

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM554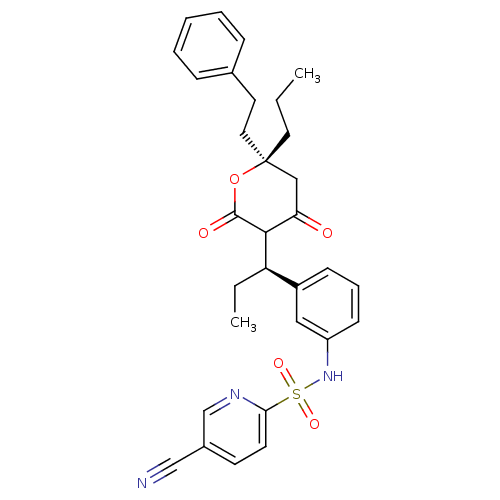 (5-cyano-N-{3-[(1R)-1-[(6R)-4-hydroxy-2-oxo-6-(2-ph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00700 | -63.0 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM558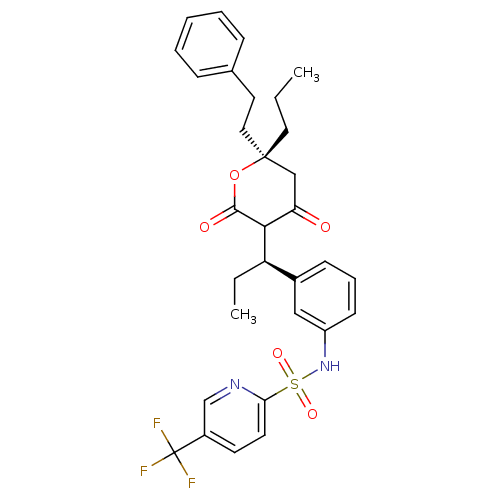 (N-{3-[(1R)-1-[(6R)-4-hydroxy-2-oxo-6-(2-phenylethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.00800 | -62.7 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM557 (N-{3-[(1R)-1-[(6S)-4-hydroxy-2-oxo-6-(2-phenylethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | -60.7 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM560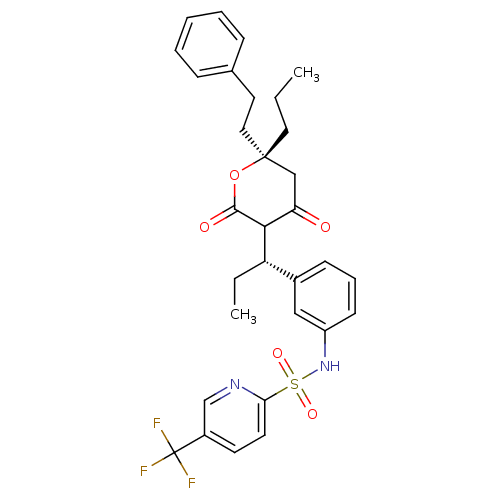 (N-{3-[(1S)-1-[(6R)-4-hydroxy-2-oxo-6-(2-phenylethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0320 | -59.3 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM553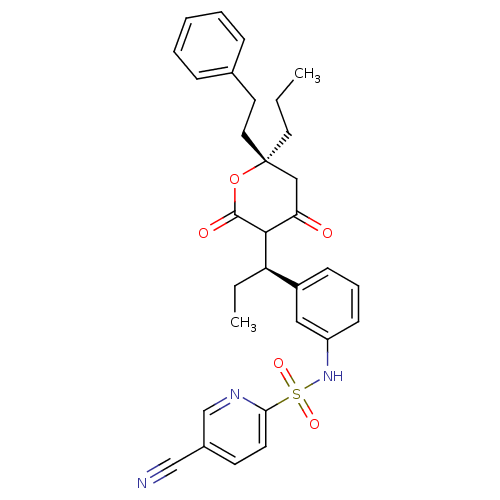 (5-cyano-N-{3-[(1R)-1-[(6S)-4-hydroxy-2-oxo-6-(2-ph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | -58.8 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM550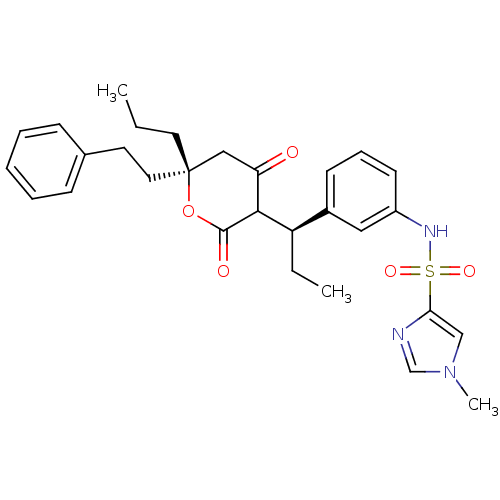 (N-{3-[(1R)-1-[(6R)-4-hydroxy-2-oxo-6-(2-phenylethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | -57.8 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM556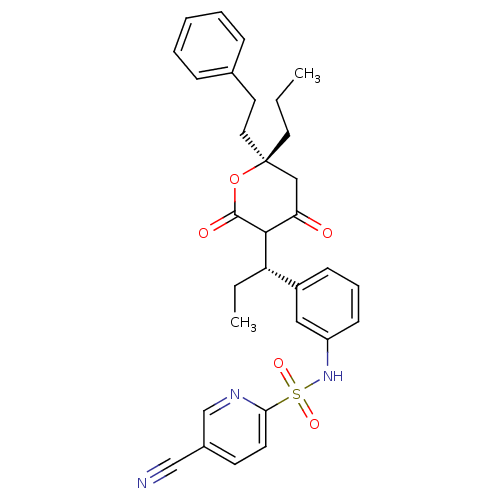 (5-cyano-N-{3-[(1S)-1-[(6R)-4-hydroxy-2-oxo-6-(2-ph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | -56.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM549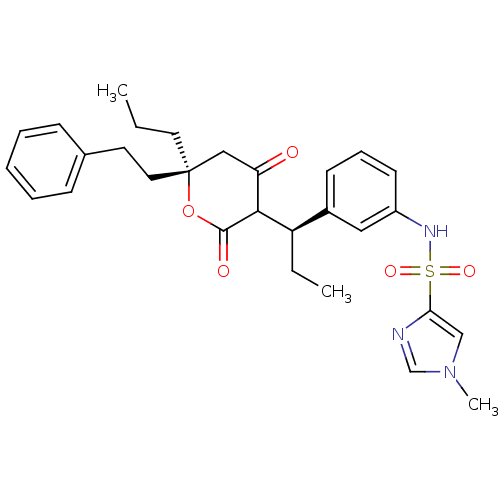 (N-{3-[(1R)-1-[(6S)-4-hydroxy-2-oxo-6-(2-phenylethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.120 | -56.1 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM555 (5-cyano-N-{3-[(1S)-1-[(6S)-4-hydroxy-2-oxo-6-(2-ph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.120 | -56.1 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM559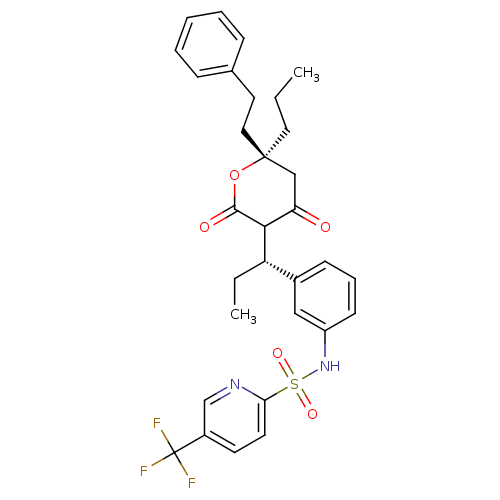 (N-{3-[(1S)-1-[(6S)-4-hydroxy-2-oxo-6-(2-phenylethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.220 | -54.6 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM552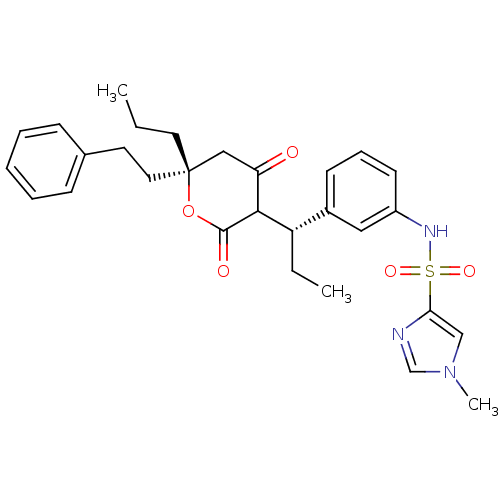 (N-{3-[(1S)-1-[(6R)-4-hydroxy-2-oxo-6-(2-phenylethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.300 | -53.8 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM548 (CHEMBL21188 | N-(3-{1-[4-hydroxy-2-oxo-6-(2-phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM551 (N-{3-[(1S)-1-[(6S)-4-hydroxy-2-oxo-6-(2-phenylethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM547 (CHEMBL20846 | N-(3-{1-[4-hydroxy-2-oxo-6-(2-phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | -48.2 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM546 (4-fluoro-N-(3-{1-[4-hydroxy-2-oxo-6-(2-phenylethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | -46.5 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM545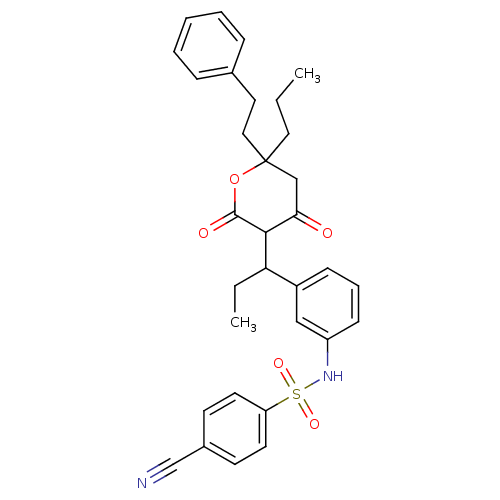 (4-cyano-N-(3-{1-[4-hydroxy-2-oxo-6-(2-phenylethyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | -45.7 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM544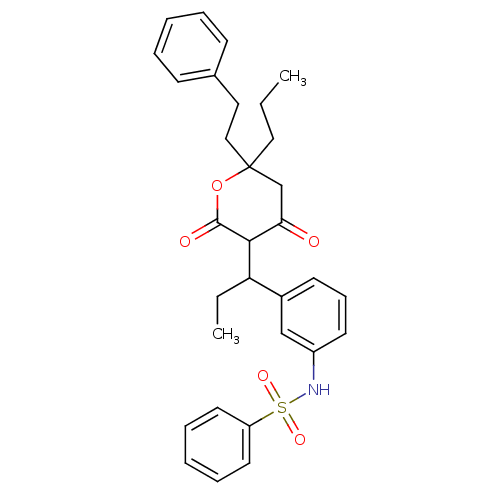 (N-(3-{1-[4-hydroxy-2-oxo-6-(2-phenylethyl)-6-propy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 17 | -43.9 | n/a | n/a | n/a | n/a | n/a | 5.0 | 22 |
Upjohn | Assay Description HIV-1 protease was purified and refolded from E. coli inclusion bodies. The substrate used spans the p17-p24 processing site (R-V-S-Q-N-Y-P-I-V-Q-N-K... | J Med Chem 39: 4349-53 (1996) Article DOI: 10.1021/jm960541s BindingDB Entry DOI: 10.7270/Q2HQ3X35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066924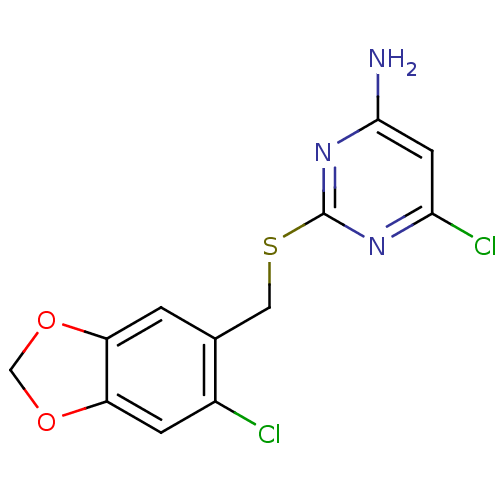 (6-Chloro-2-(6-chloro-benzo[1,3]dioxol-5-ylmethylsu...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase (P236L) | J Med Chem 41: 3793-803 (1998) Article DOI: 10.1021/jm9800806 BindingDB Entry DOI: 10.7270/Q2JS9PKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50064007 (6-CHLORO-2-(1-FURO[2,3-C]PYRIDIN-5-YL-ETHYLSULFANY...