ; Nugent, RA; Poppe, SM; Swaney, SM; Han, F; Watt, W; White, WL; Poel, TJ; Thomas, RC; Morris, J (-)-6-Chloro-2-[(1-furo[2, 3-c]pyridin-5-ylethyl)thio]-4-pyrimidinamine, PNU-142721, a new broad spectrum HIV-1 non-nucleoside reverse transcriptase inhibitor. J Med Chem41:1357-60 (1998) [PubMed] Article