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibitory activity against E233V mutant HIV-1 reverse transcriptase | J Med Chem 41: 1357-60 (1998) Article DOI: 10.1021/jm9801049 BindingDB Entry DOI: 10.7270/Q2DJ5DS1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50064007 (6-CHLORO-2-(1-FURO[2,3-C]PYRIDIN-5-YL-ETHYLSULFANY...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibitory activity against wild type HIV-1 reverse transcriptase (WT-RT) | J Med Chem 41: 1357-60 (1998) Article DOI: 10.1021/jm9801049 BindingDB Entry DOI: 10.7270/Q2DJ5DS1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50064009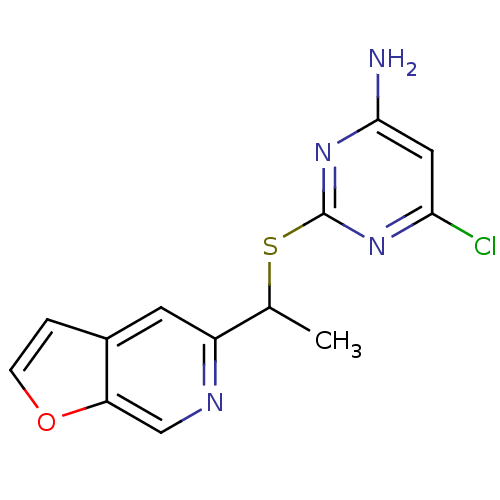 (6-Chloro-2-(1-furo[2,3-c]pyridin-5-yl-ethylsulfany...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibitory activity against P236L mutant HIV-1 reverse transcriptase | J Med Chem 41: 1357-60 (1998) Article DOI: 10.1021/jm9801049 BindingDB Entry DOI: 10.7270/Q2DJ5DS1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50064007 (6-CHLORO-2-(1-FURO[2,3-C]PYRIDIN-5-YL-ETHYLSULFANY...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibitory activity against P236L mutant HIV-1 reverse transcriptase | J Med Chem 41: 1357-60 (1998) Article DOI: 10.1021/jm9801049 BindingDB Entry DOI: 10.7270/Q2DJ5DS1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50064009 (6-Chloro-2-(1-furo[2,3-c]pyridin-5-yl-ethylsulfany...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibitory activity against wild type HIV-1 reverse transcriptase (WT-RT) | J Med Chem 41: 1357-60 (1998) Article DOI: 10.1021/jm9801049 BindingDB Entry DOI: 10.7270/Q2DJ5DS1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50064008 (6-Chloro-2-((R)-1-furo[2,3-c]pyridin-5-yl-ethylsul...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibitory activity against P236L mutant HIV-1 reverse transcriptase | J Med Chem 41: 1357-60 (1998) Article DOI: 10.1021/jm9801049 BindingDB Entry DOI: 10.7270/Q2DJ5DS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50064007 (6-CHLORO-2-(1-FURO[2,3-C]PYRIDIN-5-YL-ETHYLSULFANY...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibitory activity against L100I mutant HIV-1 reverse transcriptase | J Med Chem 41: 1357-60 (1998) Article DOI: 10.1021/jm9801049 BindingDB Entry DOI: 10.7270/Q2DJ5DS1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50064009 (6-Chloro-2-(1-furo[2,3-c]pyridin-5-yl-ethylsulfany...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibitory activity against E233V mutant HIV-1 reverse transcriptase | J Med Chem 41: 1357-60 (1998) Article DOI: 10.1021/jm9801049 BindingDB Entry DOI: 10.7270/Q2DJ5DS1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50064009 (6-Chloro-2-(1-furo[2,3-c]pyridin-5-yl-ethylsulfany...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibitory activity against L100I mutant HIV-1 reverse transcriptase | J Med Chem 41: 1357-60 (1998) Article DOI: 10.1021/jm9801049 BindingDB Entry DOI: 10.7270/Q2DJ5DS1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066931 ((E)-4-(4-Amino-6-chloro-pyrimidin-2-ylsulfanyl)-bu...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase (P236L) | J Med Chem 41: 3793-803 (1998) Article DOI: 10.1021/jm9800806 BindingDB Entry DOI: 10.7270/Q2JS9PKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066930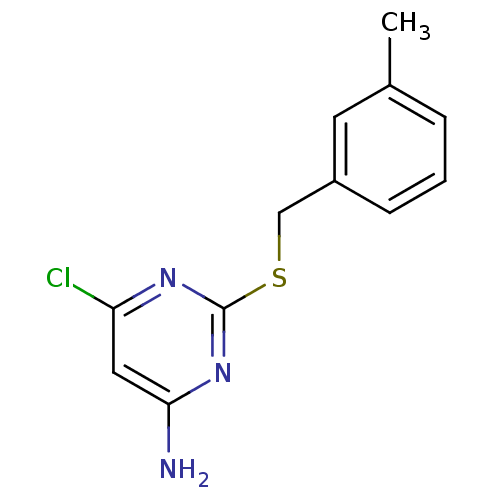 (6-Chloro-2-(3-methyl-benzylsulfanyl)-pyrimidin-4-y...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase (P236L) | J Med Chem 41: 3793-803 (1998) Article DOI: 10.1021/jm9800806 BindingDB Entry DOI: 10.7270/Q2JS9PKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50064009 (6-Chloro-2-(1-furo[2,3-c]pyridin-5-yl-ethylsulfany...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibitory activity against Y188H mutant HIV-1 reverse transcriptase | J Med Chem 41: 1357-60 (1998) Article DOI: 10.1021/jm9801049 BindingDB Entry DOI: 10.7270/Q2DJ5DS1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066931 ((E)-4-(4-Amino-6-chloro-pyrimidin-2-ylsulfanyl)-bu...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase (wild type) | J Med Chem 41: 3793-803 (1998) Article DOI: 10.1021/jm9800806 BindingDB Entry DOI: 10.7270/Q2JS9PKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066932 (6-Chloro-2-(3,4-dichloro-benzylsulfanyl)-pyrimidin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase (P236L) | J Med Chem 41: 3793-803 (1998) Article DOI: 10.1021/jm9800806 BindingDB Entry DOI: 10.7270/Q2JS9PKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066934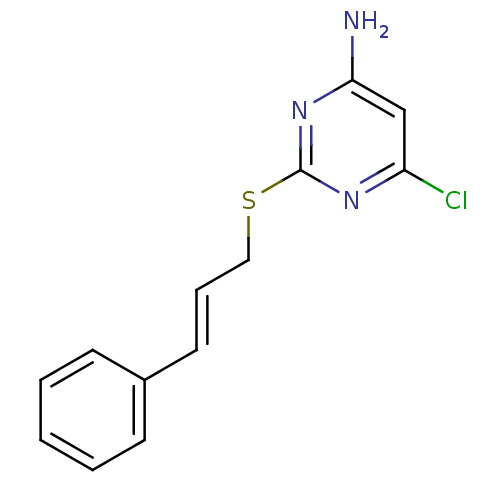 (6-Chloro-2-((E)-3-phenyl-allylsulfanyl)-pyrimidin-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase (P236L) | J Med Chem 41: 3793-803 (1998) Article DOI: 10.1021/jm9800806 BindingDB Entry DOI: 10.7270/Q2JS9PKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066937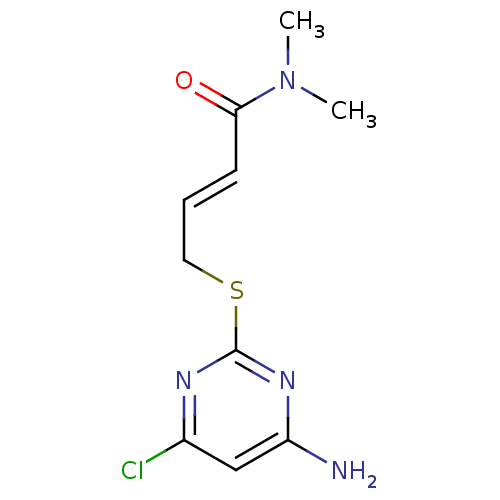 ((E)-4-(4-Amino-6-chloro-pyrimidin-2-ylsulfanyl)-bu...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase (P236L) | J Med Chem 41: 3793-803 (1998) Article DOI: 10.1021/jm9800806 BindingDB Entry DOI: 10.7270/Q2JS9PKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50064007 (6-CHLORO-2-(1-FURO[2,3-C]PYRIDIN-5-YL-ETHYLSULFANY...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibitory activity against Y188H mutant HIV-1 reverse transcriptase | J Med Chem 41: 1357-60 (1998) Article DOI: 10.1021/jm9801049 BindingDB Entry DOI: 10.7270/Q2DJ5DS1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50064008 (6-Chloro-2-((R)-1-furo[2,3-c]pyridin-5-yl-ethylsul...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibitory activity against wild type HIV-1 reverse transcriptase (WT-RT) | J Med Chem 41: 1357-60 (1998) Article DOI: 10.1021/jm9801049 BindingDB Entry DOI: 10.7270/Q2DJ5DS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066924 (6-Chloro-2-(6-chloro-benzo[1,3]dioxol-5-ylmethylsu...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description inhibitory activity against HIV-1 reverse transcriptase (wild type | J Med Chem 41: 3793-803 (1998) Article DOI: 10.1021/jm9800806 BindingDB Entry DOI: 10.7270/Q2JS9PKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066936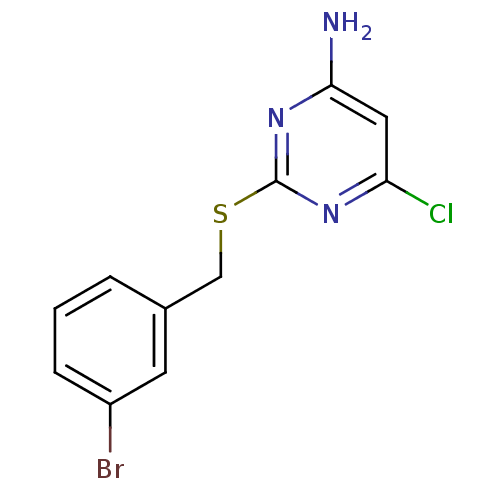 (2-(3-Bromo-benzylsulfanyl)-6-chloro-pyrimidin-4-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase (P236L) | J Med Chem 41: 3793-803 (1998) Article DOI: 10.1021/jm9800806 BindingDB Entry DOI: 10.7270/Q2JS9PKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066927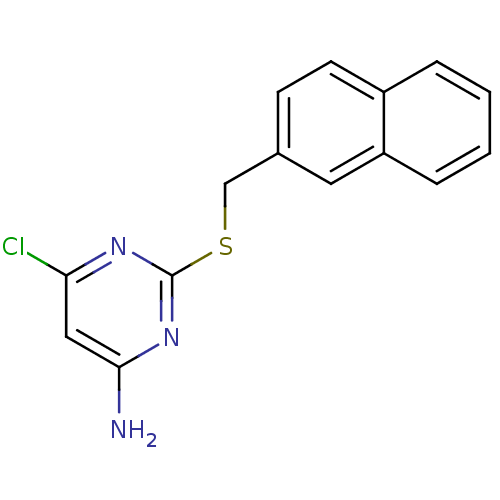 (6-Chloro-2-(naphthalen-2-ylmethylsulfanyl)-pyrimid...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase (P236L) | J Med Chem 41: 3793-803 (1998) Article DOI: 10.1021/jm9800806 BindingDB Entry DOI: 10.7270/Q2JS9PKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066925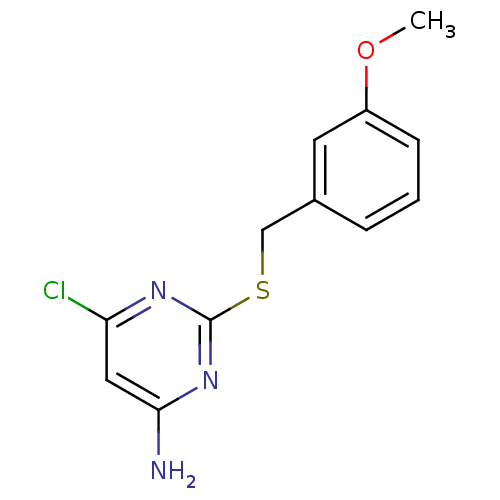 (6-Chloro-2-(3-methoxy-benzylsulfanyl)-pyrimidin-4-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase (P236L) | J Med Chem 41: 3793-803 (1998) Article DOI: 10.1021/jm9800806 BindingDB Entry DOI: 10.7270/Q2JS9PKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1944 (BHAP deriv. | CHEMBL593 | DELAVIRDINE MESYLATE | D...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase (wild type) | J Med Chem 41: 3793-803 (1998) Article DOI: 10.1021/jm9800806 BindingDB Entry DOI: 10.7270/Q2JS9PKT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50064007 (6-CHLORO-2-(1-FURO[2,3-C]PYRIDIN-5-YL-ETHYLSULFANY...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 179 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibitory activity against Y181C mutant HIV-1 reverse transcriptase | J Med Chem 41: 1357-60 (1998) Article DOI: 10.1021/jm9801049 BindingDB Entry DOI: 10.7270/Q2DJ5DS1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066934 (6-Chloro-2-((E)-3-phenyl-allylsulfanyl)-pyrimidin-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase (wild type) | J Med Chem 41: 3793-803 (1998) Article DOI: 10.1021/jm9800806 BindingDB Entry DOI: 10.7270/Q2JS9PKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066937 ((E)-4-(4-Amino-6-chloro-pyrimidin-2-ylsulfanyl)-bu...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase (wild type) | J Med Chem 41: 3793-803 (1998) Article DOI: 10.1021/jm9800806 BindingDB Entry DOI: 10.7270/Q2JS9PKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066928 (3-(4-Amino-6-chloro-pyrimidin-2-ylsulfanylmethyl)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase (P236L) | J Med Chem 41: 3793-803 (1998) Article DOI: 10.1021/jm9800806 BindingDB Entry DOI: 10.7270/Q2JS9PKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1944 (BHAP deriv. | CHEMBL593 | DELAVIRDINE MESYLATE | D...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibitory activity against wild type HIV-1 reverse transcriptase (WT-RT) | J Med Chem 41: 1357-60 (1998) Article DOI: 10.1021/jm9801049 BindingDB Entry DOI: 10.7270/Q2DJ5DS1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066930 (6-Chloro-2-(3-methyl-benzylsulfanyl)-pyrimidin-4-y...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase (wild type) | J Med Chem 41: 3793-803 (1998) Article DOI: 10.1021/jm9800806 BindingDB Entry DOI: 10.7270/Q2JS9PKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066935 (2-(Naphthalen-2-ylmethylsulfanyl)-6-trifluoromethy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase (wild type) | J Med Chem 41: 3793-803 (1998) Article DOI: 10.1021/jm9800806 BindingDB Entry DOI: 10.7270/Q2JS9PKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066928 (3-(4-Amino-6-chloro-pyrimidin-2-ylsulfanylmethyl)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase (wild type) | J Med Chem 41: 3793-803 (1998) Article DOI: 10.1021/jm9800806 BindingDB Entry DOI: 10.7270/Q2JS9PKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50064009 (6-Chloro-2-(1-furo[2,3-c]pyridin-5-yl-ethylsulfany...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description Inhibitory activity against Y181C mutant HIV-1 reverse transcriptase | J Med Chem 41: 1357-60 (1998) Article DOI: 10.1021/jm9801049 BindingDB Entry DOI: 10.7270/Q2DJ5DS1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 73 total ) | Next | Last >> |